EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document 01991L0068-20060215
Council Directive of 28 January 1991 on animal health conditions governing intra-Community trade in ovine and caprine animals (91/68/EEC)
Consolidated text: Council Directive of 28 January 1991 on animal health conditions governing intra-Community trade in ovine and caprine animals (91/68/EEC)
Council Directive of 28 January 1991 on animal health conditions governing intra-Community trade in ovine and caprine animals (91/68/EEC)
1991L0068 — EN — 15.02.2006 — 010.001
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
COUNCIL DIRECTIVE of 28 January 1991 on animal health conditions governing intra-Community trade in ovine and caprine animals (OJ L 046, 19.2.1991, p.19) |
Amended by:
|
|
|
Official Journal |
||
|
No |
page |
date |
||
|
L 74 |
42 |
17.3.1994 |
||
|
L 371 |
14 |
31.12.1994 |
||
|
L 102 |
63 |
12.4.2001 |
||
|
Directive 2001/10/EC of the European Parliament and of the Council of 22 May 2001 |
L 147 |
41 |
31.5.2001 |
|
|
L 91 |
31 |
6.4.2002 |
||
|
L 122 |
1 |
16.5.2003 |
||
|
L 169 |
51 |
8.7.2003 |
||
|
L 258 |
11 |
10.10.2003 |
||
|
L 248 |
1 |
22.7.2004 |
||
|
L 340 |
68 |
23.12.2005 |
||
Amended by:
|
C 241 |
21 |
29.8.1994 |
||
|
|
(adapted by Council Decision 95/1/EC, Euratom, ECSC) |
L 001 |
1 |
.. |
COUNCIL DIRECTIVE
of 28 January 1991
on animal health conditions governing intra-Community trade in ovine and caprine animals
(91/68/EEC)
THE COUNCIL OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Economic Community, and in particular Article 43 thereof,
Having regard to the proposal from the Commission ( 1 ),
Having regard to the opinion of the European Parliament ( 2 ),
Having regard to the opinion of the Economic and Social Committee ( 3 ),
Whereas the harmonious operation of the common organization of the market in ovine and caprine animals will not have the results expected of it as long as disparities between Member States as regards health conditions act as a restraint on intra-Community trade;
Whereas in order to encourage such trade it is advisable to remove those disparities and introduce Community-wide rules on the marketing of ovine and caprine animals in such trade; whereas that objective will also contribute to the completion of the single market;
Whereas in order to be eligible for intra-Community trade, ovine and caprine animals should meet certain animal health requirements designed to avoid the spread of infectious or contagious diseases;
Whereas the animal health requirements applicable should vary depending on the purpose for which the animals are traded;
Whereas the health situation for ovine and caprine animals is not the same throughout the territory of the Community; whereas reference should therefore be made to ‘regions’ as defined in Council Directive 64/432/EEC of 26 June 1964 on animal health problems affecting intra-Community trade in bovine animals and swine ( 4 ), as last amended by Directive 90/425/EEC ( 5 ), when dealing with parts of that territory;
Whereas there must be no barriers to trade between regions in which the animal health conditions are equivalent;
Whereas provision should be made for allowing the Commission to approve certain additional requirements in the light of the progress made by a Member State in eradicating certain diseases, provided that those requirements in no case exceed those applied nationally by the Member State concerned;
Whereas in order to avoid the spread of infectious or contagious diseases, conditions should be laid down as regards the transportation of the animals to their place of destination;
Whereas in order to ensure that the requirements applicable are complied with, provision should be made for introducing a health certificate which would be issued by an official veterinarian and which would accompany ovine and caprine animals until they reach their place of destination;
Whereas reference should be made, as regards the organization and follow up of the checks to be carried out by the Member States and as regards the protective measures to be introduced, to the general rules laid down in Council Directive 90/425/EEC of 26 June 1990 concerning veterinary and zootechnical checks applicable in intra-Community trade in certain live animals and products with a view of the completion of the internal market;
Whereas provision should be made for allowing the Commission to conduct its own checks;
Whereas a procedure should be introduced which provides for close and efficient cooperation between the Member States and the Commission within the Standing Veterinary Committee,
HAS ADOPTED THIS DIRECTIVE:
Article 1
This Directive defines the animal health conditions governing intra-Community trade in ovine and caprine animals.
Article 2
(a) The definitions given in Article 2 of Directive 90/425/EEC and in Article 2 of Directive 91/628/EEC of 19 November 1991 on the protection of animals during transport and amending Directives 90/425/EEC and 91/496/EEC ( 6 ) shall apply as far as applicable.
(b) In addition, the following definitions apply for the purposes of this Directive:
1. ‘ovine or caprine animals for slaughter’ means animals of the ovine or caprine species intended to be taken either directly or via an approved assembly centre to a slaughterhouse in order to be slaughtered;
2. ‘ovine or caprine animals for breeding’ means ovine and caprine animals other than those mentioned in points 1 and 3 intended to be transported to the place of destination, either directly or via an approved assembly centre, for breeding and production purposes;
3. ‘ovine or caprine animals for fattening’ means ovine and caprine animals other than those mentioned in points 1 and 2 intended to be transported to the place of destination, either directly or via an approved assembly centre, in order to be fattened for subsequent slaughter;
4. ‘officially brucellosis-free ovine or caprine holding’ means a holding which satisfies the conditions laid down in Section I of Chapter 1 of Annex A;
5. ‘brucellosis-free ovine or caprine holding’ means a holding which satisfies the conditions laid down in Chapter 2 of Annex A;
6. ‘compulsorily notifiable disease’ means a disease listed under Section I of Annex B;
7. ‘official veterinarian’ means a veterinarian designated by the competent central authority of the Member State;
8. ‘holding of origin’ means any holding on which the ovine and caprine animals have been continuously present as required by this Directive and on which records are maintained demonstrating the residence of the animals which may be audited by the competent authorities;
9. ‘assembly centre’ means collection centres and markets, at which under the supervision of the official veterinarian ovine and caprine animals originating from different holdings are grouped together to form consignments of animals for national movement;
10. ‘approved assembly centre’ means premises on which ovine or caprine animals originating from different holdings are grouped together to form consignments of animals intended for intra-Community trade;
11. ‘dealer’ means any natural or legal person who buys and sells animals commercially either directly or indirectly, who has a turnover of these animals and who within a maximum of 29 days of purchasing animals resells them or relocates them from the first premises to other premises or directly to a slaughterhouse not within his ownership;
12. ‘approved dealer's premises’ means premises operated by a dealer as defined in point 11 and approved by the competent authorities at which ovine or caprine animals originated from different holdings are grouped together to form consignments of animals intended for intra-Community trade;
13. ‘transporter’ means any natural or legal person referred to in Article 5 of Directive 91/628/EEC;
14. ‘region’means that part of a Member State's territory which is at least 2 000 km2 in area and which is subject to inspection by the competent authorities and includes at least one of the following administrative regions:
|
— Belgium: |
province/provincie |
|
— Germany: |
Regierungsbezirk |
|
— Denmark: |
amt or island |
|
— France: |
département |
|
— Italy: |
provincia |
|
— Luxembourg |
— |
|
— Netherlands: |
RVV-kring |
|
— United Kingdom: |
|
|
England, Wales and Northern Ireland: |
county |
|
Scotland: |
district or island area |
|
— Ireland: |
county |
|
— Greece: |
νομός |
|
— Spain: |
provincia |
|
— Portugal: |
|
|
continent: |
distrito |
|
other parts of Portugal's territory: |
região autónoma |
|
— Austria: |
Bezirk |
|
— Sweden: |
län |
|
— Finland: |
lääni/län. |
Article 3
(1) Ovine and caprine animals for slaughter may be the subject of trade only if they fulfil the conditions laid down in Articles 4, 4a, 4b and 4c.
(2) Ovine and caprine animals for fattening may be the subject of trade only if they fulfil the conditions laid down in Articles 4, 4a, 4b and 5, without prejudice to any additional guarantees which may be required pursuant to Articles 7 and 8.
(3) Ovine and caprine animals for breeding may be the subject of trade only if they fulfil the conditions laid down in Articles 4, 4a, 4b, 5 and 6, without prejudice to any additional guarantees which may be required pursuant to Articles 7 and 8.
(4) By way of derogation from the provisions in paragraphs 2 and 3, the competent authorities of Member States of destination may grant general or limited derogations in respect of movement of ovine and caprine animals for breeding and fattening, intended solely for temporary pasturing near internal borders of the Community. Member States making use of such derogation shall inform the Commission of the content of the derogations granted.
(5) Ovine and caprine animals covered by this Directive must at no time between leaving the holding of origin and arriving at destination come into contact with cloven-hoofed animals other than animals that have the same health status.
Article 4
(1) Member States shall ensure that ovine and caprine animals:
(a) are identified and registered in accordance with Community legislation;
(b) are inspected by an official veterinarian during the 24 hours preceding the loading of the animals, and show no clinical sign of disease;
(c) do not come from a holding, nor have been in contact with animals from a holding, which is the subject of a prohibition on animal health grounds; the period of such prohibition shall last after the slaughter and/or the disposal of the last animal suffering from or susceptible to one of the diseases referred to in points (i), (ii) or (iii), for at least:
(i) 42 days in the case of brucellosis,
(ii) 30 days in the case of rabies,
(iii) 15 days in the case of anthrax;
(d) do not come from a holding, nor have been in contact with animals from a holding, situated in an area which for health reasons is subject to a prohibition or restriction affecting the species involved in accordance with Community and/or national legislation;
(e) are not the subject of animal health restrictions pursuant to Community legislation on foot-and-mouth disease nor have they been vaccinated against foot-and-mouth disease.
(2) Member States shall ensure that the following animals are not the subject of trade:
(a) ovine and caprine animals which may have to be slaughtered under a national programme for the eradication of diseases not referred to in Annex C to Directive 90/425/EEC or in Chapter I of Annex B to this Directive;
(b) ovine and caprine animals which cannot be marketed on their own territory for health or animal health reasons justified by Article 30 of the Treaty.
(3) Member States shall ensure that ovine and caprine animals shall:
(a) either have been born and reared since birth in the Community, or
(b) have been imported from a third country in accordance with Community legislation.
Article 4a
(1) Member States shall ensure that ovine and caprine animals for slaughter, breeding and fattening are not dispatched to another Member State, unless the animals:
(a) have been continuously resident on the holding of origin for at least 30 days, or since birth if the animals are younger than 30 days of age,
(b) do not come from a holding into which ovine or caprine animals have been introduced during the 21 days prior to dispatch,
(c) do not come from a holding into which biungulate animals imported from a third country have been introduced during the 30 days prior to dispatch.
(2) By way of derogation from paragraphs 1(b) and (c), Member States may authorise the dispatch of ovine and caprine animals to another Member State, if the animals referred to in paragraphs 1(b) and (c) have been completely isolated from all other animals on the holding.
Article 4b
(1) Member States shall ensure that the conditions set out in paragraphs 2 to 6 are applied to intra-Community trade in all ovine and caprine animals.
(2) The animals shall not be outside their holding of origin for more than six days before being last certified for trade to the final destination in another Member State as indicated in the health certificate.
Without prejudice to Article 9(1) in the case of transport by sea, the time limit of six days shall be prolonged by the duration of the sea journey.
(3) After leaving the holding of origin the animals shall be consigned directly to the destination in another Member State.
(4) By way of derogation from paragraph 3, ovine and caprine animals may, after leaving the holding of origin and before arrival at destination in another Member State, transit through only one approved assembly centre situated in the Member State of origin.
In the case of ovine and caprine animals for slaughter, the approved assembly centre may be substituted by approved dealer's premises situated in the Member State of origin.
(5) Animals for slaughter which have been taken on arrival in the Member State of destination to a slaughterhouse, must be slaughtered there as soon as possible but at least within 72 hours of arrival.
(6) Without prejudice to Article 3(5), Member States shall ensure that the animals covered by this Directive at no time, between leaving the holding of origin and their arrival at destination, compromise the health status of ovine and caprine animals not intended for intra-Community trade.
Article 4c
(1) By way of derogation from Article 4a(1)(a), ovine and caprine animals for slaughter may be subject to trade after they have been continuously resident on the holding of origin for at least 21 days.
(2) By way of derogation from Article 4a(1)(b), and without prejudice to paragraph 1 and Article 4b(2), ovine and caprine animals for slaughter may be consigned from a holding of origin into which ovine or caprine animals have been introduced during the 21 days prior to dispatch, if they are transported directly to a slaughterhouse in another Member State for immediate slaughter without passing through an assembly centre or staging point established in accordance with Directive 91/628/EEC.
(3) By way of derogation from Article 4b(3) and (4), and without prejudice to the provisions in Article 4b(2), ovine and caprine animals for slaughter may, after leaving the holding of origin, pass through one additional assembly centre under the following alternative conditions:
(a) the animals, before passing through the approved assembly centre referred to in Article 4b(4) which is situated in the Member State of origin, comply with the following conditions:
(i) after leaving the holding of origin the animals pass through one single assembly centre under official veterinary supervision, which permits at the same time only animals of at least the same health status,
(ii) without prejudice to Community legislation on identification of sheep and goats, at the latest at that assembly centre the animals are individually identified so as to enable in each case the tracing of the holding of origin and
(iii) from the assembly centre the animals are, accompanied by an official veterinary document, transported to the approved assembly centre referred to in Article 4b(4) to be certified and consigned directly to a slaughterhouse in the Member State of destination;
or
(b) the animals may after dispatch from the Member State of origin transit through one approved assembly centre before being consigned to the slaughterhouse in the Member State of destination under the following conditions:
(i) either the approved assembly centre is situated in the Member State of destination from where the animals must be removed under the responsibility of the official veterinarian directly to a slaughterhouse to be slaughtered within five days of arrival at the approved assembly centre, or
(ii) the approved assembly centre is situated in one Member State of transit from where the animals are consigned directly to the slaughterhouse in the Member State of destination indicated in the animal health certificate issued in accordance with Article 9(6).
Article 5
Without prejudice to the additional guarantees that may be required in accordance with Articles 7 and 8, ovine and caprine animals for breeding and fattening must, in addition to the conditions laid down in Article 4, meet — in order to be introduced onto an officially brucellosis-free or brucellosis-free ovine or caprine holding — respectively the requirements of Chapter I.D or Chapter 2.D of Annex A.
Article 6
Without prejudice to the additional guarantees that may be required in accordance with Articles 7 and 8, animals for breeding must furthermore meet the following requirements:
(a) They must have been obtained from a holding and must only have been in contact with animals from such a holding:
(i) in which the following diseases have not been clinically diagnosed:
— in the previous six months, contagious agalactia of sheep (Mycoplasma agalactiae) or contagious agalactia of goats (Mycoplasma agalactiae, M. capricolum, M. Mycoïdes var. mycoïdes‘large colony’),
— in the previous 12 months, paratuberculosis or caseous lymphadenitis,
— in the previous three years, pulmonary adenomatosis, Maedi Visna or caprine viral arthritis/encephalitis. However, this period shall be reduced to 12 months if the animals infected with Maedi Visna or caprine viral arthritis/encephalitis have been slaughtered and the remaining animals have reacted negatively to two tests recognized under the procedure set out in Article 15,
or which, without prejudice to compliance with the requirements for other diseases, provides, for one or more of the abovementioned diseases, within the framework of a programme approved in accordance with Articles 7 and 8, health guarantees which are equivalent for the said disease or diseases;
(ii) where no facts suggesting that the requirements of point (i) have not been met, have been brought to the attention of the official veterinarian responsible for issuing the health certificate;
(iii) whose owner states that he has no knowledge of any such facts and, moreover, states in writing that the animal or animals intended for intra-Community trade comply with the criteria laid down in point (i);
▼M4 —————
(c) whith regard to contagious epidydimitis (B. ovis), non castrated rams, for breeding, must:
— come from a holding where no case of contagious epidydimitis (B. ovis) has been diagnosed in the preceding 12 months,
— have been continuously kept on that holding for 60 days prior to dispatch,
— in the 30 days prior to dispatch have undergone, with negative results, a serological test carried out in accordance with Annex D or satisfy equivalent health guarantees to be recognized under the procedure laid down in Article 15;
(d) the certificate corresponding to Model III of Annex E states that these requirements have been met.
Article 7
1. A Member State which has a compulsory or voluntary national control programme or a national monitoring programme for one of the infectious or contagious diseases ►M4 referred to in Annex B, Section III ◄ for all or part of its territory may submit the said programme to the Commission, outlining in particular:
— the distribution of the disease in the Member State,
— the reasons for the programme, taking into consideration the importance of the disease and the programme's likely benefit in relation to its cost,
— the geographical area in which the programme will be implemented,
— the various status categories to be applied to the holdings, the standards which must be attained in each category, and the test procedures to be used,
— the programme monitoring procedures,
— the action to be taken if, for any reason, a holding loses its status,
— the measures to be taken if the results of the tests carried out in accordance with the provisions of the programme are positive.
2. The Commission shall examine the programmes presented by the Member States. Programmes as referred to in paragraph 1 may be approved, in compliance with the criteria laid down in paragraph 1, in accordance with the procedure provided for in Article 15. According to the same procedure, the additional guarantees, general or limited, which may be required in intra-Community trade, shall be defined at the same time or at the latest three months after approval of the programmes. Such guarantees must not exceed those which the Member State implements nationally.
3. Programmes submitted by Member States may be amended or supplemented in accordance with the procedure laid down in Article 15. Amendments or additions to programmes which have already been approved or to guarantees which have been defined in accordance with paragraph 2 may be approved under the same procedure.
4. Programmes approved in accordance with this Article shall benefit from the Community funding provided for in Article 24 of Council Decision 90/424/EEC of 26 June 1990 on expenditure in the veterinary field ( 7 ) for the diseases and under the conditions laid down therein.
Article 8
1. Where a Member State considers that its territory or part of its territory is free from one of the diseases ►M4 listed in Annex B, Section III ◄ to which ovine and caprine animals are susceptible, it shall present to the Commission appropriate supporting documentation, setting out in particular:
— the nature of the disease and the history of its occurrence in its territory,
— the results of surveillance testing based on serological, microbiological, pathological or epidemiological investigation and on the fact that the disease must by law be notified to the competent authorities,
— the period over which the surveillance was carried out,
— where applicable, the period during which vaccination against the disease has been prohibited and the geographical area concerned by such prohibition,
— the arrangements for verifying the absence of the disease.
2. The Commission shall examine supporting documentation submitted by Member States. The additional guarantees, general or limited, which may be required in intra-Community trade shall be defined in accordance with the procedure laid down in Article 15. Such guarantees must not exceed those which the Member State implements nationally. Where supporting documentation is submitted before 1 January 1992, decisions on additional guarantees shall be taken before 1 July 1992.
3. The Member State concerned shall notify the Commission of any change in the supporting documentation specified in paragraph 1 which relate to the disease. The guarantees defined as laid down in paragraph 2 may, in the light of such notification, be amended or withdrawn in accordance with the procedure laid down in Article 15.
4. The Commission shall examine as quickly as possible the grounds submitted by Sweden as regards ovine paratuberculosis and ovine contagious agalactia. Following that examination and if it is justified, the provisions of paragraph 2 may be applicable. The appropriate decisions provided for in paragraph 2 shall be adopted as quickly as possible. Pending those decisions Sweden may, during a period of one year from the date of entry into force of the Accession Treaty, apply its national rules in force before that date as regards the abovementioned diseases. The period of one year may if necessary be extended in accordance with the procedure laid down in Article 15.
Article 8a
(1) Member States shall ensure that, in order to be approved by the competent authority, assembly centres must at least:
(a) be under the control of an official veterinarian who shall ensure that, in particular, the provisions of Article 3(5) are complied with;
(b) be located in an area which is not subject to prohibition or restrictions in accordance with relevant Community legislation and/or national legislation;
(c) be cleaned and disinfected before use, as required by the official veterinarian;
(d) have, taking into account the animal capacity of the assembly centre:
— a facility dedicated exclusively for this purpose when used as an assembly centre,
— appropriate facilities for loading, unloading and adequate housing of a suitable standard for the animals, for watering and feeding them, and for giving them any necessary treatment; these facilities must be easy to clean and disinfect,
— appropriate inspection facilities,
— appropriate isolation facilities,
— appropriate equipment for cleaning and disinfecting rooms and trucks,
— an appropriate storage area for fodder, litter and manure,
— an appropriate system for collecting waste water,
— the use of an office for the official veterinarian;
(e) admit only animals which are identified in accordance with Community legislation and comply with the animal health conditions set down in this Directive for the category of animals concerned. To this end, when animals are admitted, the owner or person in charge of the centre shall ensure they are accompanied by health documents or appropriate certificates for the species and categories involved;
(f) be regularly inspected by the competent authority in order to ascertain that the requirements for approval continue to be fulfilled.
(2) The owner or person in charge of the assembly centre shall be required, on the basis either of the accompanying documents for the animals or of the identification numbers or marks of the animals, to record on a register or a database and retain for a minimum period of three years the following information:
— the name of the owner, the origin, date of entry and exit, number and identification of the ovine and caprine animals or the registration number of the holding of origin of the animals entering the centre, where applicable the approval or registration number of the assembly centre through which the animals have passed prior to entering the centre and their proposed destination,
— the registration number of the transporter and the licence number of the lorry delivering or collecting animals from the centre.
(3) The competent authority shall issue an approval number to each approved assembly centre. Such approval may be limited to one or the other species covered by this Directive or to animals for breeding or fattening or to animals for slaughter. The competent authority shall notify the Commission of the list of approved assembly centres and of any updates. The Commission shall present this list to Member States in the framework of the Committee referred to in Article 15(1).
(4) The competent authority may suspend or withdraw approval in the event of failure to comply with this Article or other appropriate provisions of this Directive or other directives in respect of health restrictions. Approval may be restored when the competent authority is satisfied that that assembly centre is in full compliance with all the appropriate provisions of this Directive.
(5) The competent authority shall ensure that, when operating, assembly centres have sufficient approved veterinarians to carry out all duties.
(6) Any detailed rules required for uniform application of this Article shall be adopted in accordance with the procedure referred to in Article 15(2).
Article 8b
(1) Member States shall ensure that all dealers are registered and, for the purpose of intra-Community trade, approved and issued with an approval number by the competent authority and that approved dealers comply with at least the following conditions:
(a) they must deal only in animals which are identified and come from holdings that conform with the conditions set out in Article 3. To this end, the dealer shall ensure that the animals are properly identified and are accompanied by health documents as appropriate in accordance with this Directive;
(b) the dealer shall be required, either on the basis of the document accompanying the animals, or on the basis of identification numbers or marks on the animals, to keep a record or database and to store the following data for at least three years:
— the name of the owner, origin, date of purchase, categories, number and identification of ovine and caprine animals or registration number of the holding of origin of the animals purchased, where applicable, the approval or registration number of the assembly centre through which the animals have passed prior to purchase and their destination,
— the registration number of the transporter and/or the licence number of the lorry delivering and collecting animals,
— the name and address of the purchaser and the destination of the animal,
— copies of route plans and/or serial number of health certificates as applicable;
(c) when the dealer keeps animals on his premises he shall ensure that:
— specific training is given to the staff in charge of the animals in applying the requirements of this Directive and in the care and welfare of the animals,
— any necessary controls and tests on the animals are carried out regularly by the official veterinarian and that all necessary steps are taken to prevent the spread of disease.
(2) Member States shall ensure that all premises used by a dealer in connection with his business are registered and issued with an approval number by the competent authority and that they comply with at least the following conditions:
(a) they must be under the control of an official veterinarian;
(b) they must be located in an area which is not subject to prohibition or restrictions in accordance with relevant Community or national legislation;
(c) they must have:
— appropriate facilities of sufficient capacity and, in particular, inspection facilities and isolation facilities so that all animals can be isolated in the event of an outbreak of a contagious disease,
— appropriate facilities for unloading and where necessary adequate housing of a suitable standard for the animals, for watering and feeding them, and for giving them any necessary treatment; these facilities must be easy to clean and disinfect,
— an appropriate reception area for litter and manure,
— an appropriate system for collecting waste water;
(d) they must be cleaned and disinfected before use, as required by the official veterinarian.
(3) The competent authority may suspend or withdraw approval in the event of failure to comply with this Article or other appropriate provisions of this Directive or other Directives in respect of health restrictions. Approval may be restored when the competent authority is satisfied that the dealer is in full compliance with all the appropriate provisions of this Directive.
(4) The competent authority must carry out regular inspections in order to ascertain that the requirements of this Article are fulfilled.
Article 8c
(1) Member States shall ensure that the transporters referred to in Article 5 of Directive 91/628/EEC meet the following additional conditions:
(a) for the carriage of animals they must use means of transport which are:
— constructed in such a way that the animal faeces, litter or feed cannot leak or fall out of the vehicle,
— cleaned and disinfected immediately after every animal transport or that of any product which could affect animal health and if necessary before any new loading of animals, using disinfectants officially authorised by the competent authority;
(b) they must either have appropriate cleaning and disinfection facilities approved by the competent authority, including facilities for storing litter and dung, or they must provide documentary evidence that these operations are performed by a third party approved by the competent authority.
(2) The transporter must ensure that for each vehicle used for the transport of animals a register is kept containing at least the following information which shall be kept for a minimum period of three years:
(i) places and dates of pick-up, and the name or business name and address of the holding or assembly centre where the animals are picked up,
(ii) places and dates of delivery, and the name or business name and address of the consignee(s),
(iii) species and number of animals carried,
(iv) date and place of disinfection,
(v) details of accompanying documentation, number, etc.
(3) Transporters shall ensure that the consignment or animals do not at any time, between leaving the holdings or the assembly centre of origin and arriving at their destination, come into contact with animals of a lower health status.
(4) Member States shall ensure that transporters give a written undertaking stating in particular that:
— all the measures necessary to comply with this Directive shall be taken and in particular the provisions laid down in this Article and relating to the appropriate documentation that must accompany the animals,
— the transport of animals is entrusted to staff who possess the necessary ability, professional competence and knowledge.
(5) Article 18 of Directive 91/628/EEC shall apply in a like manner in case of infringement of this Article.
Article 9
(1) Ovine and caprine animals must be accompanied during transportation to destination by a health certificate conforming to either model I, II or III set out in Annex E, as appropriate. The certificate shall consist of a single sheet or, where more than one page is required, shall be in such a form that any two or more pages are part of an integrated whole and indivisible and shall contain a serial number. It shall be drawn up on the day of the health inspection, in one of the official languages of the country of destination at least. The certificate shall be valid for 10 days from the date of the health inspection.
(2) The health inspection for the issuing of the health certificate, including additional guarantees, for a consignment of animals may be carried out in the holding of origin or in an approved assembly centre or, in the case of animals for slaughter, on approved dealer's premises. For this purpose the competent authority shall ensure that any certificate is drawn up by the official veterinarian after inspections, visits and controls as provided by this Directive.
(3) The official veterinarian for the assembly centre shall carry out all necessary checks on animals arriving there.
(4) For ovine and caprine animals for fattening and breeding dispatched to another Member State from an approved assembly centre located in the Member State of origin, the health certificate referred to in paragraph 1, conforming to either model II or III set out in Annex E as appropriate, may only be issued on the basis of the checks provided for in paragraph 3 and of an official document containing the necessary information completed by the official veterinarian responsible for the holding of origin.
(5) For ovine and caprine animals for slaughter dispatched to another Member State from an approved assembly centre or from an approved dealer's premises located in the Member State of origin, the health certificate referred to in paragraph 1, conforming to model I set out in Annex E, may only be issued on the basis of the checks provided for in paragraph 3 and of an official document containing the necessary information completed by the official veterinarian responsible for the holding of origin or the assembly centre referred to in Article 4c(3)(a)(i).
(6) For ovine and caprine animals for slaughter passing through an approved assembly centre in accordance with Article 4c(3)(b)(ii), the official veterinarian responsible for the approved assembly centre in the Member State of transit shall provide certification to the Member State of destination by issuing a second health certificate, conforming to model I set out in Annex E, completing it with the requested data from the original health certificate(s) and attaching to it an officially endorsed copy thereof. In this case, the combined validity of the certificates shall not exceed that provided for in paragraph 1.
(7) The official veterinarian issuing a health certificate for intra-Community trade conforming to either model I, II or III set out in Annex E as appropriate, shall ensure that the movement is recorded in the ANIMO system on the day the certificate is issued.
Article 10
1. The rules laid down in Directive 90/425/EEC shall apply, in particular to checks at origin, to the organization of, and follow-up to, the checks to be carried out by the Member State of destination, and to the protective measures to be implemented.
2. In Annex A, heading I of Directive 90/425/EEC the following reference is added:
‘Council Directive 91/68/EEC of 28 January 1991 on animal health conditions governing intra-Community trade in ovine and caprine animals,
OJ No L 46, 19. 2. 1991, p. 19.’
3. In Annex B point A of Directive 90/425/EEC, the first indent is deleted.
Article 11
1. Veterinary experts from the Commission may, to the extent necessary to ensure uniform application of this Directive and in cooperation with the competent national authorities, carry out on-the-spot inspections. The Member State on whose territory inspections are carried out shall afford all necessary aid to the experts in the accomplishment of their task. The Commission shall inform the Member States of the outcome of such inspections.
2. General arrangements for the application of this Article shall be adopted in accordance with the procedure laid down in Article 15.
The rules applicable in respect of the inspections referred to in this Article shall be laid down in accordance with the same procedure.
Article 12
Member States which implement an alternative control system providing guarantees equivalent to those laid down in Article 5 and Article 6 (a) and (c) as regards movements within their territory of ovine and caprine animals may grant one another derogations from the inspection provided for under Article 4 (1) (b) and the obligation to produce a certificate provided for under Article 9 on a reciprocal basis. They shall notify the Commission thereof.
▼M7 —————
Article 14
(1) Annex A shall be amended by the Council, acting by a qualified majority on a proposal from the Commission.
(2) Annexes B, C, D and E shall be amended in accordance with the procedure referred to in Article 15(2).
(3) The rules for the implementation of this Directive shall be adopted in accordance with the procedure referred to in Article 15(2).
Article 15
1. The Commission shall be assisted by the Standing Committee on the Food Chain and Animal Health set up pursuant to Article 58 of Regulation (EC) No 178/2002 ( 8 ).
2. Where reference is made to this Article, Articles 5 and 7 of Decision 1999/468/EC ( 9 ) shall apply.
The period laid down in Article 5(6) of Decision 1999/468/EC shall be set at three months.
3. The Committee shall adopt its Rules of Procedure.
▼M7 —————
Article 17
1. Member States shall bring into force the laws, regulations and administrative provisions necessary to comply with:
(i) Articles 7 and 8 of this Directive two months after the notification date thereof, it being understood that the corresponding national provisions shall continue to apply until the approval of the programmes and in the absence of programmes until the date referred to in (ii);
(ii) the other provisions of this Directive not later than 31 December 1992.
2. When Member States adopt these measures, they shall contain a reference to this Directive or shall be accompanied by such reference on the occasion of their official publication. The methods of making such a reference shall be laid down by the Member States.
Article 18
This Directive is addressed to the Member States.
ANNEX A
CHAPTER 1
I. Officially brucellosis (B. melitensis)-free ovine or caprine holding
A. Grant of status
An officially brucellosis (B. melitensis)-free ovine or caprine holding means
1. a holding:
(a) in which all the animals which are susceptible to brucellosis (B. melitensis) have been free from clinical or any other signs of brucellosis (B. melitensis) for at least 12 months;
(b) which contains no ovine or caprine animals which have been vaccinated against brucellosis (B. melitensis), save those vaccinated at least two years previously with Rev. 1 vaccine or any other vaccine approved under the procedure laid down in Article 15 of this Directive;
(c) in which two tests separated by an interval of six months or more have been carried out, with negative results, in accordance with Annex C on all ovine and caprine animals on the holding over six months of age at the time of testing; and
(d) in which, following the tests referred to in point (c), there are only ovine or caprine animals born on the holding or which have come from an officially brucellosis-free or brucellosis-free holding under the conditions laid down in point D,
and in which, after qualification, the requirements laid down in point B continue to be fulfilled;
2. a holding situated in an officially recognized brucellosis-free Member State or region in accordance with point II.
B. Maintenance of status
1. In the case of officially brucellosis (B. melitensis)-free ovine or caprine holdings which are not situated in a part of the territory which is recognized as officially brucellosis-free, and in which, after qualification, the introduction of animals is carried out in accordance with the requirements of point D, a representative number of the ovine and caprine animals over six months old must be checked annually. The holding may retain its officially brucellosis (B. melitensis)-free status if the results of the tests are negative.
The representative number of animals to be tested must, for each holding, consist of the following:
— all non-castrated male animals over six months old,
— all animals brought onto the holding since the previous test,
— 25 % of the females which have reached the age of reproduction (i.e. which are sexually mature) or are in milk, with a minimum of 50 per holding — except in holdings where there are fewer than 50 such females, in which case all females must be tested.
2. For a region which is not officially brucellosis-free where more than 99 % of the ovine or caprine holdings are declared to be officially brucellosis (B. melitensis)-free, the frequency of checks of officially brucellosis-free ovine or caprine holdings may be extended to three years, provided that the holdings which are not officially brucellosis free are placed under official control or undergo an eradication programme.
C. Suspected or actual cases of brucellosis
1. Where, on an officially brucellosis-free ovine or caprine holding,
(a) one or more ovine or caprine animals are suspected of having brucellosis (B. melitensis), the holding's officially brucellosis-free status must be withdrawn by the competent authority. However, that status may be provisionally suspended if the animal or animals are immediately destroyed or isolated, pending official confirmation of the disease or an official quashing of the suspicion of that disease;
(b) brucellosis (B. melitensis) is confirmed, the provisional suspension may be lifted by the competent authority only if all animals infected or all the animals of species susceptible to infection are slaughtered and two tests, separated by an interval of at least three months or more, and carried out in accordance with Annex C on all the animals of the holding over six months old, give negative results.
2. If the holding referred to in paragraph 1 is in a region which is recognized as officially free from brucellosis (B. melitensis), the Member State concerned must immediately inform the Commission and the other Member States.
The competent authority of the Member State concerned must:
(a) slaughter all infected animals and all animals of species susceptible to infection on the holding concerned. The Member State concerned must keep the Commission and the other Member States informed of the development of the situation;
(b) conduct an epidemiological enquiry, and the herds linked epidemiologically to the infected herd must undergo the tests laid down in point 1 (b).
3. Should an outbreak of brucellosis be confirmed in accordance with point 2, the Commission after having assessed the circumstances of the renewed outbreak of brucellosis (B. melitensis) shall adopt, if that assessment so justifies, under the procedure laid down in Article 15, a decision suspending or withdrawing the status of that region. If the status is withdrawn, the conditions for a new qualification shall be specified in accordance with the same procedure.
D. Introduction of animals onto an officially brucellosis (B. melitensis)-free ovine or caprine holding
Ovine or caprine animals may not be introduced into an ovine or caprine holding which is officially free from brucellosis unless they either:
— come from an officially brucellosis-free ovine or caprine holding;
2. or:
— come from a brucellosis-free holding and,
— are identified individually in accordance with Article 4 (1) (a) of this Directive,
— have never been vaccinated against brucellosis or if they have been vaccinated, were so vaccinated more than two years previously. However, females over two years old which were vaccinated before the age of seven months may also be brought onto the holding, and
— were isolated under official supervision on the holding of origin and, during such isolation underwent, with negative results, two tests separated by an interval of at least six weeks in accordance with Annex C.
II. Officially brucellosis-free Member State or region
Any Member State or region within the meaning of Article 2 (10) of this Directive may be recognized, under the procedure laid down in Article 15, as being officially brucellosis-free:
(a) in which at least 99,8 % of the ovine or caprine holdings are officially brucellosis-free holdings;
or
(b) which fulfils the following conditions:
(i) ovine or caprine brucellosis is a disease that has been compulsorily notifiable for at least five years;
(ii) no case of ovine or caprine brucellosis has been officially confirmed for at least five years;
(iii) vaccination has been prohibited for at least three years; and
(c) for which compliance with these conditions has been established under the procedure set out in Article 15 of this Directive;
2. in which the conditions set out in point 1 have been satisfied; and
— the first year following recognition of a Member State or region as brucellosis-free (Br. melitensis), random checks carried out at either holding or slaughterhouse level show with a confidence rating of 99 % that less than 0,2 % of the holdings were infected, or at least 10 % of the ovine and caprine animals over six months of age have undergone a test carried out in accordance with Annex C with negative results;
— annually, from the second year following recognition of a Member State or region as brucellosis-free (Br. melitensis), random checks carried out at either holding or slaughterhouse level show with a confidence rating of 95 % that less than 0,2 % of the holdings were infected, or at least 5 % of the ovine and caprine animals over six months of age have undergone a test carried out in accordance with Annex C with negative results;
— the provisions laid down in the above two indents may be amended in accordance with the procedure laid down in Article 15;
This provision shall be reviewed before the entry into force of the Accession Treaty with a view to its possible amendment, to be carried out in accordance with the procedure laid down in Article 15.
(ii) the conditions for qualification continue to be fulfilled.
CHAPTER 2
Brucellosis (B. melitensis)-free ovine or caprine holding
A. Grant of status
A. An ovine or caprine holding is considered to be brucellosis (B. melitensis)-free:
1. in which:
(a) all the animals susceptible to brucellosis (B. melitensis) have been free from clinical or other signs of brucellosis for at least 12 months;
(b) all or some of the ovine or caprine animals have been vaccinated with Rev. 1 vaccine or any other vaccine approved under the procedure laid down in Article 15 of this Directive. The vaccinated animals must have been vaccinated before the age of seven months;
(c) two tests separated by an interval of six months or more have been carried out, with negative results, in accordance with Annex C, on all vaccinated ovine and caprine animals on the holding which are over 18 months old at the time of testing;
(d) two tests separated by an interval of six months or more have been carried out, with negative results, in accordance with Annex C, on all non-vaccinated ovine and caprine animals on the holding which are over six months old at the time of testing; and
(e) after the tests referred to under points (c) or (d) have been carried out, all the ovine and caprine animals on the holding were either born there or come from a brucellosis-free holding under the conditions laid down in section D; and
2. in which the requirements laid down under B continue to be fulfilled once it has qualified as brucellosis-free.
B. Maintenance of status
An annual test must be carried out on a representative number of the ovine and caprine animals on each holding. The holding may retain its status only if the tests are negative.
The representative number of animals to be tested must, for each holding, consist of:
— all non-castrated male animals over six months old which have not been vaccinated,
— all non-castrated male animals over 18 months which have been vaccinated,
— all animals brought onto the holding since the previous test,
— 25 % of females which are of reproductive age (sexually mature) or in milk, with a minimum of 50 per holding — except in holdings where there are fewer than 50 such females, in which case all these females must be tested.
C. Suspected or actual cases of brucellosis
1. The brucellosis-free status of an ovine or caprine holding must be withdrawn if the holding contains one or more ovine or caprine animals which are suspected of having brucellosis (B. melitensis). However, that status may be provisionally suspended if the animal or animals are immediately destroyed or isolated pending official confirmation of the disease or an official quashing of the suspicion of that disease.
2. If brucellosis (B. melitensis) is confirmed, the provisional suspension may be lifted only if all animals infected or all the animals of the species susceptible to infection are slaughtered and two tests, separated by an interval of three months or more and carried out in accordance with Annex C on,
— all vaccinated animals over 18 months old,
— all non-vaccinated animals over six months old, and
both give negative results.
D. Introduction of animals into a brucellosis (B. melitensis)-free ovine or caprine holding
The following animals only may be introduced into an ovine or caprine holding which is free from brucellosis:
1. ovine or caprine animals which come from an ovine or caprine holding which is free from or officially free from brucellosis (B. melitensis);
2. until the date laid down for holdings to qualify as brucellosis-free in accordance with the eradication plans adopted under Decision 90/242/EEC ( 10 ) ovine or caprine animals from holdings other than those referred to in point 1, provided that they meet the following conditions:
(a) they must be individually identified in accordance with Article 4 (1) (a) of this Directive;
(b) they must originate in a holding on which all animals belonging to species which are susceptible to brucellosis (B. melitensis) have shown no clinical or other signs of brucellosis (B. melitensis) for at least 12 months;
— they must not have been vaccinated during the previous two years;
— they must have been kept under isolation under veterinary supervision on the holding of origin and, during that period, must have undergone, with negative results, two tests separated by an interval of at least six weeks in accordance with Annex C; or
(ii) they must have been vaccinated with Rev. 1 vaccine or any other vaccine approved in accordance with the procedure laid down in Article 15 of this Directive before the age of seven months and not less than 15 days before entering the holding of destination.
E. Change of status
A brucellosis (B. melitensis)-free ovine or caprine holding may qualify as an officially brucellosis (B. melitensis)-free herd after a minimum period of two years if:
(a) it contains no animal which has been vaccinated against brucellosis (B. melitensis) during the preceding two years at least;
(b) the conditions laid down in point D.2. have been complied with throughout that period;
(c) at the end of the second year, a test carried out, in accordance with Annex C, on all animals aged over six months has in each case given a negative result.
ANNEX B
I ( 11 )
— Foot-and-mouth disease
— Brucellosis (B. melitensis)
— Contagious epidydimitis (B. ovis)
— Anthrax
— Rabies
▼M4 —————
III
— Contagious agalactia
— Paratuberculosis
— Caseous lymphadenitis
— Pulmonary adenomatosis
— Maedi Visna
— Caprine viral arthritis/encephalitis.
ANNEX C
Brucellosis (B. melitensis) tests
For a holding to qualify for brucellosis-free status, testing for brucellosis (B. melitensis) is performed by means of the Rose Bengal method or by the complement-fixation method described in the Annex to Decision 90/242/EEC or by any other method recognized in accordance with the procedure laid down in Article 15 of this Directive. The complement-fixation method is used for tests on individual animals.
When carrying out the Rose Bengal test, if more than 5 % of the animals on a holding show a positive reaction, a further test is carried out on every animal on the holding by means of the complement-fixation method.
Serum containing 20 or more ICFT units/ml must be regarded as positive in the complement-fixation test.
The antigens used must be approved by the national laboratory and must be standardized against the second international standard anti-brucella abortus serum.
ANNEX D
Official contagious epidydimitis (Brucella ovis) test
Complement-fixing test:
The specific antigen used must be approved by the national laboratory and must be standardized against the international standard anti-brucella ovis serum.
The working serum must be standardized with the international standard anti-brucella ovis serum prepared by the Central Veterinary Laboratory, Weybridge, Surrey, United Kingdom.
A serum containing 50 or more International Units per/ml must be regarded as positive.
ANNEX E





12. Health information
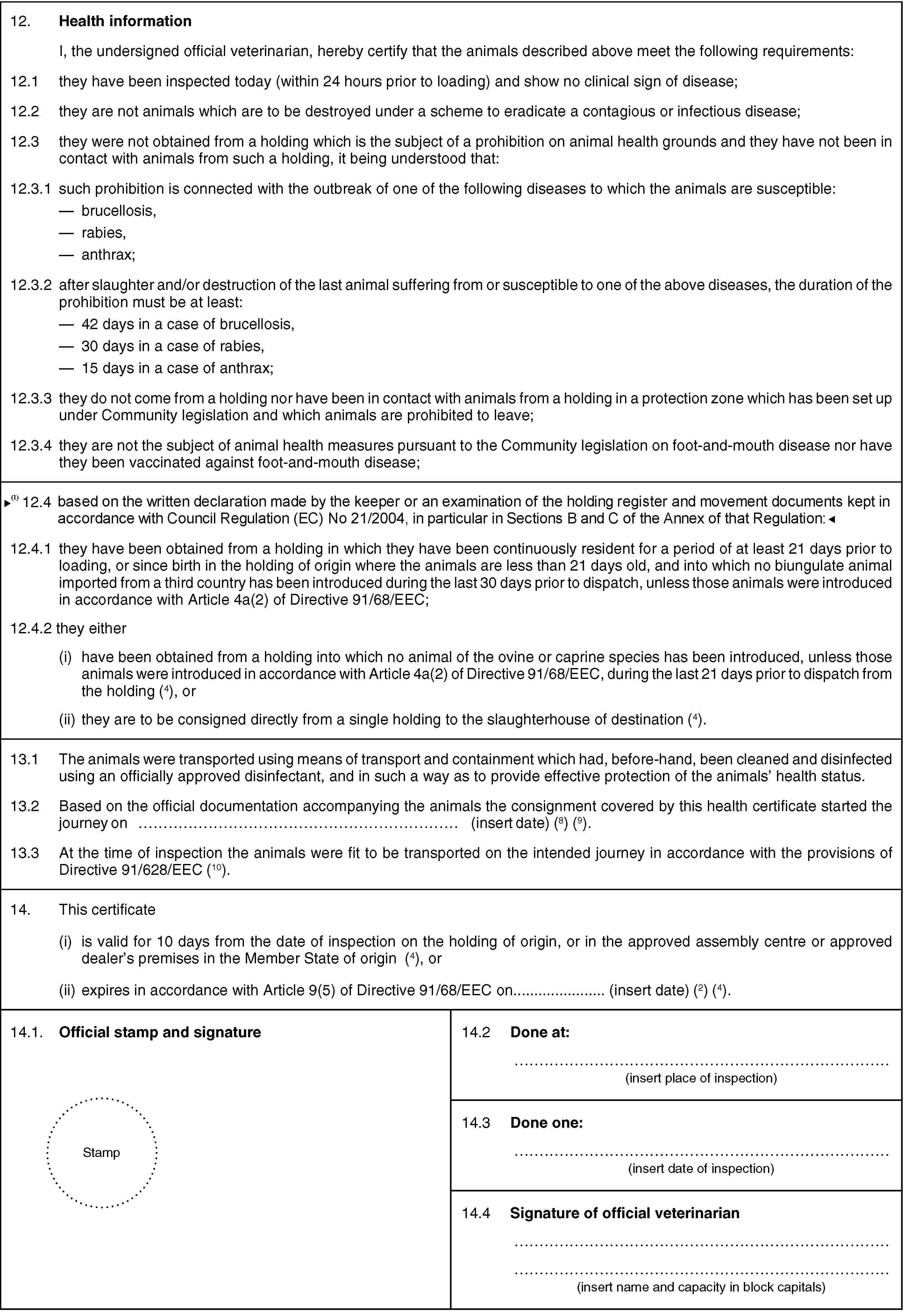
I, the undersigned official veterinarian, hereby certify, that the animals described above meet the following requirements:
12.1. they have been inspected today (within 24 hours prior to loading) and show no clinical sign of disease;
12.2. they are not animals which are to be destroyed under a scheme to eradicate a contagious or infectious disease;
12.3. they were not obtained from a holding which is the subject of a prohibition on animal health grounds and they have not been in contact with animals from such a holding, it being understood that:
12.3.1. such prohibition is connected with the outbreak of one of the following diseases to which the animals are susceptible:
- brucellosis,
- rabies,
- anthrax;
12.3.2. after slaughter and/or destruction of the last animal suffering from or susceptible to one of the above diseases, the duration of the prohibition must be at least:
- 42 days in a case of brucellosis,
- 30 days in a case of rabies,
- 15 days in a case of anthrax;
12.3.3. they do not come from a holding nor have been in contact with animals from a holding in a protection zone which has been set up under Community legislation and which animals are prohibited to leave;
12.3.4 they are not the subject of animal health measures pursuant to Community legislation on foot-and-mouth disease nor have they been vaccinated against foot-and-mouth disease;
12.4. they have remained on a single holding of origin for a period of at least 30 days prior to loading, or since birth in the holding of origin where the animals are less than 30 days old, and no animal of the ovine or caprine species has been introduced into the holding of origin during the last 21 days prior to loading and no biungulate animal imported from a third country has been introduced into the holding of origin during the 30 days prior to dispatch from the holding of origin, unless those animals were introduced in accordance with Article 4a(2) of Directive 91/68/EEC;
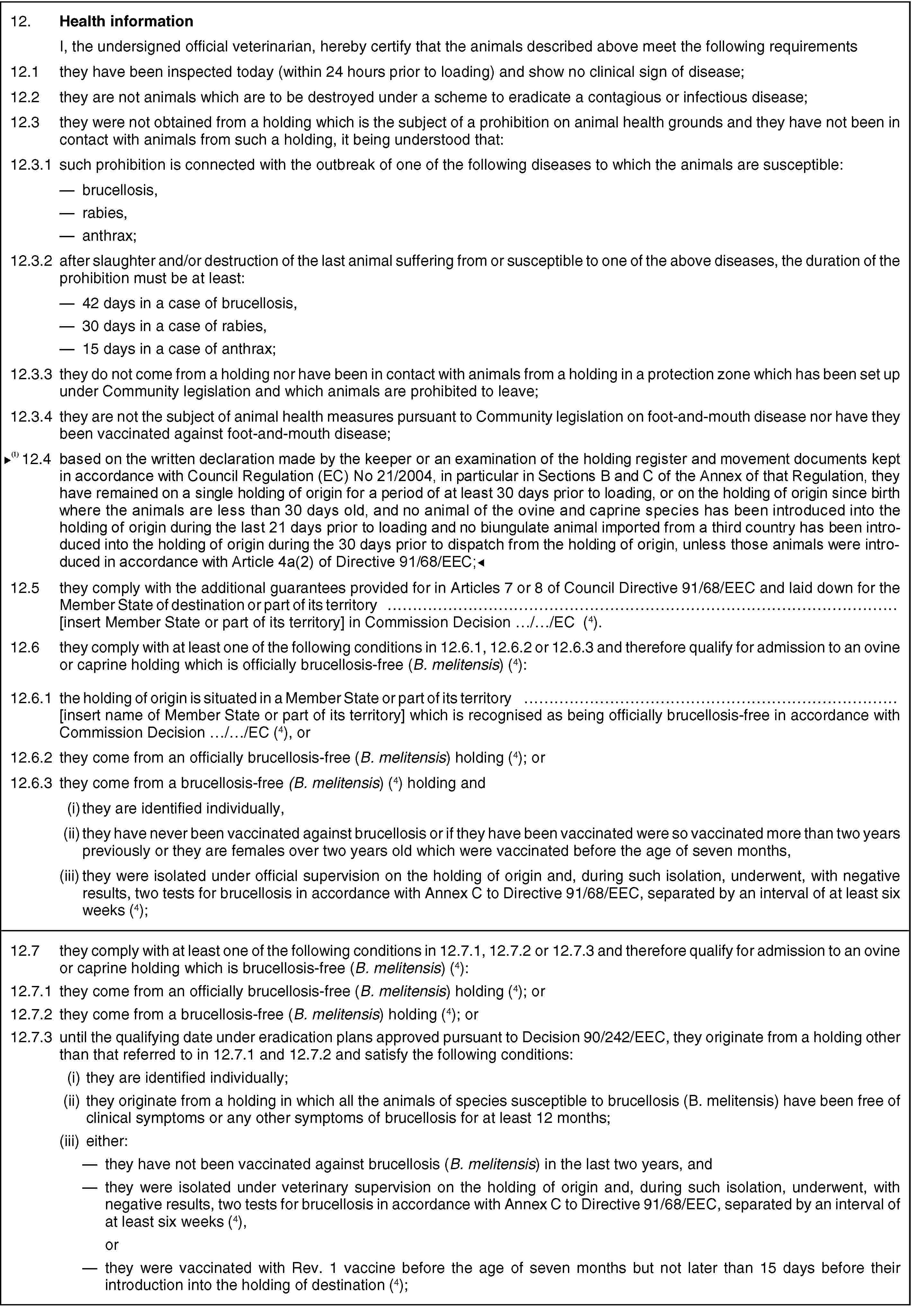
12.5. they comply with the additional guarantees provided for in Articles 7 or 8 of Directive 91/68/EEC and laid down for the Member State of destination or part of its territory … [insert Member State or part of its territory] in Commission Decision …/…/EC (4).
12.6. they comply with at least one of the following conditions in 12.6.1, 12.6.2 or 12.6.3 and therefore qualify for admission to an ovine or caprine holding which is officially brucellosis-free, (B. melitensis) (4):
12.6.1. the holding of origin is situated in a Member State or part of its territory … [insert name of Member State or part of its territory] which is recognised as being officially brucellosis-free in accordance with Commission Decision…/…/EC (4) ; or
12.6.2. they come from an officially brucellosis-free (B. melitensis) holding(4); or
12.6.3. they come from a brucellosis-free (B. melitensis) holding and
(i) they are identified individually, and
(ii) they have never been vaccinated against brucellosis or if they have been vaccinated were so vaccinated more than two years previously or they are females over two years old which were vaccinated before the age of seven months, and
(iii) they were isolated under official supervision on the holding of origin and, during such isolation, underwent, with negative results, two tests for brucellosis in accordance with Annex C to Directive 91/68/EEC, separated by an interval of at least six weeks (4);

( 1 ) OJ No C 48, 27. 2. 1989, p. 21.
( 2 ) OJ No C 96, 17. 4. 1989, p. 187.
( 3 ) OJ No C 194, 31. 7. 1989, p. 9.
( 4 ) OJ No 121, 29. 7. 1964, p. 1977/64.
( 5 ) OJ No L 224, 18. 8. 1990, p. 29.
( 6 ) OJ L 340, 11.12.1991, p. 17. Directive as last amended by Directive 95/29/EC (OJ L 148, 30.6.1995, p. 52).
( 7 ) OJ No L 224, 18. 8. 1990, p. 19.
( 8 ) OJ L 31, 1.2.2002, p. 1.
( 9 ) OJ L 184, 17.7.1999, p. 23.
( 10 ) OJ No L 140, 1. 6. 1990, p. 123.
( 11 ) Compulsorily notifiable diseases

