

This document is an excerpt from the EUR-Lex website
Document 31993L0085
Council Directive 93/85/EEC of 4 October 1993 on the control of potato ring rot
Council Directive 93/85/EEC of 4 October 1993 on the control of potato ring rot
Council Directive 93/85/EEC of 4 October 1993 on the control of potato ring rot
IO L 259, 18.10.1993, p. 1–25
(ES, DA, DE, EL, EN, FR, IT, NL, PT) Foilsíodh an doiciméad seo in eagrán speisialta
(FI, SV, CS, ET, LV, LT, HU, MT, PL, SK, SL, BG, RO, HR)
 No longer in force, Date of end of validity: 31/12/2021; Arna aisghairm le 32016R2031
No longer in force, Date of end of validity: 31/12/2021; Arna aisghairm le 32016R2031
|
18.10.1993 |
EN |
Official Journal of the European Communities |
L 259/1 |
COUNCIL DIRECTIVE 93/85/EEC
of 4 October 1993
on the control of potato ring rot
THE COUNCIL OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Economic Community, and in particular Article 43 thereof,
Having regard to the proposal from the Commission (1),
Having regard to the opinion of the European Parliament (2),
Having regard to the opinion of the Economic and Social Committee (3),
Whereas potato production occupies an important place in Community agriculture; whereas the potato yield is constantly threatened by harmful organisms;
Whereas, through the protection of potato cultivation against such harmful organisms, not only should productive capacity be maintained but agricultural productivity should also be increased;
Whereas protective measures to prevent the introduction of harmful organisms into the territory of a Member State would have only a limited effect were such organisms not controlled simultaneously and methodically throughout the Community and not prevented from spreading;
Whereas one of the harmful organisms on potatoes is Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann et Kotthoff) Davis et al., the pathogenic agent of the potato ring rot disease; whereas this disease has occurred in some parts of the Community and some limited sources of infection still exist;
Whereas there is a considerable risk to potato cultivation throughout the Community if effective measures are not taken to locate this disease and determine its distribution, to prevent it from occurring and spreading, and, if found, to prevent its spread and to control it with the aim of eradication;
Whereas, in order to ensure this, certain measures must be taken within the Community; whereas Member States must, in addition, be able to take additional or stricter measures where necessary, provided that there is no hindrance to the movement of potatoes within the Community, except in so far as laid down in Council Directive 77/93/EEC of 21 December 1976 on protective measures against the introduction into the Member States of organisms harmful to plants or plant products (4); whereas such measures must be notified to the other Member States and to the Commission;
Whereas Council Directive 80/665/EEC of 24 June 1980 on the control of potato ring rot (5), laid down minimum measures to be taken by the Member States against potato ring rot;
Whereas, since then, there have been significant developments in the understanding of potato ring rot disease and the detection of the potato ring rot pathogen;
Whereas the application of the Community plant health regime to the Community as an area without internal frontiers has called for the re-examination and revision of some provisions of Directive 80/665/EEC;
Whereas, as a result of such re-examination, the provisions of Directive 80/665/EEC have been found insufficient, and further specification of measures is necessary;
Whereas, in that situation, Directive 80/665/EEC should be repealed and the necessary measures adopted;
Whereas the measures have to take into account, first, that the disease can remain latend and unobserved both in the growing crop and in stored tubers, and so can be effectively prevented only by production and use of seed potatoes free from infection and, secondly, that systematic official surveys are necessary to locate it; whereas spread of the pathogen within the growing crop is not the most important factor, but whereas the pathogen can exist through the winter in self-sown (volunteer) potato plants and these are the major source of infection being carried from one season to the next; whereas the pathogen is spread mainly by the contamination of potatoes through contact with infected potatoes and through contact with planting, harvesting and handling equipment or transport and storage containers which have become contaminated with the organism by previous contact with infected potatoes; whereas such contaminated objects can remain infectious for some time after such contamination; whereas spread of the pathogen can be reduced or prevented by disinfection of such objects; whereas any such contamination of seed potatoes poses a major risk for the spread of the pathogen;
Whereas, for the determination of the details of such general measures, as well as for those stricter or additional measures taken by Member States to prevent the introduction of the pathogen into their territory, it is desirable for Member States to cooperate closely with the Commission within the Standing Committee of Plant Health (hereinafter referred to as ‘the Committee’),
HAS ADOPTED THIS DIRECTIVE:
Article 1
The Directive concerns the measures to be taken within the Member States against Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann et Kotthoff) Davis et al., the cause of potato ring rot (hereinafter referred to as ‘the organism’), in order to:
|
(a) |
locate it and determine its distribution; |
|
(b) |
prevent its occurrence and spread; and |
|
(c) |
if found, to prevent its spread and to control it with the aim of eradication. |
Article 2
1. Member States shall conduct systematic official surveys for the organism on tubers and, where appropriate, on plants of potato (Solanum tuberosum L.) originating in their territory, for the confirmation of absence of the organism.
For these surveys, in the case of tubers samples of both seed and other potatoes shall be taken, preferably from lots in store and subjected to official or officially supervised laboratory testing using the method set out in Annex I for the detection and diagnosis of the organism. In addition, where appropriate, official or officially supervised visual inspection by cutting of tubers on other samples may be done.
In the case of plants, these surveys shall be carried out according to appropriate methods and the samples shall be subjected to appropriate official or officially supervised testing.
The number, origin, stratification and timing of collection of samples shall be decided by the responsible official bodies within the meaning of Directive 77/93/EEC based on sound scientific and statistical principles and the biology of the organism, and taking into account the particular potato production systems of the Member States concerned. The details thereof shall be submitted annually to the other Member States and the Commission, with a view to ensuring comparable levels of assurance between Member States for confirmation of the absence of the organism.
2. The results of the official surveys provided for in paragraph 1 shall be notified at least once a year to the other Member States and to the Commission. The details of this notification shall be confidential. They may be submitted to the Committee in accordance with the procedure laid down in Article 16a of Directive 77/93/EEC.
3. The following provisions may be adopted in accordance with the procedure laid down in Article 16a of Directive 77/93/EEC;
|
— |
the details of surveys provided for in paragraph 1 above, to be carried out in accordance with sound scientific and statistical principles, |
|
— |
the details of the notification provided for in paragraph 2 above. |
4. The following provisions shall be adopted in accordance with the procedure laid down in Article 16a of Directive 77/93/EEC:
|
— |
the appropriate method for the surveys and the testing provided for in the third subparagraph of paragraph 1 above. |
Article 3
Member States shall ensure that the suspected occurrence or confirmed presence of the organism, in potato plants and tubers or harvested, stored or marketed tubers in their territory shall be reported to their own responsible official bodies.
Article 4
1. In cases of suspected occurrence, the responsible official bodies of the Member State in which these cases have been reported shall ensure completion of official or officially supervised laboratory testing, using the method set out in Annex I, and in accordance with the conditions specified in point 1 of Annex II, in order to confirm or refute the suspected occurrence. In the former case, the requirements laid down in point 2 of Annex II shall apply.
2. Pending the confirmation or refutation of the suspected occurrence under paragraph 1, in those cases of suspect occurrence where, either:
|
(i) |
suspect diagnostic visual symptoms of the disease have been seen; or, |
|
(ii) |
a positive immunofluorescence test as specified in Annex I or other appropriate positive test has been identified, |
the responsible official bodies of the Member States shall:
|
(a) |
prohibit the movement of all lots or consignments from which the samples have been taken, except under their control and provided that it has been established that there is no identifiable risk of the organism spreading; |
|
(b) |
take steps to trace the origin of the suspected occurrence; |
|
(c) |
introduce appropriate additional precautionary measures based on the level of estimated risk, in order to prevent any spread of the organism. These measures may include the official control of the movement of all other tubers or plants within or off premises associated with the suspected occurrence. |
3. The following provision may be adopted in accordance with the procedure laid down in Article 16a of Directive 77/93/EEC:
|
— |
the measures referred to in paragraph 2 (c) above. |
4. The following provision shall be adopted in accordance with the procedure laid down in Article 16a of Directive 77/93/EEC:
|
— |
the other appropriate test provided for in paragraph 2 (ii) above; |
Article 5
1. If official or officially supervised laboratory testing using the method set out in Annex I confirms the presence of the organism in a sample of tubers, plants, or parts of plants, the responsible official bodies of a Member State, having regard to sound scientific principles, the biology of the organism and the particular production, marketing and processing systems in that Member State shall:
|
(a) |
designate as contaminated the tubers or plants, consignment and/or lot, and the machinery, vehicle, vessel, store, or units thereof, and any other objects including packaging material, from which the sample was taken, and, where appropriate, the place(s) of production and field(s) from which the tubers or plants were harvested; |
|
(b) |
determine, taking into account the provisions of point 1 of Annex III, the extent of probable contamination through pre- or post-harvest contact or through production link with the designated contamination; |
|
(c) |
demarcate a zone on the basis of the designation of contamination under (a), the determination of the extent of probable contamination under (b), and the possible spread of the organism, taking into account the provisions of point 2 of Annex III. |
2. Member States shall immediately notify the other Member States and the Commission, in accordance with the provisions of point 3 of Annex III, of any contamination designated under paragraph 1 (a) and the details of the zone demarcation under paragraph 1 (c).
The details of this notification shall be confidential. They may be submitted to the Committee in accordance with the procedure laid down in Article 16a of Directive 77/93/EEC.
3. As a result of the notification under paragraph 2 and the elements mentioned therein, other Member States detailed in the notification shall, as appropriate, designate contamination, determine the extent of probable contamination and demarcate a zone, in accordance with paragraph 1 (a), (b) and (c) respectively.
Article 6
Member States shall prescribe that where tubers or plants have been designated to be contaminated under Article 5 (1) (a), testing in accordance with Article 4 (1) shall be carried out on potato stocks which are clonally related to those involved in the contamination. The testing shall be carried out on as many such tubers or plants as are needed to determine the probable primary source of infection and the extent of the probable contamination, preferably in order of degree of risk.
As a result of the testing, further designation of contamination, determination of the extent of probable contamination and demarcation of a zone shall be conducted, as appropriate, under Articles 5 (1) (a), (b) and (c) respectively.
Article 7
1. Member States shall prescribe that tubers or plants, designated to be contaminated under Article 5 (1) (a) may not be planted and that, under the control of their responsible official bodies, they shall be:
|
— |
destroyed, or |
|
— |
otherwise disposed of, subject to officially supervised measure(s), in accordance with the provisions of point 1 of Annex IV, provided that it is established that there is no identifiable risk of the organism spreading. |
2. Member States shall prescribe that tubers or plants determined as probably contaminated under Article 5(1) (b) may not be planted and, without prejudice to the outcome of the testing referred to in Article 6 for clonally related stocks shall, under the control of their responsible official bodies, be put to appropriate use or disposal as specified in point 2 of Annex IV, in such a way that it is established that there is no identifiable risk of the organism spreading.
3. Member States shall prescribe that any machinery, vehicle, vessel, store, or units thereof, and any other objects including packaging material, designated as contaminated under Article 5 (1) (a) or determined as probably contaminated under Article 5(1) (b), shall either be destroyed or cleansed and disinfected using appropriate methods as specified in point 3 of Annex IV. After disinfection, any such objects shall no longer be considered contaminated.
4. Without prejudice to the measures implemented under paragraphs 1, 2 and 3, Member States shall prescribe that, in the zone demarcated under Article 5 (1) (c), a series of measures, as specified in point 4 of Annex IV, shall be implemented.
Article 8
1. Member States shall prescribe that seed potatoes shall meet the requirements of Directive 77/93/EEC and shall derive in direct line from material obtained under an officially approved programme which has been found free of the organism in official or officially supervised testing using the method set out in Annex I.
The aforesaid testing shall be carried out:
|
— |
in cases where the contamination affects seed potato production, on the plants of the initial clonal selection, |
|
— |
in other cases, either on the plants of the initial clonal selection or on representative samples of the basic seed potatoes or earlier propagations. |
2. The following provisions may be adopted in accordance with the procedure laid down in Article 16a of Directive 77/93/EEC:
|
— |
the detailed rules of application of the first indent of the second subparagraph of paragraph 1 of this Article, |
|
— |
the rules concerning the representative samples provided for in the second indent of the second subparagraph of paragraph 1 of this Article. |
Article 9
Member States shall ban the holding and handling of the organism.
Article 10
Without prejudice to the provisions of Directive 77/93/EEC, Member States may authorize derogations from the measures referred to in Articles 6, 7 and 9 of this Directive for experimental or scientific purposes, and for work on varietal selection, provided that such derogations do not prejudice the control of the organism and create no risk of spread of the organism.
Article 11
Member States may adopt such additional or stricter measures as may be required to combat the organism or to prevent it from spreading, in so far as they are in compliance with the provisons of Directive 77/93/EEC.
The additional measures mentioned in the first subparagraph may include the prescription that only seed potatoes may be planted that are either officially certified or officially inspected to meet the required plant health standards. The latter may apply in particular in case farmers are authorized to use, on their own holding, seed potatoes which they have obtained from their own harvest and in other cases that own-produced seed potatoes are planted.
The details of these measures shall be notified to the other Member States and to the Commission.
Article 12
Amendments to the Annexes to this Directive, to be made in the light of developments in scientific or technical knowledge, shall be adopted in accordance with the procedure laid down in Article 16a of Directive 77/93/EEC.
Article 13
1. By 15 November 1993 Member States shall adopt and publish the provisions necessary to comply with this Directive. They shall immediately inform the Commission thereof.
When Member States adopt these provisions, they shall contain a reference to this Directive or shall be accompanied by such reference at the time of their official publication. The procedure for making such reference shall be adopted by Member States.
Member States shall apply these provisions from 16 November 1993.
2. Member States shall immediately communicate to the Commission all provisions of national law which they adopt in the field covered by this Directive. The Commission shall inform the other Member States thereof.
Article 14
Directive 80/665/EEC is hereby repealed with effect from 16 November 1993.
Article 15
This Directive is addressed to the Member States.
Done at Luxembourg, 4 October 1993.
For the Council
The President
W. CLAES
(1) OJ No C 93, 2. 4. 1993, p. 12.
(2) OJ No C 176, 28. 6. 1993, p. 210.
(3) OJ No C 161, 14. 6. 1993, p. 18.
(4) OJ No L 26, 31. 1. 1977, p. 20. Directive as last amended by Commission Directive 92/103/EEC (OJ No L 363, 11. 12. 1992, p. 1).
(5) OJ No L 180, 14. 7. 1980, p. 30.
ANNEX I
METHOD FOR THE DETECTION AND DIAGNOSIS OF THE RING ROT BACTERIUM, CLAVIBACTER MICHIGANENSIS (Smith) Davis et al. ssp. SEPEDONICUS (Spieckermann et Kotthof) Davis et al. IN BATCHES OF POTATO TUBERS
1. Removal of heel-end cores
|
1.1. |
Wash 200 tubers in running tap water and remove the epidermis around the heel end of each tuber using a regularly disinfected scalpel or potato peeler; disinfection may be achieved by dipping the peeler in 70 % ethanol and flaming. |
|
1.2. |
Carefully remove conical tissue cores from the heel ends with a knife or potato peeler. Keep the excess non-vascular tissue to a minimum. Once removed, heel ends should be processed within 24 hours (see paragraph 3) or conserved at -20oC for no longer than two weeks. |
2. Visual examination for ring rot symptoms
After removal of heel ends, cut each tuber transversely and observe for the presence of ring rot symptoms.
Squeeze the tubers and look for expression of macerated tissues from the vascular tissue.
The earliest symptoms are a slight glassiness or translucence of the tissue without softening round the vascular system, particularly near the heel end. The vascular ring at the heel end may be slightly darker in colour than normal. The first readily identifiable symptom is one whereby the vascular ring has a yellowish coloration and when the tuber is gently squeezed, pillars of cheese-like material emerge from the vessels. This exudation contains millions of bacteria. Browning of the vascular tissue may develop at this stage. At first, these symptoms may be restricted to one part of the ring, not necessarily close to the heel end and may gradually extend to the whole ring. As the infection progresses, destruction of the vascular tissue occurs; the outer cortex may become separated from the inner cortex. In advanced stages of infection, cracks appear on the surface of the tuber, which are often reddish-brown at their margins. Secondary fungal or bacterial invasion may mask the symptoms and it may be difficult, if not impossible, to distinguish advanced ring rot symptoms from other tuber rots.
3. Preparation of samples for Gram staining, immunofluorescence staining (IF) and eggplant test
|
3.1. |
Homogenize the heel ends until complete maceration has just been achieved in a diluent known to be non-toxic to Corynebacterium sepedonicum (for example, 0,05 M phosphate buffered saline (PBS) pH 7,0) at a temperature of less than 30oC; the addition of a non-toxic deflocculant is advisable and non-toxic antifoam agent may be needed (Appendices 1 and 2). Excessive maceration should be avoided. |
|
3.2. |
Extract bacteria from the homogenate by one of the methods as follows (1):
|
|
3.3. |
Suspend the pellet in sterile 0,01 M phosphate buffer pH 7,2 (Appendix 2) to give a total volume of approximately 1 ml. Divide in two equal parts and retain one part for reference purposes by freezing at - 20 oC (2) or by lyophilization. Divide the other part into halves using one half for the IF test and Gram stain and the other for the eggplant test. |
|
3.4. |
It is imperative that all positive C. sepedonicum controls and samples are treated separately to avoid contamination. This applies to IF slides and to eggplant tests. |
4. Gram staining
|
4.1. |
Prepare Gram stains for all pellet dilutions (5.2.1) and for any cut tubers (2) which show glassiness, rotting or other suspect symptoms. Samples should be taken from the edge of diseased tissues. |
|
4.2. |
Prepare Gram stains for known C. sepedonicum cultures and, if possible, for naturally infected tissue (5.1). |
|
4.3. |
Determine which samples contain typical Gram positive coryneform cells. In general, C. sepedonicum cells are 0,8 to l,2μm long and 0,4 to 0,60μm wide. An appropriate staining procedure is given in Appendix 3. Preparations from natural infections or recently isolated cultures often show a predominance of coccoid rods which are usually slightly smaller than cells from oder agar cultures. On most culture media, C. sepedonicum cells are pleomorphic coryneform rods and may give a variable Gram reaction. Cells are single, in pairs with characteristic ‘elbows’ typical of bending division, and occasionally in irregular groups often referred to as palisades and Chinese letters. |
5. Scheme for IF-testing
5.1. Use antiserum to a known strain of C. sepedonicum — ATCC 33113 (NCPPB 2137), or NCPPB 2140. This should have an IF titre of more than 1:600. Include one PBS control on the test slide to determine whether the fluorescein isothiocyanate anti-rabbit immunoglobulin conjugate (FITC) combines non-specifically with bacterial cells. Corynebacterium sepedonicum (ATCC 33113 (NCPPB 2137), NCPPB 2140) should be used as homologous antigen controls on a separate slide. Naturally infected tissue (maintained by lyophilization or freezing at - 20oC) should be used where possible as a similar control on the same slide (Figure 2).
5.2. Procedure
|
5.2.1. |
Prepare three serial ten fold dilutions (101, 102, 103) of the final pellet in distilled water (Figure 1). |
|
5.2.2. |
Pipette a measured standard volume sufficient to cover the window (approximately 25 μl) of each pellet dilution or C. sepedonicum suspension (approximately 106 cells/ml) to windows of a multispot slide as shown in Figure 1. |
Figure 1
Sample and PBS control slide
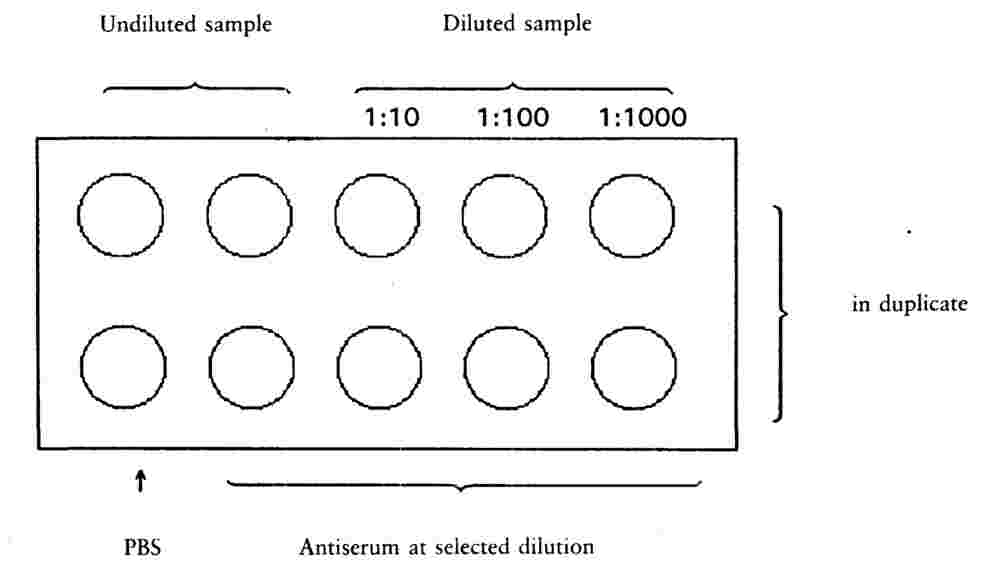
Figure 2
Positive control slide

|
5.2.4. |
Cover appropriate windows which C. sepedonicum antiserum at the recommended dilutions, 0,01 M PBS pH 7,2 (Appendix 2), as shown in Figure 1. (Use PBS for the FITC control.) The working dilution of the antiserum should be approximately half that of the IF titre. If other antiserum dilutions are to be included, separate slides should be prepared for each dilution to be used. |
|
5.2.5. |
Incubate in a humid chamber at ambient temperature for 30 minutes. |
|
5.2.6. |
Rinse carefully with 0,01 M PBS pH 7,2. Wash for five minutes in three changes of 0,01 M PBS pH 7,2. |
|
5.2.7. |
Carefully remove excess moisture. |
|
5.2.8. |
Cover each window with FITC conjugate at the same dilution used to determine the titre and incubate in a dark humid chamber at ambient temperature for 30 minutes. |
|
5.2.9. |
Rinse and wash as before. |
|
5.2.10. |
Apply approximately 5 to 10 μl of 0,1 M phosphate buffered glycerine pH 7,6 (or a similar mountant with a pH not less than 7,6) to each window and cover with a coverglass (Appendix 2). |
|
5.2.11. |
Examine with a microscope fitted with an epifluorescent light source and filters suitable for working with FITC. A magnification of 400 to 1 000 is suitable. Scan replicated windows across two diameters at right angles and around the window perimeters. Observe for fluorescing cells in the positive controls and determine the titre. Observe for fluorescing cells in the FITC/PBS control window and, if absent, proceed to the test windows. Determine in a minimum of 10 microscope fields the mean number of morphologically typical fluorescing cells per field and calculate the number per ml of undiluted pellet (Appendix 4). There are several problems inherent to the immunofluorescence test.
A negative immunofluorescence test is identified for any sample where morphologically typical fluorescing cells are not found. The samples shall be considered as ‘not contaminated’ with Clavibacter michiganensis ssp. sepedonicus. The eggplant test is not required. A positive immunofluorescence test is identified for any sample where morphologically typical fluorescing cells are found. Samples for which a positive immunofluorescence test have been identified with both antisera shall be considered as ‘potentially contaminated’ with Clavibacter michiganensis ssp. sepedonicus. The eggplant test is required for all samples considered as potentially contaminated. |
6. Eggplant test
For cultural details, see Appendix 5.
6.1. Distribute the pellet from 3.3 between at least 25 eggplants at leaf stage 3 (Appendix 5) by one of the methods given below (6.2, 6.3 or 6.4).
6.2. Slit inoculation I
|
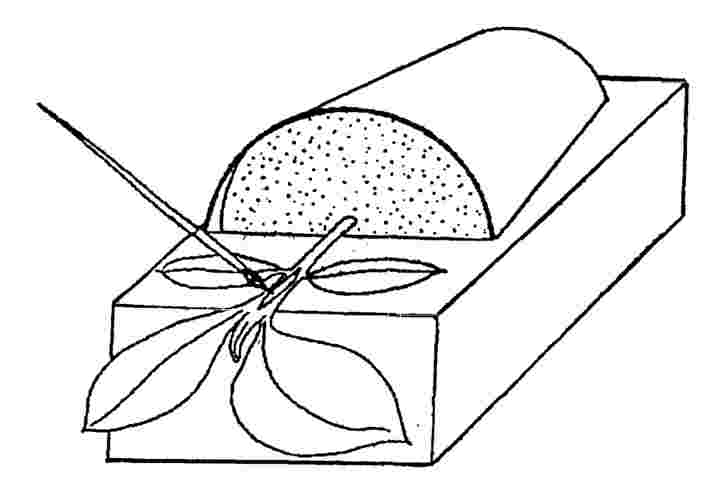
6.2.1. |
Support each pot horizontally (a block of expanded polystyrene with a piece 5 cm deep x 10 cm wide x 15 cm long, removed from one surface (Figure 3) is adequate for a 10 cm pot). A strip of sterile aluminium foil should be placed between the stem and the block for each sample tested. The plant may be held in place by a rubber band around the block. |
|
6.2.2. |
Using a scalpel, make a longitudinal or slightly diagonal cut 0,5 to 1,0 cm long and approximately three quarters of the stem diameter deep, between the cotyledons and the first leaf. |
|
6.2.3. |
Hold the slit open with the scalpel blade point and paint the inoculum into it using an eyeliner or fine artist's brush charged with the pellet. Distribute the remainder of the pellet between the eggplants. |
|
6.2.4. |
Seal the cut with sterile vaseline from a 2 ml syringe barrel. |
Figure 3
6.3. Slit inoculation II
|
6.3.1. |
Holding the plant between two fingers, pipette a drop (approximately 5 to 10 µl) of the suspended pellet on the stem between the cotyledons and the first leaf. |
|
6.3.2. |
Using a sterile scalpel, make a diagonal (at an angle of approximately 5o) slit, 1,0 cm long and approximately 2/3 of the stem thickness deep, starting the cut from the pellet drop. |
|
6.3.3. |
Seal the cut with sterile vaseline from a syringe barrel. |
6.4. Syringe inoculation
|
6.4.1. |
Do not water eggplants for one day prior to inoculation to reduce turgor pressure. |
|
6.4.2. |
Inoculate the eggplant stems just above the cotyledons using a syringe fitted with a hypodermic needle (not less than 23G). Distribute the pellet between the eggplants. |
6.5. Inoculate 25 plants with a known C. sepedonicum culture and, where possible, naturally infected tuber tissue (5.1) by the same inoculation method (6.2, 6.3 or 6.4).
6.6. Inoculate 25 plants with sterile 0,05 M PBS by the same inoculation method (6.2, 6.3 or 6.4).
6.7. Incubate the plants in appropriate conditions (Appendix 5) for 40 days. Examine regularly for symptoms after eight days. Count the number of plants showing symptoms. C. sepedonicum causes leaf wilting in eggplants which may commence as a marginal or interveinal flaccidity. Wilted tissue may initially appear dark green or mottled but turns paler before becoming necrotic. Interveinal wilts often have a greasy water-soaked appearance. Necrotic tissue often has a bright yellow margin. Plants are not necessarily killed; the longer the period before symptoms develop, the greater the chance of survival. Plants may outgrow the infection. Susceptible young eggplants are much more sensitive to low populations of C. sepedonicum than are older plants, hence the necessity to use plants at or just before leaf stage 3.
Wilts may also be induced by populations of other bacteria or fungi present in the tuber tissue pellet. These include Erwinia carotovora, subsp. carotovora and E. carotovora subsp. atroseptica, Phoma exigua var. foveata, as well as large populations of saprophytic bacteria. Such wilts can be distinguished from those caused by C. sepedonicum since whole leaves or whole plants wilt rapidly.
6.8. Prepare a Gram stain (4) for all batches of eggplants showing symptoms, using sections of wilted leaf tissue and stem tissue from plants and isolate on to suitable nutrient media (7). Surface disinfect the eggplant leaves and sterns by wiping with 70 % ethanol.
6.9. Under certain circumstances, in particular where growing conditions are not optimal, it may be possible for C. sepedonicum to exist as a latent infection within eggplants even after incubation for 40 days. Such infections may possibly result in stunting and lack of vigour in the inoculated plants. If the IF test is considered positive, it may be considered necessary to test further. It is, therefore, essential to compare the growth rates of all eggplant test plants with the sterile 0,05 M PBS inoculated controls and to monitor the environmental conditions of the glasshouse.
Recommendations for further testing are as follows:
|
6.9.1. |
excise the stems above the inoculation site and remove the leaves; |
|
6.9.2. |
macerate the stems in 0,05 M PBS pH 7,0, as in 3.1 to 3.2; |
|
6.9.3. |
use half of the pellet to perform a Gram stain (4) and an IF test (5); |
|
6.9.4. |
use the other half to perform a further eggplant test (6) if the Gram stain and/or IF tests are positive. Use a known C. sepedonicum culture and sterile 0,05M PBS controls. If symptoms are not observed in the subsequent test, the sample must be considered negative. |
7. Isolation of C. sepedonicum
Diagnosis can only be confirmed if C. sepedonicum is isolated and so identified (8). Although C. sepedonicum is a fastidious organism, it can be isolated from symptomatic tissue. However, it may be outgrown by rapidly growing saprophytic bacteria and, therefore, isolations directly from the tuber tissue pellet (3.3) are not recommended. Eggplants provide an excellent selective enrichment medium for the growth of C. sepedonicum and also provide an excellent confirmatory host test.
Isolations should be made from all symptomatic potato tubers and eggplants (4, 6). Maceration of eggplant stems when necessary should be carried out as in 3. and 6.9.
|
7.1. |
Streak suspensions on to one of the following media: (formulae are given in Appendix 6): nutrient dextrose agar (for subculture only), yeast peptone glucose agar, nutrient yeast dextrose agar, yeast extract mineral salts agar. Incubate at 21 oC for up to 20 days. C. sepedonicum is slow-growing, usually producing pin-point, cream, domed colonies within 10 days. Re-streak to establish purity. Growth rates are improved with subculture. Typical colonies are creamy-white or ivory, rounded, smooth, raised, convex-domed, mucoid-fluidal, with entire edges and usually 1 to 3 mm in diameter. |
Identification
Many Gram positive coryneform bacteria, with colonial characters similar to those of C. sepedonicum, may be isolated from healthy or diseased potatoes and eggplants. In this context C. sepedonicum must be identified by the following tests:
IF test (5.1),
eggplant test,
nutrition and physiological tests (Appendix 7),
|
— |
oxidation/fermentation test (O/F), |
|
— |
oxidase test, |
|
— |
growth at 37oC, |
|
— |
urease production, |
|
— |
aesculin hydrolysis, |
|
— |
starch hydrolysis, |
|
— |
tolerance of 7 % sodium chloride solution, |
|
— |
indole test, |
|
— |
catalase test, |
|
— |
H28 production, |
|
— |
citrate utilization, |
|
— |
gelatin hydrolysis |
|
— |
acid from: glycerol, lactose, rhamnose and salicin, |
|
— |
Gram stain. |
All tests should include a known C. sepedonicum control. Nutritional and physiological tests should be made using inocula from nutrient agar subcultures. Morphological comparisons should be made from nutrient dextrose agar cultures.
For the IF test, cell populations should be adjusted to 106 cells/ml. The IF titre should be similar to that of the known C. sepedonicum culture.
For the eggplant test cell populations should be adjusted to c 107 cells/ml. Eggplant tests should be made using 10 plants for each of the test organisms, again using known C. sepedonicum culture and sterile water controls; with pure cultures typical wilting should be obtained within 20 days but plants not showing symptons after this time should be incubated for a total of 30 days at temperatures conducive to eggplant growth but not exceeding 30oC (Appendix 5). If after 30 days symptoms are not present, the culture cannot be confirmed as being a pathogenic form of Corynebacterium sepedonicum.
|
Test |
C. sepedonicum |
|
O/F |
Inert or weakly oxidative |
|
Oxidase |
- |
|
Catalase |
+ |
|
Nitrate reduction |
- |
|
Urease activity |
- |
|
H2S production |
- |
|
Indole production |
- |
|
Citrate utilization |
- |
|
Starch hydrolysis |
- or weak |
|
Growth at 37o |
- |
|
Growth in 7 % NaCl |
- |
|
Gelatin hydrolysis |
- |
|
Aesculin hydrolysis |
+ |
|
Acid from: |
|
|
— Glycerol |
- |
|
— Lactose |
- or weak |
|
— Rhamnose |
- |
|
— Salicin |
|
(1) An alternative method for extraction is given by Dinesen, 1984.
(2) There is evidence (Janse and Van Vaerenberg, 1987) that freezing may reduce viability of Corynebacterium sepedonicum. Suspension of the pellet in 10 % glycerol may overcome this problem.
Appendix 1
FORMULATION OF MACERATING FLUID RECOMMENDED BY LELLIOTT AND SELLAR, 1976
|
D C silicone antifoam MS A compound (Hopkins & Williams Ltd, Cat. No 9964-25, Chadwell Heath, Essex, England) |
10 ml |
|
Lubrol W flakes (ICI Ltd) |
0,5 g |
|
Tetra-sodium pyrophosphate |
1 g |
|
0,05 M phosphate buffered saline pH 7,0 (Appendix 2) |
1 litre |
Appendix 2
BUFFERS
0,05 M phosphate buffered saline pH 7,0
This buffer can be used for tuber tissue maceration (2.1)
|
Na2HPO4 |
4,26 g |
|
KH2PO4 |
2,72 g |
|
NaCl |
8,0 g |
|
Distilled water to |
1 litre |
0,01 M phosphate buffered saline pH 7,2
This buffer is used for diluting antisera and washing IF slides
|
Na2HPO4 12 H2O |
2,7 g |
|
NaH2PO4 2 H2O |
0,4 g |
|
NaCl |
8,0 g |
|
Distilled water to |
1 litre |
0,1 M phosphate buffered glycerine pH 7,6
This buffer is used as a mountant to enhance fluorescence in the IF test
|
Na2HPO4 12 H2O |
3,2 g |
|
NaH2PO4 2 H2O |
0,15 g |
|
Glycerol |
50 ml |
|
Distilled water |
100 ml |
Appendix 3
GRAM STAIN PROCEDURE (HUCKER'S MODIFICATION) (DOETSCH, 1981)
Crystal violet solution
Dissolve 2 g crystal violet in 20 ml 95 % ethanol.
Dissolve 0,8 g ammonium oxalate in 80 ml distilled water.
Mix the two solutions.
Lugol's iodine
|
Iodine |
1 g |
|
Potassium iodide |
2 g |
|
Distilled water |
300 ml. |
Grind the solids together in a pestle and mortar. Add to the water and stir to dissolve in a closed container.
Safranin counterstain solution
Stock solution:
|
Safranin O |
2,5 g |
|
95 % ethanol |
100 ml. |
Mix and store.
Dilute: 1:10 to obtain a working solution.
Staining procedure
|
1. |
Prepare smears, air dry and heat fix. |
|
2. |
Flood slide with crystal violet solution for one minute. |
|
3. |
Wash briefly with tap water. |
|
4. |
Flood with Lugol's iodine for one minute. |
|
5. |
Wash with tap water and blot dry. |
|
6. |
Decolourize with 95 % ethanol, added dropwise, until no further colour is removed or immerse with gentle agitation for 30 seconds. |
|
7. |
Wash in tap water and blot dry. |
|
8. |
Flood with safranin solution for 10 seconds. |
|
9. |
Wash with tap water and blot dry. Gram positive bacteria stain violet-blue; Gram negative bacteria stain pink-red. |
Appendix 4
DETERMINATION OF POPULATION OF IF-POSITIVE CELLS
Surface area (S) of window of multispot slide
![]()
Where D = diameter of window. Surface area(s) of objective field
![]()
where d = diameter of field.
Calculate d either by direct measurement or from the following formule:

|
where |
|
from (2)
from (3)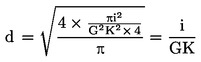 (4)
(4)
Count the number of typical fluorescent cells per field (c).
Calculate the number of typical fluorescent cells per window (C).
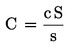
Calculate the number of typical fluorescent cells per ml pellet (N)

|
where |
|
|||
|
where |
|
Appendix 5
EGGPLANT CULTURE
Sow seeds of eggplant ( Solarium melongena cv. Black Beauty) in pasteurized seed compost. Transplant seedlings with fully expanded cotyledons (10 to 14 days) into pasteurized potting compost.
Use eggplants at leaf stage 3 when two, but not more than three, leaves are fully uncurled.
Eggplants should be grown in a glasshouse with the following environmental conditions:
|
day length: |
14 hours or natural day length if greater; |
|
temperature: day: |
21 to 24oC, |
|
night: |
15oC. |
NB: C. sepedonicum will not grow at temperatures >30oC. If night temperatures do not fall to 15oC, chromophore damage (silvery necrosis) may occur.
Root damage caused by sciarid larvae can be overcome by the application of an appropriate insecticide.
Eggplant cv. Black Beauty may be obtained from:
|
1. |
|
|
2. |
|
|
3. |
|
|
4. |
|
Appendix 6
MEDIA FOR GROWTH AND ISOLATION OF C. SEPEDONICUM
Nutrient agar (NA)
Difco bacto nutrient agar in distilled water at manufacturer's rate. Sterilize by autoclaving at 121 oC for 15 minutes.
Nutrient dextrose agar (NDA)
Difco bacto nutrient agar containing 1 % D( + ) glucose (monohydrate). Sterilize by autoclaving at 115 oC for 20 minutes.
Yeast peptone glucose agar (YPGA)
|
Difco bacto yeast extract (No 0127) |
5 g |
|
Difco bacto peptone (No 0118) |
5 g |
|
D( + )-glucose (monohydrate) |
10 g |
|
Difco bacto purified agar (No 0560) |
15 g |
|
Distilled water |
1 litre |
Sterilize 0,5 litre volumes of medium by autoclaving at 115 oC for 20 minutes.
Yeast extract mineral salts medium (YGM)
|
Difco bacto yeast extract |
2,0 g |
|
D( + )-glucose (monohydrate) |
2,5 g |
|
K2HPO4 |
0,25 g |
|
KH2PO4 |
0,25 g |
|
MgSO4. 7H2O |
0,1 g |
|
MnSO4. H2O |
0,015 g |
|
NaCl |
0,05 g |
|
FeSO4. 7H2O |
0,005 g |
|
Difco bacto purified agar |
18 g |
|
Distilled water |
1 litre |
Sterilize 0,5 litre volumes of medium by autoclaving at 115 oC for 20 minutes.
Appendix 7
NUTRITIONAL AND PHYSIOLOGICAL TESTS FOR THE IDENTIFICATION OF C. SEPEDONICUM
All media should be incubated at 21 oC and examinated after six days. If no growth has occurred, incubate for up to 20 days.
— Oxidative and fermentative test (Hugh & Leifson), 1953) - O/F-test.
Basal medium:
|
KCl |
0,2 g |
|
MgSO4. 7H2O |
0,2 g |
|
NH4H2PO4 |
1,0 g |
|
Difco bacto peptone |
1,0 g |
|
Difco bacto purified agar |
3,0 g |
|
D( + )-glucose (monohydrate) |
10,0 g |
|
Bromothymol blue |
0,03 g |
|
Distilled water |
1 litre |
Mix and adjust to pH 7,0 to 7,2 with 1N KOH.
Dispense in Pyrex culture tubes 16 mm x 100 mm (12 ml capacity) in 5 ml and 10 ml volumes.
Sterilize by autoclaving at 115 oC for 10 minutes.
Stab inoculate 5 ml and 10 ml tubes for each culture. Aseptically add 1 to 2 ml sterile liquid paraffin to the 10 ml tube. Incubate.
Positive reaction:
|
Tube |
Colour |
Interpretation |
|
|
Open |
Yellow |
|
Fermentative |
|
Closed |
Yellow |
||
|
Open |
Yellow |
|
Oxidative |
|
Closed |
Blue-green |
||
|
Open |
Greenish |
|
Oxidative or inert |
|
Closed |
Blue-green |
||
— Oxidase test (Kovacs, 1956)
Kovacs'oxidase reagent:
1 % aqueous solution of tetramethyl paraphenylenediamine dihydrochloride (BDH No 30386) in distilled water.
This reagent should be freshly made up in 1 ml volumes or can be stored in a brown glass bottle at 5 oC for 1 to 4 weeks.
Place a drop of reagent on filter paper in a clean Petri dish. Immediately rub some of the test culture from nutrient agar using a platinum loop.
Positive reaction: development of purple coloration within 10 seconds. Cultures with times of 10 to 30 seconds are weakly positive.
NB: It is essential to use a platinum loop and NA cultures, since traces of iron or high sugar content in the growth medium may give false positive results.
— Acid production from lactose, rhamnose, salicin, glycerol
Prepare Hugh & Leifson's O/F medium without the glucose. Distribute into 5 ml volumes in tubes. Sterilize by autoclaving at 115 oC for 10 minutes. To the molten base at 45 oC, aseptically add 0,5 ml of filter sterilized 10 % aqueous solutions of either glycerol, lactose, rhamnose or salicin. Mix carefully.
Positive reaction: colour change from blue-green to yellow indicates production of acid.
— Catalase test
Place a drop of hydrogen peroxide (30 volume) onto a clean slide, and emulsify with a loopful of culture using a platinum loop.
Positive reaction: production of oxygen bubbles in the drop indicates the presence of catalase.
— Nitrate reductase activity and denitrification (Bradbury, 1970)
Culture medium:
|
KNO3 (nitrite free) |
1 g |
|
Difco bacto yeast extract |
1 g |
|
K2HPO4 |
5 g |
|
Distilled water |
1 litre |
Dispense into 10 ml volumes in 20 ml bottles. Sterilize by autoclaving at 121 oC for 15 minutes.
Reagent A:
|
H2SO4 |
8g |
|
5N acetic acid |
1 litre |
Reagent B:
|
naphthylamine |
5 g |
|
5N acetic acid |
1 litre |
Inoculate the nitrate medium in duplicate. Test after 10 and 20 days, by adding one drop of Lugol's iodine, 0,5 ml reagent A and 0,5 ml reagent B. If medium does not turn reddish, add approximately 50 mg zinc powder. Observe the colour reaction.
Positive reaction:
|
|
Colour reaction |
|
|
|
Stage 1 |
Stage 2 |
|
No reduction of nitrate |
colourless |
red |
|
Reduction of nitrate as far as nitrite (nitrate reductase only) |
red |
— |
|
Reduction of nitrate beyond nitrite (denitrification — nitrate and nitrite reductase) |
colourless |
colourless |
— Urease production (Lelliott, 1966)
Basal medium:
|
Oxoid urea agar base (CM53) |
2,4 g |
|
Distilled water |
95 ml |
Sterilize by autoclaving at 115 oC for 20 minutes. Cool the molten base to 50 oC and aseptically add 5 ml of filter-sterilized 40 % aqueous urea solution (Oxoid SR20). Mix well.
Distribute into 6 ml volumes in sterile tubes (16. x 100 mm) and allow to set as slopes with a good butt.
Positive reaction: the yellow-orange medium develops a cherry red or magenta pink coloration if urease activity has occurred.
Utilization of citrate (Christensen) (Skerman, 1967)
|
Citrate agar base (Merck 2503) |
23 g |
|
Distilled water |
1 litre |
Mix and dissolve by heating. Dispense into 6 ml volumes as for urea medium. Sterilize by autoclaving at 121 oC for 15 minutes and allow to set as slopes.
Positive reaction: citrate utilization is indicated by a change in the colour of the medium from orange to red.
— Hydrogen sulphide production (Ramamurthi, 1959)
Medium:
|
Difco bacto tryptone (No 0123) |
10 g |
|
K2HPO4 |
1 g |
|
NaCl |
5 g |
|
Distilled water |
1 litre. |
Dissolve and dispense into 6 ml volumes in 16 x 100 mm tubes. Sterilize by autoclaving at 115 oC for 10 minutes.
Inoculate and aseptically suspend a lead acetate paper (Merck 9511) from the lip if the tube. Hold in place with the cap. Incubate for up to 20 days.
Positive reaction: H2S production from tryptone is indicated by the development of a black-brown coloration of the test paper.
— Indole production (Ramamurthi, 1959)
Medium:
As for H2S test.
Remove the lead acetate paper and add 1 to 2 ml of diethyl ether and shake gently. Allow the layers to separate (five minutes). Add 0,5 ml Kovacs' reagent (Merck 9293) carefully down the inside of the sloped tube.
Positive reaction: the presence of indole is indicated by the development of a red colour in the yellow layer between the ether and aqueous fractions.
— Growth ar 37 oC (Ramamurthi, 1959)
Medium:
|
Difco bacto nutrient broth (No 0003) |
8 g |
|
Distilled water |
1 litre |
Mix, dissolve and distribute into 6 ml volumes in tubes.
Sterilize by autoclaving at 121 oC for 15 minutes.
Inoculate and incubate at 37 oC.
Positive reaction: look for growth.
— Growth in 7 % sodium chloride (Ramamurthi, 1959)
Medium:
|
Difco bacto nutrient broth |
8g |
|
NaCl |
70 g |
|
Distilled water |
1 litre |
Mix, dissolve and distribute into 6 ml volumes in tubes.
Sterilize by autoclaving at 121 oC for 15 minutes.
Positive reaction: look for growth.
— Gelatin hydrolysis (Lelliott, Billing & Hayward, 1966)
Medium:
|
Difco bacto gelatine (No 0143) |
120 g |
|
Distilled water |
1 litre |
Mix, dissolve by heating and distribute into 6 ml volumes in tubes.
Sterilize by autoclaving at 121 oC for 15 minutes.
Positive reaction: liquefaction of gelating even when held at 5 oC for 30 minutes.
— Starch hydrolysis
Medium:
|
Difco bacto nutrient agar (molten) |
1 litre |
|
Difco bacto soluble starch (No 0178) |
2 g |
Mix, sterilize by autoclaving at 115 oC for 10 minutes.
Pour plates. Spot inoculate the plates.
After good growth has occurred (10 to 20 days), remove part of the growth and flood with Lugol's iodine.
Positive reaction: starch hydrolysis is indicated by clear zones under or around the bacterial growth; the remainder of the medium stains purple.
— Aesculin hydrolase activity (Sneath & Collins, 1974)
Medium:
|
Difco bacto peptone |
10 g |
|
Aesculin |
1 g |
|
Ferric citrate (Merck 3862) |
0,05 g |
|
Sodium citrate |
1 g |
|
Distilled water |
1 litre |
Mix to dissolve and distribute into 6 ml volumes in tubes.
Sterilize by autoclaving at 115 oC for 10 minutes.
The medium is clear but has a bluish fluorescence.
Positive reaction: aesculin hydrolysis is indicated by the development of a brown colour together with a disappearance of fluorescence. This can be checked using an ultraviolet lamp.
REFERENCES
Bradbury, J. F., 1970. Isolation and preliminary study of bacteria from plants. Rev. PI. Path., 49, 213-218.
Dinesen, I. G., 1984. The extraction and diagnosis of Corynebacterium sepedonicum from diseased potato tubers. EPPO Bull. 14 (2), 147-152.
Doetsch, R. N., 1981. Determinative methods of light microscopy. In: Manual of methods for general bacteriology, American Society for Microbiology, Washington, 21-23.
Hugh, R. and Leifson, F., 1953. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram-negative bacteria. J. Bact., 66, 24-26.
Janse, J. D. and J. Van Vaerenbergh. The interpretation of the EC method for the detection of latent ring rot infections (Corynebacterium sepedonicum) in potato. EPPO Bull., No 17, 1987, pp. 1-10.
Kovacs, N., 1956. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature, Lond., 178, 703.
Lelliott, R. A., 1966. The plant pathogenic coryneform bacteria. J. appl. Bact., 29, 114-118.
Lelliott, R. A., E. Billing and A. C. Hayward, 1966. A determinative scheme for the fluorescent plant pathogenic pseudomonads J. appl. Bact., 29, 470-489.
Lelliott, R. A. and P. W., Sellar, 1976. The detection of latent ring rot (Corynebacterium sepedonicum (Spiek. et Kotth.) Skapt. et Burkh.) in potato stocks. EPPO Bull., 6 (2), 101-106.
Ramamurthi, C. S., 1959. Comparative studies on some Gram-positive phytopathogenic bacteria and their relationship to the Corynebacteria. Mem. Cornell agric. Exp. Sta., 366, 52 pp.
Skerman, V. B. D., 1967. A guide to the identification of the genera of bacteria. 2nd ed., William and Wilkins Company, Baltimore.
Sneath, P. H. A. and V. G. Collins, 1974. A study in test reproductibility between laboratories: report of Pseudomonas working party. Antonie van Leeuwenhoek, 40, 481-527.
ANNEX II
|
1. |
For each suspected occurrence for which a positive immunofluorescence test has been identified according to the method set out in Annex I, and confirmation or refutation by completion of the said method is awaited, there should be the retention and appropriate conservation of:
|
|
2. |
In the case of positive confirmation of the organism, there should be retention and appropriate conservation of:
until at least one month after the notification procedure under Article 5 (2). |
ANNEX III
|
1. |
The elements to be considered in the determination of the extent of probable contamination under Article 5 (1) (b), shall include:
|
|
2. |
The elements to be considered in the determination of the possible spread under Article 5 (1) (c) shall include:
|
|
3. |
The details of the notification referred to in the first subparagraph of Article 5 (2) shall include:
|
ANNEX IV
|
1. |
The officially supervised measures referred to in Article 7 (1) for the disposal of tubers or plants designated to be contaminated under Article 5 (1) (a) shall be:
|
|
2. |
The appropriate use or disposal of tubers or plants determined as probably contaminated under Article 5 (1) (b) and referred to in Article 7 (2), under the control of the responsible official bodies of the Member States, shall be:
|
|
3. |
The appropriate methods for cleansing and disinfecting of the objects referred to in Article 7 (3) shall be those for which it has been established that there is no identifiable risk of the organism spreading and shall be employed under the supervision of the responsible official bodies of the Member States. |
|
4. |
The series of measures to be implemented by Member States within the demarcated zone established under Article 5 (1) (c) and referred to in Article 7 (4) shall include:
|