

This document is an excerpt from the EUR-Lex website
Document 02010R0605-20180902
Commission Regulation (EU) No 605/2010 of 2 July 2010 laying down animal and public health and veterinary certification conditions for the introduction into the European Union of raw milk, dairy products, colostrum and colostrum-based products intended for human consumption (Text with EEA relevance)
Consolidated text: Commission Regulation (EU) No 605/2010 of 2 July 2010 laying down animal and public health and veterinary certification conditions for the introduction into the European Union of raw milk, dairy products, colostrum and colostrum-based products intended for human consumption (Text with EEA relevance)
Commission Regulation (EU) No 605/2010 of 2 July 2010 laying down animal and public health and veterinary certification conditions for the introduction into the European Union of raw milk, dairy products, colostrum and colostrum-based products intended for human consumption (Text with EEA relevance)
02010R0605 — EN — 02.09.2018 — 007.001
This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document
|
►M6 COMMISSION REGULATION (EU) No 605/2010 of 2 July 2010 laying down animal and public health and veterinary certification conditions for the introduction into the European Union of raw milk, dairy products, colostrum and colostrum-based products intended for human consumption ◄ (OJ L 175 10.7.2010, p. 1) |
Amended by:
|
|
|
Official Journal |
||
|
No |
page |
date |
||
|
COMMISSION IMPLEMENTING REGULATION (EU) No 914/2011 of 13 September 2011 |
L 237 |
1 |
14.9.2011 |
|
|
COMMISSION IMPLEMENTING REGULATION (EU) No 957/2012 of 17 October 2012 |
L 287 |
5 |
18.10.2012 |
|
|
COMMISSION IMPLEMENTING REGULATION (EU) No 300/2013 of 27 March 2013 |
L 90 |
71 |
28.3.2013 |
|
|
L 158 |
74 |
10.6.2013 |
||
|
COMMISSION IMPLEMENTING REGULATION (EU) No 556/2013 of 14 June 2013 |
L 164 |
13 |
18.6.2013 |
|
|
COMMISSION IMPLEMENTING REGULATION (EU) No 209/2014 of 5 March 2014 |
L 66 |
11 |
6.3.2014 |
|
|
COMMISSION IMPLEMENTING REGULATION (EU) 2018/83 of 19 January 2018 |
L 16 |
6 |
20.1.2018 |
|
|
COMMISSION IMPLEMENTING REGULATION (EU) 2018/1120 of 10 August 2018 |
L 204 |
31 |
13.8.2018 |
|
Corrected by:
COMMISSION REGULATION (EU) No 605/2010
of 2 July 2010
laying down animal and public health and veterinary certification conditions for the introduction into the European Union of raw milk, dairy products, colostrum and colostrum-based products intended for human consumption
(Text with EEA relevance)
Article 1
Subject matter and scope
This Regulation lays down:
(a) the public and animal health conditions and certification requirements for the introduction into the European Union of consignments of raw milk, dairy products, colostrum and colostrum-based products;
(b) the list of third countries from which the introduction into the European Union of such consignments is authorised.
This Regulation shall apply without prejudice to any specific certification requirements laid down in other Union acts or in agreements concluded by the Union with third countries.
Article 2
Importation of raw milk, dairy products, colostrum and colostrum-based products from third countries or parts thereof listed in column A of Annex I
Member States shall authorise the importation of consignments of raw milk, dairy products, colostrum and colostrum-based products from the third countries or parts thereof listed in column A of Annex I.
Article 3
Imports of certain dairy products from third countries or parts thereof listed in column B of Annex I
Member States shall authorise the importation of consignments of dairy products derived from raw milk of cows, ewes, goats or buffaloes from the third countries or parts thereof not at risk from foot-and-mouth disease listed in column B of Annex I, provided that such dairy products have undergone, or been produced from raw milk which has undergone a pasteurisation treatment involving a single heat treatment:
(a) with a heating effect at least equivalent to that achieved by a pasteurisation process of at least 72 °C for 15 seconds;
(b) where applicable, sufficient to ensure a negative reaction to an alkaline phosphatase test applied immediately after the heat treatment.
Article 4
Imports of certain dairy products from third countries or parts thereof listed in column C of Annex I
Member States shall authorise the importation of consignments of dairy products derived from raw milk of cows, ewes, goats, buffaloes or, where specifically authorised in Annex I, from camels of the species Camelus dromedarius from the third countries or parts thereof at risk of foot-and-mouth disease listed in column C of Annex I, provided that such dairy products have undergone, or been produced from raw milk which has undergone, a heat treatment involving:
◄
(a) a sterilisation process, to achieve an F0 value equal to or greater than three;
(b) an ultra high temperature (UHT) treatment at not less than 135 °C in combination with a suitable holding time;
(c)
(i) a high temperature short time pasteurisation treatment (HTST) at 72 °C for 15 seconds applied twice to milk with a pH equal to or greater than 7.0 achieving, where applicable, a negative reaction to a alkaline phosphatase test, applied immediately after the heat treatment; or
(ii) a treatment with an equivalent pasteurisation effect to point (i) achieving, where applicable, a negative reaction to an alkaline phosphatase test, applied immediately after the heat treatment;
(d) a HTST treatment of milk with a pH below 7.0; or
(e) a HTST treatment combined with another physical treatment by either:
(i) lowering the pH below 6 for one hour, or
(ii) additional heating equal to or greater than 72 °C, combined with desiccation.
2. Member States shall authorise the importation of consignments of dairy products derived from raw milk of animals other than those referred to in paragraph 1, from the third countries or parts thereof at risk of foot-and-mouth disease listed in column C of Annex I, provided that such dairy products have undergone, or been produced from raw milk which has undergone a treatment involving:
(a) a sterilisation process, to achieve an F0 value equal to or greater than three; or
(b) an ultra high temperature (UHT) treatment at not less than 135 °C in combination with a suitable holding time.
Article 5
Certificates
Consignments authorised for importation in accordance with Articles 2, 3 and 4 shall be accompanied by a health certificate drawn up in accordance with the appropriate model set out in Part 2 of Annex II for the commodity concerned and completed in accordance with the explanatory notes set out in Part 1 of that Annex.
However, the requirements laid down in this Article shall not preclude the use of electronic certification or of other agreed systems, harmonised at European Union level.
Article 6
Transit and storage conditions
The introduction into the European Union of consignments of raw milk, dairy products, colostrum and colostrum-based products not intended for importation into the European Union but destined for a third country either by immediate transit or after storage in the Union, in accordance with Articles 11, 12 or 13 of Directive 97/78/EC, shall only be authorised if the consignments comply with the following conditions:
(a) they come from a third country or part thereof listed in Annex I for the introduction into the European Union of consignments of raw milk, dairy products, colostrum or colostrum-based products and comply with the appropriate treatment conditions for such consignments, as provided for in Articles 2, 3 and 4;
(b) they comply with the specific animal health conditions for importation into the European Union of the raw milk, dairy products, colostrum or colostrum-based products concerned, as laid down in the animal health attestation in point II.1 of the relevant model health certificate set out in Part 2 of Annex II;
(c) they are accompanied by a health certificate drawn up in accordance with the appropriate model set out in Part 3 of Annex II for the consignment concerned and completed in accordance with the explanatory notes set out in Part 1 of that Annex;
(d) they are certified as acceptable for transit, including for storage as appropriate, on the Common Veterinary Entry Document referred to in Article 2(1) of Regulation (EC) No 136/2004, signed by the official veterinarian of the border inspection post of introduction into the Union.
Article 7
Derogation concerning transit and storage conditions
1. By way of derogation from Article 6, the transit by road or by rail through the European Union, between designated border inspection posts in Latvia, Lithuania and Poland listed in Commission Decision 2009/821/EC ( 1 ), of consignments coming from and destined to Russia directly or via another third country shall be authorised provided that the following conditions are complied with:
(a) the consignment is sealed with a serially numbered seal at the border inspection post of introduction into the European Union by the veterinary services of the competent authority;
(b) the documents accompanying the consignment and referred to in Article 7 of Directive 97/78/EC are stamped ‘ONLY FOR TRANSIT TO RUSSIA VIA THE EU’ on each page by the official veterinarian of the competent authority responsible for the border inspection post of introduction into the European Union;
(c) the procedural requirements provided for in Article 11 of Directive 97/78/EC are complied with;
(d) the consignment is certified as acceptable for transit on the common veterinary entry document by the official veterinarian of the border inspection post of introduction into the European Union.
2. Unloading or storage, as defined in Article 12(4) or in Article 13 of Directive 97/78/EC, of such consignments on European Union territory shall not be allowed.
3. Regular audits shall be made by the competent authority to ensure that the number of consignments and the quantities of products leaving the European Union territory matches the number and quantities entering the European Union.
Article 7a
Derogation for transit through Croatia of consignments coming from Bosnia and Herzegovina and destined to third countries
1. By way of derogation from Article 6, the direct transit by road through the Union, between the border inspection post of Nova Sela and the border inspection post of Ploče, of consignments coming from Bosnia and Herzegovina and destined to third countries shall be authorised provided that the following conditions are complied with:
(a) the consignment is sealed with a serially numbered seal by the official veterinarian at the border inspection post of entry;
(b) the documents accompanying the consignment and referred to in Article 7 of Directive 97/78/EC are stamped ‘ONLY FOR TRANSIT TO THIRD COUNTRIES VIA THE EU’ on each page by the official veterinarian at the border inspection post of entry;
(c) the procedural requirements provided for in Article 11 of Directive 97/78/EC are complied with;
(d) the consignment is certified as acceptable for transit on the Common Veterinary Entry Document referred to in Article 2(1) of Regulation (EC) No 136/2004 by the official veterinarian at the border inspection post of entry.
2. Unloading or storage, as defined in Article 12(4) or in Article 13 of Directive 97/78/EC, of such consignments on Union territory shall not be allowed.
3. Regular audits shall be made by the competent authority to ensure that the number of consignments and the quantities of products leaving the Union matches the number and quantities entering the Union.
Article 8
Specific treatment
Consignments of dairy products and colostrum-based products authorised for introduction into the European Union in accordance with Articles 2, 3, 4,6 or 7 from third countries or parts thereof where an outbreak of foot-and-mouth disease has occurred within the period of 12 months preceding the date of signature of the health certificate, or which have carried out vaccination against that disease during that period, shall only be authorised for introduction into the European Union if such products have undergone one of the treatments listed in Article 4.
Article 9
Repeal
Decision 2004/438/EC is repealed.
References to Decision 2004/438/EC shall be construed as references to this Regulation.
Article 10
Transitional provisions
For a transitional period until 30 November 2010, consignments of raw milk and milk-based products as defined in Decision 2004/438/EC in respect of which the relevant health certificates have been issued in accordance Decision 2004/438/EC may continue to be introduced into the European Union.
Article 11
Entry into force and applicability
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
It shall apply from 1 August 2010.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
ANNEX I
List of third countries or parts thereof authorised for the introduction into the European Union of consignments of raw milk, dairy products, colostrum (1) and colostrum-based products (1) and indicating the type of heat treatment required for such commodities
|
ISO code of third country |
Third country or part thereof |
Column A |
Column B |
Column C |
|
AE |
The Emirates of Abu Dhabi and Dubai of the United Arab Emirates (1) |
0 |
0 |
+ (2) |
|
AD |
Andorra |
+ |
+ |
+ |
|
AL |
Albania |
0 |
0 |
+ |
|
AR |
Argentina |
0 |
0 |
+ |
|
AU |
Australia |
+ |
+ |
+ |
|
BR |
Brazil |
0 |
0 |
+ |
|
BW |
Botswana |
0 |
0 |
+ |
|
BY |
Belarus |
0 |
0 |
+ |
|
BZ |
Belize |
0 |
0 |
+ |
|
BA |
Bosnia and Herzegovina |
+ |
+ |
+ |
|
CA |
Canada |
+ |
+ |
+ |
|
CH |
Switzerland (2) |
+ |
+ |
+ |
|
CL |
Chile |
0 |
+ |
+ |
|
CN |
China |
0 |
0 |
+ |
|
CO |
Colombia |
0 |
0 |
+ |
|
CR |
Costa Rica |
0 |
0 |
+ |
|
CU |
Cuba |
0 |
0 |
+ |
|
DZ |
Algeria |
0 |
0 |
+ |
|
ET |
Ethiopia |
0 |
0 |
+ |
|
GL |
Greenland |
0 |
+ |
+ |
|
GT |
Guatemala |
0 |
0 |
+ |
|
HK |
Hong Kong |
0 |
0 |
+ |
|
HN |
Honduras |
0 |
0 |
+ |
|
IL |
Israel |
0 |
0 |
+ |
|
IN |
India |
0 |
0 |
+ |
|
IS |
Iceland |
+ |
+ |
+ |
|
KE |
Kenya |
0 |
0 |
+ |
|
MA |
Morocco |
0 |
0 |
+ |
|
ME |
Montenegro |
+ |
+ |
+ |
|
MG |
Madagascar |
0 |
0 |
+ |
|
MK (3) |
former Yugoslav Republic of Macedonia |
0 |
+ |
+ |
|
MR |
Mauritania |
0 |
0 |
+ |
|
MU |
Mauritius |
0 |
0 |
+ |
|
MX |
Mexico |
0 |
0 |
+ |
|
NA |
Namibia |
0 |
0 |
+ |
|
NI |
Nicaragua |
0 |
0 |
+ |
|
NZ |
New Zealand |
+ |
+ |
+ |
|
PA |
Panama |
0 |
0 |
+ |
|
PY |
Paraguay |
0 |
0 |
+ |
|
RS (4) |
Serbia |
0 |
+ |
+ |
|
RU |
Russia |
0 |
0 |
+ |
|
SG |
Singapore |
0 |
0 |
+ |
|
SV |
El Salvador |
0 |
0 |
+ |
|
SZ |
Swaziland |
0 |
0 |
+ |
|
TH |
Thailand |
0 |
0 |
+ |
|
TN |
Tunisia |
0 |
0 |
+ |
|
TR |
Turkey |
0 |
0 |
+ |
|
UA |
Ukraine |
0 |
0 |
+ |
|
US |
United States |
+ |
+ |
+ |
|
UY |
Uruguay |
0 |
0 |
+ |
|
ZA |
South Africa |
0 |
0 |
+ |
|
ZW |
Zimbabwe |
0 |
0 |
+ |
|
(*1) The colostrum and colostrum-based products can only be introduced into the European Union from countries authorised in column A. (*2) Certificates in accordance with the Agreement between the European Community and the Swiss Confederation on trade in agricultural products (OJ L 114, 30.4.2002, p. 132). (*3) The former Yugoslav Republic of Macedonia; the definitive nomenclature for this country will be agreed following the conclusion of the negotiations currently taking place on this subject at UN level. (*4) Not including Kosovo which is at present under international administration pursuant to United Nations Security Council Resolution 1244 of 10 June 1999. (1) Only dairy products from camels of the species Camelus dromedarius. (2) Dairy products from camels of the species Camelus dromedarius are authorised. |
||||
ANNEX II
PART 1
Models of health certificates
|
‘Milk-RM’ |
: |
Health certificate for raw milk from third countries or parts thereof authorised in column A of Annex I intended for further processing in the European Union before being used for human consumption. |
|
‘Milk-RMP’ |
: |
Health certificate for dairy products derived from raw milk for human consumption, from third countries or parts thereof authorised in column A of Annex I intended for importation into the European Union. |
|
‘Milk-HTB’ |
: |
Health certificate for dairy products derived from milk of cows, ewes, goats and buffaloes for human consumption from third countries or parts thereof authorised in column B of Annex I intended for importation into the European Union. |
|
‘Milk-HTC’ |
: |
Health certificate for dairy products for human consumption from third countries or parts thereof authorised in column C of Annex I intended for importation into the European Union. |
|
‘Colostrum-C/CPB’ |
: |
Health certificate for colostrum of cows, ewes, goats and buffaloes and colostrum-based products derived from colostrum of the same species from third countries or parts thereof listed in column A of Annex I for human consumption intended for importation into the European Union. |
|
‘Milk/ Colostrum-T/S’ |
: |
Animal health certificate for raw milk, colostrum, dairy products or colostrum-based products for human consumption, intended for transit through or storage in the European Union. |
Explanatory notes
(a) The health certificates shall be issued by the competent authorities of the third country of origin, in accordance with the appropriate model set out in Part 2 of this Annex, according to the layout of the model that corresponds to the raw milk, colostrum, dairy products or colostrum-based products concerned. They shall contain, in the numbered order that appears in the model, the attestations that are required for any third country and, as the case may be, those supplementary guarantees that are required for the exporting third country concerned.
(b) The original of the health certificate shall consist of a single sheet printed on either pages or, where more text is required, such that all the sheets form a whole and cannot be separated.
(c) A separate, single health certificate must be presented for each consignment of the commodity concerned, exported to the same destination from a third country listed in the table in Annex I and transported in the same railway wagon, road vehicle, aircraft or ship.
(d) The original of the health certificate and the labels referred to in the model certificate shall be drawn up in at least one official language of the Member State where border inspection takes place and of the Member State of destination. However, those Member States may allow it to be drawn up in another official language of the European Union instead of their own, accompanied, if necessary, by an official translation.
(e) Where additional sheets are attached to the health certificate for the purpose of identifying the commodities making up the consignment, such additional sheets shall also be considered to form part of the original certificate, provided the signature and stamp of the certifying official veterinarian appear on each page.
(f) Where the health certificate comprises more than one page, each page shall be numbered ‘–x(page number) of y(total number of pages)–’ on the bottom of the page and shall bear the certificate reference number allocated by the competent authority on the top of the page.
(g) The original of the health certificate must be completed and signed by a representative of the competent authority responsible for verifying and certifying that the raw milk, colostrum, dairy products or colostrum-based products meet the health conditions laid down in Section IX of Chapter I of Annex III to Regulation (EC) No 853/2004 and in Directive 2002/99/EC.
(h) The competent authorities of the exporting third country shall ensure that principles of certification equivalent to those laid down in Council Directive 96/93/EC ( 2 ) are complied with.
(i) The colour of the signature of the official veterinarian shall be different from that of the printing on the health certificate. That requirement shall also apply to stamps other than embossed stamps or watermarks.
(j) The original of the health certificate must accompany the consignment until it reaches the border inspection post of introduction into the European Union.
(k) Where the model certificate states that certain statements shall be kept as appropriate, statements which are not relevant, may be crossed out and initialled and stamped by the certifying officer, or completely deleted from the certificate.
PART 2
Model Milk-RM
Health Certificate for raw milk from third countries or parts thereof authorised in column A of Annex I to Regulation (EU) No 605/2010 intended for further processing in the European Union before being used for human consumption



Model Milk-RMP
Health Certificate for dairy products derived from raw milk for human consumption from third countries or parts thereof authorised in column A of Annex I to Regulation (EU) No 605/2010 intended for importation into the European Union



Model Milk-HTB
Health Certificate for dairy products derived from milk of cows, ewes, goats and buffaloes for human consumption from third countries or parts thereof authorised in column B of Annex I to Regulation (EU) No 605/2010 intended for importation into the European Union



Model Milk-HTC
Health certificate for dairy products for human consumption from third countries or parts thereof authorised in column C of Annex I to Regulation (EU) No 605/2010 intended for importation into the European Union






PART 3
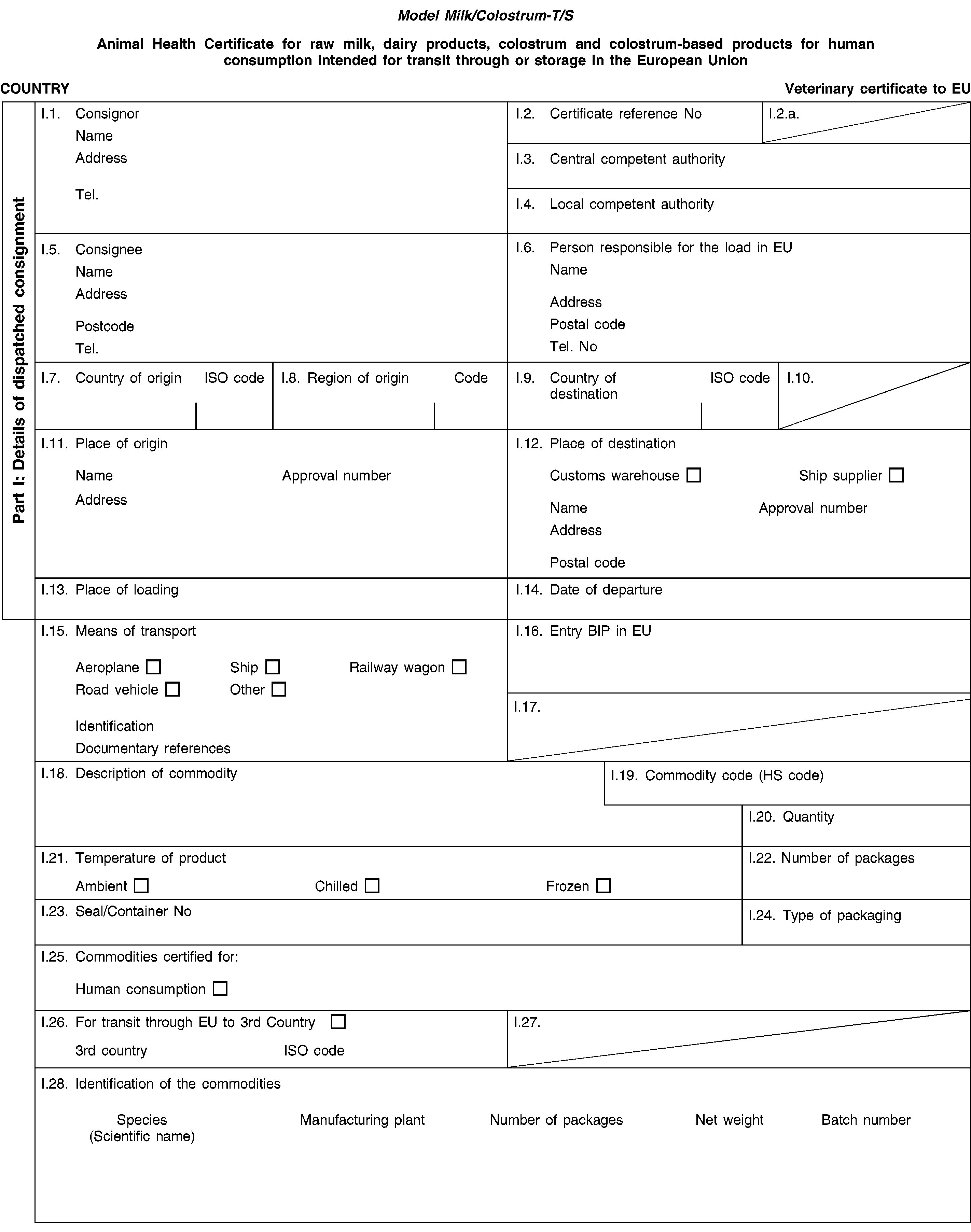


( 1 ) OJ L 296, 12.11.2009, p. 1
( 2 ) OJ L 13, 16.1.1997, p. 28.
