

This document is an excerpt from the EUR-Lex website
Document 01999L0045-20060213
Directive 1999/45/EC of the European Parliament and of the Council of 31 May 1999 concerning the approximation of the laws, regulations and administrative provisions of the Member States relating to the classification, packaging and labelling of dangerous preparations
Consolidated text: Directive 1999/45/EC of the European Parliament and of the Council of 31 May 1999 concerning the approximation of the laws, regulations and administrative provisions of the Member States relating to the classification, packaging and labelling of dangerous preparations
Directive 1999/45/EC of the European Parliament and of the Council of 31 May 1999 concerning the approximation of the laws, regulations and administrative provisions of the Member States relating to the classification, packaging and labelling of dangerous preparations
- Date of document:
- 13/02/2006
- Date of effect:
- 13/02/2006
- Author:
- European Parliament, Council of the European Union
- Form:
- Consolidated text
- Additional information:
- LASTMODIN 32006L0008
- Link
- Link
- Link
- Select all documents mentioning this document
- Consolidation: basic act:
- 31999L0045
1999L0045 — EN — 13.02.2006 — 004.001
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
DIRECTIVE 1999/45/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 May 1999 (OJ L 200, 30.7.1999, p.1) |
Amended by:
|
|
|
Official Journal |
||
|
No |
page |
date |
||
|
COMMISSION DIRECTIVE 2001/60/EC Text with EEA relevance of 7 August 2001 |
L 226 |
5 |
22.8.2001 |
|
|
REGULATION (EC) No 1882/2003 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 29 September 2003 |
L 284 |
1 |
31.10.2003 |
|
|
L 168 |
35 |
1.5.2004 |
||
|
COMMISSION DIRECTIVE 2006/8/EC Text with EEA relevance of 23 January 2006 |
L 19 |
12 |
24.1.2006 |
|
Corrected by:
DIRECTIVE 1999/45/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
of 31 May 1999
concerning the approximation of the laws, regulations and administrative provisions of the Member States relating to the classification, packaging and labelling of dangerous preparations
THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION,
Having regard to the Treaty establishing the European Community, and in particular Article 95 thereof,
Having regard to the proposal of the Commission ( 1 ),
Having regard to the opinion of the Economic and Social Committee ( 2 ),
Acting in accordance with the procedure laid down in Article 251 of the Treaty ( 3 ),
|
(1) |
Whereas Council Directive 88/379/EEC of 7 June 1988 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the classification, packaging and labelling of dangerous preparations ( 4 ) has been amended on several occasions; whereas on the occasion of further amendments, the said Directive should, for reasons of clarity, be recast; |
|
(2) |
Whereas, in spite of Community provisions, the rules applying to certain dangerous preparations in the Member States exhibit considerable differences as regards classification, packaging and labelling; whereas these differences constitute a barrier to trade, create unequal competition conditions and directly affect the functioning of the internal market; whereas it is therefore necessary to remove this barrier to trade by approximating the relevant legislation existing in the Member States; |
|
(3) |
Whereas measures for the approximation of the provisions of the Member States affecting the establishment and functioning of the internal market must, in so far as they concern health, safety and protection of man and the environment, adopt a high level of protection as a basis; whereas this Directive must, at the same time, ensure protection for the general public, and, in particular, persons who come into contact with dangerous preparations in the course of their work or in the pursuit of a hobby, protection for consumers and for the environment; |
|
(4) |
Whereas containers containing certain categories of dangerous preparations offered or sold to the general public must be fitted with child-resistant fastenings and/or carry a tactile warning of danger; whereas certain preparations not falling within these categories of danger may nevertheless, owing to their composition, present a danger for children; whereas the packaging of such preparations should therefore be equipped with child-resistant fastenings; |
|
(5) |
Whereas it is necessary to provide concentration limits expressed as a volume/volume percentage in the case of preparations marketed in gaseous form; |
|
(6) |
Whereas this Directive contains special labelling provisions applicable to certain preparations; whereas, to ensure an adequate level of protection for man and the environment, special labelling provisions must also be introduced for certain preparations which, although not dangerous within the meaning of this Directive, may nevertheless present a danger to the user; |
|
(7) |
Whereas on 30 April 1992 the Council adopted Directive 92/32/EEC amending for the seventh time Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances ( 5 ); whereas on 27 April 1993 the Commission adopted Directive 93/21/EEC ( 6 ) adapting to technical progress for the 18th time Council Directive 67/548/EEC; whereas new criteria developed for classifying and labelling substances dangerous for the environment were introduced by those Directives, together with the appropriate symbols, indications of danger, risk phrases and safety advice required to appear on labelling; whereas provisions should be adopted at Community level on the classification and labelling of preparations to take account of their effects on the environment and whereas it is therefore necessary to introduce a method for assessing the hazards of a given preparation for the environment either by a calculation method, or by determining the ecotoxicological properties by test methods under certain conditions; |
|
(8) |
Whereas the number of animals used for experiments should be reduced to a minimum, in accordance with the provisions of Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes ( 7 ); whereas Article 7(2) of that Directive stipulates that an experiment shall not be performed if another scientifically satisfactory method of obtaining the results sought, not entailing the use of an animal, is reasonably and practically available; whereas, therefore, this Directive makes use of the results of assessments of toxicological and ecotoxicological properties only when these are already known and entails no obligation to conduct further experiments on animals; |
|
(9) |
Whereas it is necessary to define what human experience might be considered for the evaluation of the health hazards of a preparation; whereas, if clinical studies may be accepted, it is taken as given that such studies comply with the Helsinki Declaration and OECD Guidelines for Good Clinical Practice; |
|
(10) |
Whereas the characteristics of alloys are such that it may not be possible accurately to determine their properties using currently available conventional methods; whereas it is therefore necessary to develop a specific method of classification which takes into account their particular chemical properties; whereas the Commission, in consultation with Member States, will examine this need and submit a proposal, if appropriate, before the implementation date of this Directive; |
|
(11) |
Whereas classification, packaging and labelling of plant protection products covered by Council Directive 78/631/EEC of 26 June 1978 on the approximation of the laws of the Member States relating to the classification, packaging and labelling of dangerous preparations (pesticides) ( 8 ) need to be revised taking into account technical and scientific developments as well as regulatory developments following implementation of Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market ( 9 ); |
|
(12) |
Whereas Directive 91/414/EEC and Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market ( 10 ), in contrast to the provisions applicable to chemical preparations covered by this Directive, provide for an authorisation procedure for each product on the basis of a dossier presented by the applicant and an assessment carried out by the competent authority in each Member State; whereas furthermore that authorisation procedure includes a control relating specifically to the classification, packaging and labelling of each product before it is placed on the market; whereas it is appropriate, as part of a clear and transparent information process, to classify and label plant protection products according to the provisions of this Directive, and also to provide instructions for use in accordance with the results of the evaluation carried out in the framework of Directive 91/414/EEC and to ensure that the labelling satisfies the high level of protection sought by both this Directive and Directive 91/414/EEC; whereas, in addition, a safety data sheet has to be established for plant protectioon products in accordance with this Directive; |
|
(13) |
Whereas it is appropriate to provide, in relation to environmental labelling, that specific exemptions or specific provisions may be decided upon in specific cases where it can be demonstrated that the overall environmental impact of the product types in question is lower than that of corresponding product types; |
|
(14) |
Whereas, although munitions are not covered by this Directive, explosives marketed to produce an explosive or pyrotechnic effect may, through their chemical composition, present dangers to health; whereas it is therefore necessary as part of a transparent information process to classify them and assign to them a safety data sheet in accordance with the provisions of this Directive and also to label them in accordance with the international rules used for the transport of dangerous goods; |
|
(15) |
Whereas, in order to take account of certain preparations which, although they are not considered dangerous under this Directive, may nevertheless present a danger for users, it is necessary to extend certain provisions of this Directive to cover such preparations; |
|
(16) |
Whereas the label constitutes a basic tool for users of the dangerous preparations in so far as it provides them with the initial essential concise information; whereas it nevertheless needs to be supplemented by a two-fold system of more detailed information, consisting firstly of the safety data sheet, intended for professional users as defined by Commission Directive 91/155/EEC of 5 March 1991 defining and laying down the detailed arrangements for the system of specific information relating to dangerous preparations in implementation of Article 10 of Directive 88/379/EEC ( 11 ) and secondly of the bodies appointed by the Member States which are responsible for the provision of information solely for medical purposes, both preventive and curative; |
|
(17) |
Whereas, on the basis of information to be supplied by the Member States and the various parties concerned, the Commission will submit a report to the European Parliament and the Council within two years of the entry into force of this Directive on experience with the present overall approach to labelling of dangerous preparations and in particular on its understanding and application by users, experience with publicity campaigns and educational and training programmes; whereas, on the basis of this report, the Commission will, if appropriate, submit the necessary proposals; |
|
(18) |
Whereas it is necessary to require safety data sheets providing proportionate information on the dangers to man and the environment arising from preparations not classified as dangerous within the meaning of this Directive but containing substances classified as dangerous or having a Community exposure limit; whereas the Commission, on the basis of information submitted by Member States, will review Directive 91/155/EEC and submit proposals, if appropriate, before the expiry of the date for implementation of this Directive; |
|
(19) |
Whereas, in the case of preparations classified as dangerous within the meaning of this Directive, it is appropriate to permit Member States to allow certain derogations with respect to labelling where the packaging is too small, or otherwise unsuitable for labelling, or where such small packaging or such small quantities are involved that there is no reason to fear any danger to man or the environment; whereas in such cases appropriate consideration should also be given to the approximation of the relevant provisions at Community level; whereas the Commission will therefore examine the needs for harmonisation and, if appropriate, submit proposals; |
|
(20) |
Whereas the confidentiality of certain substances contained in the preparations should be guaranteed and whereas it is therefore necessary to institute a system which allows the person responsible for placing the preparation on the market to request confidentiality for such substances; |
|
(21) |
Whereas the provisions of this Directive will have regard to the commitment entered into by the Community and its Member States, in accordance with the goals for sustainable development set under Agenda 21, Chapter 19, at the UNCED conference held in June 1992 in Rio de Janeiro, to strive for the future harmonisation of systems for the classification of dangerous substances and preparations; |
|
(22) |
Whereas the Commission should be given the powers necessary to adapt all the Annexes to this Directive to technical progress; |
|
(23) |
Whereas the adoption of this Directive should not affect the obligations of the Member States concerning the deadlines for transposition into national law and for application of the Directives indicated in Annex VIII; |
|
(24) |
Whereas the Directives indicated in Annex VIII should be repealed, subject to certain conditions; whereas the conditions for repealing the Directives indicated in Annex VIII should be specified for Austria, Finland and Sweden in order to take account of the present level of their legislation, in particular as regards the protection of health and the protection of the environment, |
HAVE ADOPTED THIS DIRECTIVE:
Article 1
Objectives and scope
1. This Directive aims at the approximation of the laws, regulations and administrative provisions of the Member States relating to:
— the classification, packaging and labelling of dangerous preparations, and to
— the approximation of specific provisions for certain preparations which may present hazards, whether or not they are classified as dangerous within the meaning of this Directive,
when such preparations are placed on the market of the Member States.
2. This Directive shall apply to preparations which:
— contain at least one dangerous substance within the meaning of Article 2,
— and
— are considered dangerous within the meaning of Article 5, 6 or 7.
3. The specific provisions set out:
— in Article 9 and defined in Annex IV,
— in Article 10 and defined in Annex V, and
— in Article 14
shall also apply to preparations which are not considered dangerous within the meaning of Articles 5, 6 or 7 but may nevertheless present a specific hazard.
4. Without prejudice to Directive 91/414/EEC, the articles on classification, packaging, labelling and safety data sheets of this Directive shall apply to plant protection products.
5. This Directive shall not apply to the following preparations in the finished state, intended for the final user:
(a) medicinal products for human or veterinary use, as defined in Directive 65/65/EEC ( 12 );
(b) cosmetic products as defined in Directive 76/768/EEC ( 13 );
(c) mixtures of substances which, in the form of waste, are covered by Directives 75/442/EEC ( 14 ) and 78/319/EEC ( 15 );
(d) foodstuffs;
(e) animal feedingstuffs;
(f) preparations containing radioactive substances as defined by Directive 80/836/Euratom ( 16 );
(g) medical devices which are invasive or used in direct physical contact with the human body in so far as Community measures lay down provisions for the classification and labelling of dangerous substances and preparations which ensure the same level of information provision and protection as this Directive.
6. This Directive shall not apply to:
— the carriage of dangerous preparations by rail, road, inland waterway, sea or air,
— preparations in transit which are under customs supervision, provided they do not undergo any treatment or processing.
Article 2
Definitions
1. For the purposes of this Directive:
(a) ‘substances’ means chemical elements and their compounds in the natural state or obtained by any production process, including any additive necessary to preserve the stability of the products and any impurity deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition;
(b) ‘preparations’ means mixtures or solutions composed of two or more substances;
(c) ‘polymer’ means a substance consisting of molecules characterised by the sequence of one or more types of monomer units and comprising a simple weight majority of molecules containing at least three monomer units which are covalently bound to at least one other monomer unit or other reactant and consists of less than a simple weight majority of molecules of the same molecular weight. Such molecules must be distributed over a range of molecular weights wherein differences in the molecular weight are primarily attributable to differences in the number of monomer units. In the context of this definition a ‘monomer unit’ means the reacted form of a monomer in a polymer;
(d) (………);
(e) ‘placing on the market’ means making available to third parties. Importation into the Community customs territory shall be deemed to be placing on the market for the purposes of this Directive;
(f) ‘scientific research and development’ means scientific experimentation, analysis or chemical research carried out under controlled conditions; it includes the determination of intrinsic properties, performance and efficacy as well as scientific investigation related to product development;
(g) ‘process-orientated research and development’ means the further development of a substance in the course of which pilot plant or production trials are used to test the fields of application of the substance;
(h) ‘Einecs’ means the European Inventory of Existing Commercial Chemical Substances. This inventory contains the definitive list of all chemical substances deemed to be on the Community market on 18 September 1981.
2. The following are ‘dangerous’ within the meaning of this Directive:
(a) explosive substances and preparations: solid, liquid, pasty or gelatinous substances and preparations which may also react exothermically without atmospheric oxygen thereby quickly evolving gases, and which, under defined test conditions, detonate, quickly deflagrate or upon heating explode when partially confined;
(b) oxidising substances and preparations: substances and preparations which give rise to a highly exothermic reaction in contact with other substances, particularly flammable substances;
(c) extremely flammable substances and preparations: liquid substances and preparations having an extremely low flash-point and a low boiling-point and gaseous substances and preparations which are flammable in contact with air at ambient temperature and pressure;
(d) highly flammable substances and preparations:
— substances and preparations which may become hot and finally catch fire in contact with air at ambient temperature without any application of energy, or
— solid substances and preparations which may readily catch fire after brief contact with a source of ignition and which continue to burn or to be consumed after removal of the source of ignition, or
— liquid substances and preparations having a very low flash-point, or
— substances and preparations which, in contact with water or damp air, evolve extremely flammable gases in dangerous quantities;
(e) flammable substances and preparations: liquid substances and preparations having a low flash-point;
(f) very toxic substances and preparations: substances and preparations which in very low quantities cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin;
(g) toxic substances and preparations: substances and preparations which in low quantities cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin;
(h) harmful substances and preparations: substances and preparations which may cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin;
(i) corrosive substances and preparations: substances and preparations which may, on contact with living tissues, destroy them;
(j) irritant substances and preparations: non-corrosive substances and preparations which, through immediate, prolonged or repeated contact with the skin or mucous membrane, may cause inflammation;
(k) sensitising substances and preparations: substances and preparations which, if they are inhaled or if they penetrate the skin, are capable of eliciting a reaction of hypersensitisation such that on further exposure to the substance of preparation, characteristic adverse effects are produced;
(l) carcinogenic substances and preparations: substances or preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce cancer or increase its incidence;
(m) mutagenic substances and preparations: substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce heritable genetic defects or increase their incidence;
(n) substances and preparations which are toxic for reproduction: substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may produce, or increase the incidence of, non-heritable adverse effects in the progeny and/or an impairment of male or female reproductive functions or capacity;
(o) substances and preparations which are dangerous for the environment: substances and preparations which, were they to enter the environment, would or could present an immediate or delayed danger for one or more components of the environment.
Article 3
Determination of dangerous properties of preparations
1. The evaluation of the hazards of a preparation shall be based on the determination of:
— physico-chemical properties,
— properties affecting health,
— environmental properties.
These different properties shall be determined in accordance with the provisions laid down in Articles 5, 6 and 7.
Where laboratory tests are conducted, they shall be carried out on the preparation as placed on the market.
2. Where the determination of dangerous properties is carried out in accordance with Articles 5, 6 and 7, all dangerous substances within the meaning of Article 2 and in particular those which:
— are listed in Annex I to Directive 67/548/EEC,
— are listed in Elincs in accordance with Article 21 of Directive 67/548/EEC,
— are classified and labelled provisionally by the person responsible for the placing on the market in accordance with Article 6 of Directive 67/548/EEC,
— are classified and labelled in accordance with Article 7 of Directive 67/548/EEC and are not yet included in Elincs,
— are covered by Article 8 of Directive 67/548/EEC,
— are classified and labelled in accordance with Article 13 of Directive 67/548/EEC,
shall be taken into consideration in accordance with the provisions laid down in the method used.
3. For preparations covered by this Directive, dangerous substances as referred to in paragraph 2 which are classified as dangerous on the basis of their health and/or environmental effects, whether they are present as impurities or additives, shall be taken into consideration when their concentrations are equal to, or greater than, those defined in the following table unless lower values are given in Annex I to Directive 67/548/EEC, or in Part B of Annex II to this Directive or in Part B of Annex III thereto, unless otherwise specified in Annex V to this Directive.
|
Category of danger of the substance |
Concentration to take into consideration for |
|
|
gaseous preparations % vol/vol |
other preparations % w/w |
|
|
Very toxic |
≥ 0,02 |
≥ 0,1 |
|
Toxic |
≥ 0,02 |
≥ 0,1 |
|
Carcinogenic Category 1 or 2 |
≥ 0,02 |
≥ 0,1 |
|
Mutagenic Category 1 or 2 |
≥ 0,02 |
≥ 0,1 |
|
Toxic for reproduction Category 1 or 2 |
≥ 0,02 |
≥ 0,1 |
|
Harmful |
≥ 0,2 |
≥ 1 |
|
Corrosive |
≥ 0,02 |
≥ 1 |
|
Irritant |
≥ 0,2 |
≥ 1 |
|
Sensitising |
≥ 0,2 |
≥ 1 |
|
Carcinogenic Category 3 |
≥ 0,2 |
≥ 1 |
|
Mutagenic Category 3 |
≥ 0,2 |
≥ 1 |
|
Toxic for reproduction Category 3 |
≥ 0,2 |
≥ 1 |
|
Dangerous for the environment N Dangerous for the environment N |
≥ 0,1 |
|
|
Dangerous for the environment ozone |
≥ 0,1 |
≥ 0,1 |
|
Dangerous for the environment |
≥ 1 |
|
Article 4
General principles of classification and labelling
1. The classification of dangerous preparations according to the degree and specific nature of the hazards involved shall be based on the definitions of categories of danger laid down in Article 2.
2. The general principles of the classification and labelling of preparations shall be applied in accordance with the criteria laid down in Annex VI to Directive 67/548/EEC, save where alternative criteria referred to in Article 5, 6, 7 or 10 and the relevant Annexes of this Directive are applied.
Article 5
Evaluation of the hazards deriving from physico-chemical properties
1. The hazards of a preparation deriving from its physico-chemical properties shall be assessed by determining, by means of the methods specified in Part A of Annex V to Directive 67/548/EEC, the physico-chemical properties of the preparation necessary for appropriate classification and labelling in accordance with the criteria laid down in Annex VI to that Directive.
2. By way of derogation from paragraph 1:
the determination of the explosive, oxidising, extremely flammable, highly flammable, or flammable properties is not necessary provided that:
— none of the constituents possesses such properties and that, on the basis of the information available to the manufacturer, the preparation is unlikely to present hazards of this kind,
— in the event of a change in the composition of a preparation of known composition, scientific evidence indicates that a reassessment of the hazards will not lead to a change in classification,
— preparations placed on the market in the form of aerosols satisfy the provisions of Article 9a of Directive 75/324/EEC ( 17 ).
3. For certain cases for which the methods laid down in Part A of Annex V to Directive 67/548/EEC are not appropriate, alternative calculation methods are laid down in Part B of Annex I to this Directive.
4. Certain exemptions from the application of the methods laid down in Part A of Annex V to Directive 67/548/EEC are referred to in Part A of Annex I to this Directive.
5. The hazards deriving from the physico-chemical properties of a preparation covered by Directive 91/414/EEC shall be assessed by determining the physico-chemical properties of the preparation necessary for appropriate classification in accordance with the criteria set out in Annex VI to Directive 67/548/EEC. These properties shall be determined by means of the methods laid down in Part A of Annex V to Directive 67/548/EEC unless other internationally recognised methods are acceptable in accordance with the provisions of Annexes II and III to Directive 91/414/EEC.
Article 6
Evaluation of health hazards
1. The health hazards of a preparation shall be assessed by one or more of the following procedures:
(a) by a conventional method described in Annex II;
(b) by determining the toxicological properties of the preparation necessary for appropriate classification in accordance with the criteria in Annex VI to Directive 67/548/EEC. These properties shall be determined by means of the methods laid down in Part B of Annex V to Directive 67/548/EEC, unless, in the case of plant protection products, other internationally recognised methods are acceptable in accordance with the provisions of Annexes II and III to Directive 91/414/EEC.
2. Without prejudice to the requirements of Directive 91/414/EEC, only where it can be scientifically demonstrated by the person responsible for placing the preparation on the market that the toxicological properties of the preparation cannot correctly be determined by the method outlined in paragraph 1(a), or on the basis of existing test results on animals, the methods outlined in paragraph 1(b) may be used, provided they are justified or specifically authorised under Article 12 of Directive 86/609/EEC.
When a toxicological property is established by the methods outlined in paragraph 1(b) to obtain new data, the test shall be conducted in compliance with the principles of good laboratory practice provided for in Council Directive 87/18/EEC of 18 December 1986 on the harmonisation of laws, regulations and administrative provisions relating to the application of the principles of good laboratory practice and the verification of their applications for tests on chemical substances ( 18 ) and the provisions of Directive 86/609/EEC, in particular Articles 7 and 12 thereof.
Subject to the provisions of paragraph 3, where a toxicological property has been established on the basis of both the methods outlined in paragraphs 1(a) and (b), the results from the methods outlined in paragraph 1(b) shall be used for classifying the preparation, except in the case of carcinogenic, mutagenic or toxic effects for reproduction for which only the method outlined in 1(a) shall be used.
Any of the toxicological properities of the preparation which are not assessed by the method outlined in paragraph 1(b) shall be assessed in accordance with the method outlined in paragraph 1(a).
3. Furthermore, where it can be demonstrated:
— by epidemiological studies, by scientifically valid case studies as specified by Annex VI to Directive 67/548/EEC or by statistically backed experience, such as the assessment of data from poison information units or concerning occupational diseases, that toxicological effects on man differ from those suggested by the application of the methods outlined in paragraph 1, then the preparation shall be classified according to its effects on man,
— that, owing to effects such as potentiation, a conventional assessment would underestimate the toxicological hazard, those effects shall be taken into account in classifying the preparation,
— that, owing to effects such as antagonism, a conventional assessment would overestimate the toxicological hazard, those effects shall be taken into account in classifying the preparation.
4. For preparations of a known composition, with the exception of those covered by Directive 91/414/EEC, classified in accordance with paragraph 1(b), a new evaluation of health hazard by the methods outlined in either paragraph 1(a) or (b) shall be performed whenever:
— changes of composition of the initial concentration, as a weight/weight or volume/volume percentage, of one or more of the dangerous constituents are introduced by the manufacturer, in accordance with the following table:
—
|
Initial concentration range of the constituent |
Permitted variation in initial concentration of the constituent |
|
≤ 2,5 % |
± 30 % |
|
> 2,5 ≤ 10 % |
± 20 % |
|
> 10 ≤ 25 % |
± 10 % |
|
> 25 ≤ 100 % |
± 5 % |
— changes of composition involving the substitution or addition of one or more constituents, which may or may not be dangerous within the meaning of the definitions set out in Article 2, are introduced by the manufacturer.
This new evaluation will apply unless there is valid scientific justification for considering that a re-evaluation of the hazard will not result in a change of classification.
Article 7
Evaluation of environmental hazards
1. The hazards of a preparation for the environment shall be assessed by one or more of the following procedures:
(a) by a conventional method described in Annex III to this Directive;
(b) by determining the hazardous properties of the preparation for the environment necessary for appropriate classification in accordance with the criteria set out in Annex VI to Directive 67/548/EEC. These properties shall be determined by means of the methods laid down in Part C of Annex V to Directive 67/548/EEC unless, in the case of plant protection products, other internationally recognised methods are acceptable in accordance with the provisions of Annexes II and III to Directive 91/414/EEC. Without prejudice to the testing requirements set out in Directive 91/414/EEC, the conditions for application of the test methods are described in Part C of Annex III to this Directive.
2. Where an ecotoxicological property is established by one of the methods outlined in paragraph 1(b) to obtain new data, the test shall be conducted in compliance with the principles of good laboratory practice provided for in Directive 87/18/EEC and with the provisions of Directive 86/609/EEC.
Where the environmental hazards have been assessed in compliance with both the procedures mentioned above, the results of the methods referred to in paragraph 1(b) shall be used for classifying the preparation.
3. For preparations of a known composition, with the exception of those covered by Directive 91/414/EEC, classified in accordance with the method outlined in paragraph 1(b), a new evaluation of environmental hazard either by the method outlined in paragraph 1(a) or that outlined in paragraph 1(b) shall be performed whenever:
— changes of composition of the initial concentration, as a weight/weight or volume/volume percentage, of one or more of the dangerous constituents are introduced by the manufacturer, in accordance with the following table:
—
|
Initial concentration range of the constituent |
Permitted variation in initial concentration of the constituent |
|
≤ 2,5 % |
± 30 % |
|
> 2,5 ≤ 10 % |
± 20 % |
|
> 10 ≤ 25 % |
± 10 % |
|
> 25 ≤ 100 % |
± 5 % |
— changes of composition involving the substitution or addition of one or more constituents, which may or may not be dangerous within the meaning of the definitions set out in Article 2, are introduced by the manufacturer.
This new evaluation will apply unless there is valid scientific justification for considering that a re-evaluation of the hazard will not result in a change of classification.
Article 8
Obligations and duties of the Member States
1. Member States shall take all necessary measures to ensure that the preparations covered by this Directive cannot be placed on the market unless they comply with it.
2. In order to ensure compliance with this Directive, the authorities of the Member States may request information on the composition of the preparation and any other pertinent information from any person responsible for placing the preparation on the market.
3. Member States shall take all necessary measures to ensure that those responsible for placing the preparation on the market hold at the disposal of the authorities of the Member States:
— the data used for the classification and labelling of the preparation,
— any pertinent information relating to packaging requirements in accordance with Article 9(1.3), including the test certificate issued in accordance with Part A of Annex IX to Directive 67/548/EEC,
— the data used for establishing the safety data sheet, in accordance with Article 14.
4. Member States and the Commission shall exchange information concerning the name and full address of the national authority (authorities) responsible for communicating and exchanging information relating to the practical application of this Directive.
Article 9
Packaging
|
1. |
Member States shall take all necessary measures to ensure that: 1.1. preparations within the meaning of Article 1(2) and preparations covered by Annex IV pursuant to Article 1(3) cannot be placed on the market unless their packaging satisfies the following requirements: — it shall be so designed and constructed that its contents cannot escape; this requirement shall not apply where special safety devices are prescribed, — the materials constituting the packaging and fastenings must not be susceptible to adverse attack by the contents, or liable to form dangerous compounds with the contents, — packaging and fastenings must be strong and solid throughout to ensure that they will not loosen and will safely meet the normal stresses and strains of handling, — containers fitted with replaceable fastening devices shall be so designed that the packaging can be refastened repeatedly without the contents escaping; 1.2. containers which contain preparations within the meaning of Article 1(2) and preparations covered by Annex IV pursuant to Article 1(3) offered or sold to the general public do not have: — either a shape and/or graphic decoration likely to attract or arouse the active curiosity of children or to mislead consumers, or — a presentation and/or a designation used for foodstuffs or animal feedingstuffs or medicinal or cosmetic products. 1.3. containers which contain certain preparations offered or sold to the general public covered by Annex IV to this Directive: — are fitted with child-resistant fastenings, — and/or — carry a tactile warning of danger. The devices must conform to the technical specifications given in Parts A and B of Annex IX to Directive 67/548/EEC. |
|
2. |
The packaging of preparations shall be deemed to satisfy the requirements of paragraph 1.1, first, second and third indents, if it complies with the requirements for carriage of dangerous goods by rail, road, inland waterway, sea or air. |
Article 10
Labelling
1.1. Member States shall take all necessary measures to ensure that:
(a) preparations within the meaning of Article 1(2) cannot be placed on the market unless the labelling on their packaging satisfies all the requirements of this Article and the specific provisions of Part A and B of Annex V;
(b) preparations within the meaning of Article 1(3) as defined in Parts B and C of Annex V cannot be placed on the market unless the labelling on their packaging satisfies the requirements of paragraphs 2.1 and 2.2 and the specific provisions of Parts B and C of Annex V.
1.2. With respect to plant protection products subject to Directive 91/414/EEC, the labelling requirements in accordance with this Directive shall be accompanied by the following wording: ‘To avoid risks to man and the environment, comply with the instructions for use.’
This labelling shall be without prejudice to the information required in accordance with Article 16 of, and Annex V to, Directive 91/414/EEC.
|
2. |
The following information shall be clearly and indelibly marked on any package: 2.1. the trade name or designation of the preparation; 2.2. the name, full address and telephone number of the person established in the Community who is responsible for placing the preparation on the market, whether it be the manufacturer, the importer or the distributor; 2.3. the chemical name of the substance or substances present in the preparation in accordance with the following detailed rules: 2.3.1. for preparations classified T+, T, Xn in accordance with Article 6, only the substances T+, T, Xn present in concentrations equal to, or greater than, the lowest limit (limit Xn) for each of them laid down in Annex I to Directive 67/548/EEC or, failing that, Part B of Annex II to this Directive have to be taken into consideration; 2.3.2. for preparations classified C in accordance with Article 6, only C substances present in concentrations equal to, or greater than, the lowest limit (limit Xi) laid down in Annex I to Directive 67/548/EEC or, failing that, Part B of Annex II to this Directive have to be taken into consideration; 2.3.3. the name of the substances which have given rise to the classification of the preparation in one or more of the following danger categories: — carcinogen category 1, 2 or 3, — mutagen category 1, 2 or 3, — toxic for reproduction category 1, 2 or 3, — very toxic, toxic or harmful due to non-lethal effects after a single exposure, — toxic or harmful due to severe effects after repeated or prolonged exposure, — sensitising; shall be mentioned on the label. The chemical name shall be one of the designations listed in Annex I to Directive 67/548/EEC or an internationally recognised chemical nomenclature if no corresponding designation is yet listed in that Annex. 2.3.4. As a consequence of the above provisions the name of any substance which led to the classification of the preparation in the following danger categories: — explosive, — oxidising, — extremely flammable, — highly flammable, — flammable, — irritant, — dangerous for the environment, need not be mentioned on the label unless the substance has to be mentioned pursuant to paragraphs 2.3.1, 2.3.2 or 2.3.3. 2.3.5. As a general rule, a maximum of four chemical names shall suffice to identify the substances primarily responsible for the major health hazards which have given rise to the classification and the choice of the corresponding phrases referring to the risk involved. In some cases, more than four chemical names may be necessary. 2.4. The danger symbol(s) and indication(s) of danger The danger symbols, where specified in this Directive, and indications of the dangers involved in the use of the preparation, shall be in accordance with the wording of Annexes II and VI to Directive 67/548/EEC and shall be applied in accordance with the evaluation of the hazards carried out in accordance with Annexes I, II and III to this Directive. Where more than one danger symbol must be assigned to a preparation the obligation to apply the symbol: — T shall make the symbols C and X optional unless otherwise specified in Annex I to Directive 67/548/EEC, — C shall make the symbol X optional, — E shall make the symbols F and O optional, — Xn shall make the symbol Xi optional. The symbol(s) shall be printed in black on an orange-yellow background. 2.5. The risk phrases (R phrases) The indications concerning special risks (R phrases) shall comply with the wording in Annexes III and VI to Directive 67/548/EEC and shall be assigned in accordance with the results of the hazard evaluation carried out in accordance with Annexes I, II, and III to this Directive. As a general rule, a maximum of six R phrases shall suffice to describe the risks; for this purpose, the combined phrases listed in Annex III to Directive 67/548/EEC shall be regarded as single phrases. However, if the preparation falls within more than one danger category, those standard phrases shall cover all the principal hazards associated with the preparation. In some cases more than six R phrases may be necessary. The standard phrases ‘extremely flammable’ or ‘highly flammable’ need not be used where they describe an indication of danger used in accordance with 2.4. 2.6. The safety advice (S phrases) The indications giving safety advice (S phrases) shall comply with the wording in Annex IV and with Annex VI to Directive 67/548/EEC and shall be assigned in accordance with the results of the hazard evaluation carried out in accordance with Annexes I, II and III to this Directive. As a general rule, a maximum of six S phrases shall suffice to formulate the most appropriate safety advice; for this purpose the combined phrases listed in Annex IV to Directive 67/548/EEC shall be regarded as single phrases. However, in some cases more than six S phrases may be necessary. Where it is physically impossible to include the advice on the label or package itself, the package shall be accompanied by safety advice on the use of the preparation.
|
|
3. |
For certain preparations classified as dangerous within the meaning of Article 7, by way of derogation from paragraphs 2.4, 2.5, and 2.6 of this Article, exemptions to certain provisions on environmental labelling or specific provisions in relation to environmental labelling may be determined in accordance with the procedure referred to in Article 20, where it can be demonstrated that there would be a reduction in the environmental impact. These exemptions or specific provisions are defined and laid down in Part A or B of Annex V. |
4. If the contents of the package do not exceed 125 ml:
— in the case of preparations that are classified as highly flammable, oxidising, irritant, with the exception of those assigned R41, or dangerous for the environment and assigned the N symbol it shall not be necessary to indicate the R phrases or the S phrases,
— in the case of preparations that are classified as flammable or dangerous for the environment and not assigned the N symbol it shall be necessary to indicate the R phrases but it shall not be necessary to indicate the S phrases.
5. Without prejudice to Article 16(4) of Directive 91/414/EC, indications such as ‘non-toxic’, ‘non-harmful’, ‘non-polluting’, ‘ecological’ or any other statement indicating that the preparation is not dangerous or likely to lead to underestimation of the dangers of the preparation in question shall not appear on the packaging or labelling of any preparation subject to this Directive.
Article 11
Implementation of the labelling requirements
1. Where the particulars required by Article 10 appear on a label, that label shall be firmly affixed to one or more surfaces of the packaging so that those particulars can be read horizontally when the package is set down normally. The dimensions of the label are laid down in Annex VI to Directive 67/548/EEC and the label is intended solely for provision of the information required by this Directive and if necessary of any supplementary health or safety information.
2. A label shall not be required when the particulars are clearly shown on the package itself, as specified in paragraph 1.
3. The colour and presentation of the label — or, in the case of paragraph 2, of the package — shall be such that the danger symbol and its background stand out clearly from it.
4. The information required on the label under Article 10 shall stand out clearly from its background and shall be of such size and spacing as to be easily read.
Specific provisions regarding the presentation and format of this information shall be laid down in Annex VI to Directive 67/548/EEC.
5. Member States may make the placing on the market of preparations covered by this Directive within their territories subject to use of their official language or languages in respect of the labelling thereof.
6. For the purposes of this Directive, labelling requirements shall be deemed to be satisfied:
(a) in the case of an outer package containing one or more inner packages, if the outer package is labelled in accordance with international rules on the transport of dangerous goods and the inner package or packages are labelled in accordance with this Directive;
(b) in the case of a single package:
— if such a package is labelled in accordance with international rules on the transport of dangerous goods and with Article 10(2.1), (2.2), (2.3), (2.5) and (2.6); for preparations classified according to Article 7, the provisions of Article 10(2.4) shall additionally apply with respect to the property in question when it has not been so identified on the label, or
— where appropriate, for particular types of packaging such as mobile gas cylinders, if the specific requirements referred to in Annex VI to Directive 67/548/EEC are complied with.
Where dangerous preparations do not leave the territory of a Member State, labelling may be permitted which complies with national rules instead of with international rules on the transport of dangerous goods.
Article 12
Exemptions from the labelling and packaging requirements
1. Articles 9, 10 and 11 shall not apply to explosives placed on the market with a view to obtaining an explosive or pyrotechnic effect.
2. For certain dangerous preparations within the meaning of Article 5, 6 or 7 defined in Annex VII which, in the form in which they are placed on the market, do not present any physico-chemical risk, or risk to health or to the environment, Articles 9, 10 and 11 shall not apply.
3. Member States may also:
(a) permit the labelling required by Article 10 to be applied in some other appropriate manner on packages which are either too small or otherwise unsuitable for labelling in accordance with Article 11(1) and (2);
(b) by way of derogation from Articles 10 and 11 permit the packaging of dangerous preparations which are classified as harmful, extremely flammable, highly flammable, flammable, irritant or oxidising to be unlabelled or to be labelled in some other way, if they contain such small quantities that there is no reason to fear any danger to persons handling such preparations or to other persons;
(c) by way of derogation from Articles 10 and 11, for preparations classified according to Article 7, permit the packaging of dangerous preparations to be unlabelled or labelled in some other way if they contain such small quantities that there is no reason to fear any dangers to the environment;
(d) by way of derogation from Articles 10 and 11 permit the packaging of dangerous preparations which are not mentioned in (b) or (c) above to be labelled in some other appropriate way, if the packages are too small for the labelling provided for in Articles 10 and 11 and there is no reason to fear any danger to persons handling such preparations or to other persons.
Where this paragraph is applied, the use of symbols, indications of danger, risk (R) phrases or safety (S) phrases different to those laid down in this Directive shall not be permitted.
4. If a Member State makes use of the options provided for in paragraph 3, it shall forthwith inform the Commission and Member States thereof. Where it is appropriate, measures shall be decided upon in the framework of Annex V and in accordance with the provisions of article 20.
Article 13
Distance selling
Any advertisement for a preparation within the meaning of this Directive which enables a member of the general public to conclude a contract for purchase without first having sight of the label for that preparation must make mention of the type or types of hazard indicated on the label. This requirement is without prejudice to Directive 97/7/EC of the European Parliament and of the Council of 20 May 1997 on the protection of consumers in respect of distance contracts ( 19 ).
Article 14
Safety data sheet
1. The safety data sheet information is principally intended for use by professional users and must enable them to take the necessary measures as regards the protection of health, safety and the environment at the place of work.
2.1. Member States shall take all the necessary measures to ensure that:
(a) the person responsible for placing on the market a preparation within the meaning of Article 1(2) provides a safety data sheet;
(b) the person responsible for placing on the market a preparation provides on request of a professional user a safety data sheet providing proportionate information for preparations not classified as dangerous within the meaning of Articles 5, 6 and 7 but containing in an individual concentration of ≥ 1 % by weight for non-gaseous preparations and ≥ 0,2 % by volume for gaseous preparations at least:
— one substance posing health or environmental hazards, or
— one substance for which there are Community workplace exposure limits.
2.2. The safety data sheet and its supply must comply with the provisions of Directive 91/155/EEC.
2.3. The necessary amendments required to adapt to technical progress Directive 91/155/EEC shall be adopted in accordance with the procedure laid down in Article 20 of this Directive.
In particular, the necessary amendments to take account of provisions in paragraph 2.1(b) shall be adopted before the date specified in Article 22(1).
2.4. The safety data sheet may be supplied on paper or electronically, provided that the addressee has the necessary means of receiving it.
Article 15
Confidentiality of chemical names
Where the person responsible for placing the preparation on the market can demonstrate that the disclosure on the label or safety data sheet of the chemical identity of a substance which is exclusively classified as:
— irritant with the exception of those assigned R41 or irritant in combination with one or more of the other properties mentioned in point 2.3.4 of Article 10, or
— harmful or harmful in combination with one or more of the properties mentioned in point 2.3.4 of Article 10 presenting acute lethal effects alone
will put at risk the confidential nature of his intellectual property, he may, in accordance with the provisions of Annex VI, be permitted to refer to that substance either by means of a name that identifies the most important functional chemical groups or by means of an alternative name. This procedure may not be applied where the substance concerned has been assigned a Community exposure limit.
Where the person responsible for placing a preparation on the market wishes to take advantage of confidentiality provisions, he shall make a request to the competent authority of the Member State in which the preparation is to be first placed on the market.
This request must be made in accordance with the provisions of Annex VI and must provide the information required in the form in Part A of that Annex. The competent authority may nevertheless request further information from the person responsible for placing the preparation on the market if such information appears necessary in order to evaluate the validity of the request.
The authority of the Member State receiving a request for confidentiality shall notify the applicant of its decision. The person responsible for placing the preparation on the market shall forward a copy of this decision to each of the Member States where he wishes to market the product.
Confidential information brought to the attention of the authorities of a Member State or of the Commission shall be treated in accordance with Article 19(4) of Directive 67/548/EEC.
Article 16
Rights of Member States regarding safety of workers
This Directive shall not affect the right of Member States to specify, in compliance with the Treaty, the requirements they deem necessary to ensure that workers are protected when using the dangerous preparations in question, provided that this does not mean that the classification, packaging, and labelling of dangerous preparations are modified in a way not provided for in this Directive.
Article 17
Bodies responsible for receiving information relating to health
Member States shall appoint the body or bodies responsible for receiving information, including chemical composition, relating to preparations placed on the market and considered dangerous on the basis of their health effects or on the basis of their physico-chemical effects.
Member States shall take the necessary steps to ensure that the appointed bodies provide all the requisite guarantees for maintaining the confidentiality of the information received. Such information may only be used to meet any medical demand by formulating preventive and curative measures, in particular in case of emergency.
Member States shall ensure that the information is not used for other purposes.
Member States shall ensure that the appointed bodies have at their disposal all the information required from the manufacturers or persons responsible for marketing to carry out the tasks for which they are responsible.
Article 18
Free movement clause
Without prejudice to the provisions set out in other Community legislation, Member States may not prohibit, restrict or impede the placing on the market of preparations because of their classification, packaging, labelling or safety data sheets if such preparations comply with the provisions laid down in this Directive.
Article 19
Safeguard clause
1. Where a Member State has detailed evidence that a preparation, although satisfying the provisions of this Directive, constitutes a hazard for man or the environment on grounds relating to the provisions of this Directive, it may provisionally prohibit the placing on the market of that preparation or subject it to special conditions in its territory. It shall immediately inform the Commission and the other Member States of such action and give reasons for its decision.
2. In the case referred to in paragraph 1, the Commission shall consult the Member States as soon as possible.
3. The Commission shall take a decision in accordance with the procedure laid down in Article 20.
Article 20
1. Amendments required to adapt the Annexes to this Directive to technical progress shall be adopted in accordance with the procedure laid down in Article 29(4)(a) of Directive 67/548/EEC.
2. The Commission shall be assisted by a committee.
3. Where reference is made to this Article, Articles 5 and 7 of Decision 1999/468/EC ( 20 ) shall apply, having regard to the provisions of Article 8 thereof.
The period laid down in Article 5(6) of Decision 1999/468/EC shall be set at three months.
4. The Committee shall adopt its rules of procedure.
Article 21
Repeal of Directives
1. The Directives listed in Part A of Annex VIII are hereby repealed, without prejudice to the obligation of the Member States concerning the deadlines for transposition into national law and for application of the Directives indicated in Part B of Annex VIII.
2. The Directives listed in Part A of Annex VIII shall apply to Austria, Finland and Sweden subject to provisions laid down in Part C of that Annex and pursuant to the Treaty.
3. References to the repealed Directives shall constitute references to this Directive and should be read in accordance with the correlation table set out in Annex IX.
Article 22
Transposition
1. Member States shall adopt and publish before 30 July 2002 the laws, regulations and administrative provisions necessary to comply with this Directive. They shall forthwith inform the Commission thereof.
2. Member States shall apply the laws, regulations and administrative provisions referred to in paragraph 1:
(a) to preparations not within the scope of Directive 91/414/EEC or Directive 98/8/EC as from 30 July 2002; and
(b) to preparations within the scope of Directive 91/414/EEC or Directive 98/8/EC as from 30 July 2004.
3. When Member States adopt such measures, they shall contain a reference to this Directive or shall be accompanied by such reference at the time of their official publication. The methods of making such reference shall be laid down by Member States.
Article 23
Entry into force
This Directive shall enter into force on the day of its publication in the Official Journal of the European Communities.
Article 21(2) shall apply from 1 January 1999.
Article 24
Addressees
This Directive is addressed to the Member States.
ANNEX I
METHODS FOR THE EVALUATION OF PHYSICO-CHEMICAL PROPERTIES OF PREPARATIONS IN ACCORDANCE WITH ARTICLE 5
PART A
Exemptions to test methods of Annex V — Part A to Directive 67/548/EEC
See 2.2.5 of Annex VI to Directive 67/548/EEC.
PART B
Alternative calculation methods
B.1. Non-gaseous preparations
1. Method for the determination of oxidising properties of preparations containing organic peroxides.
See point 2.2.2.1 of Annex VI to Directive 67/548/EEC.
B.2. Gaseous preparations
1. Method for the determination of oxidising properties
See 9.1.1.2 of Annex VI to Directive 67/548/EEC.
2. Method for the determination of flammability properties
See 9.1.1.1 of Annex VI to Directive 67/548/EEC.
ANNEX II
METHODS FOR THE EVALUATION OF HEALTH HAZARDS OF PREPARATIONS IN ACCORDANCE WITH ARTICLE 6
Introduction
An assessment must be made for all the health effects corresponding to the health effects of substances contained in a preparation. This conventional method described in Parts A and B of this Annex is a calculation method which is applicable to all preparations and which takes into consideration all the health hazards of substances contained in the preparation. For that purpose the dangerous health effects have been subdivided into:
1. acute lethal effects;
2. non-lethal irreversible effects after a single exposure;
3. severe effects after repeated or prolonged exposure;
4. corrosive effects, irritant effects;
5. sensitising effects;
6. carcinogenic effects, mutagenic effects, toxic effects for reproduction.
The health effects of a preparation are to be assessed in accordance with Article 6(1)(a) by the conventional method described in parts A and B of this Annex using individual concentration limits.
(a) where the dangerous substances listed in Annex I to Directive 67/548/EEC are assigned concentration limits necessary for the application of the method of assessment described in part A of this Annex, these concentration limits must be used;
(b) where the dangerous substances do not appear in Annex I to Directive 67/548/EEC or appear there without the concentration limits necessary for the application of the method of evaluation described in part A of this Annex, the concentration limits must be assigned in accordance with the specifications in part B of this Annex.
The procedure for classification is set out in Part A of this Annex.
The classification of the substance(s) and the resulting classification of the preparation are expressed:
— either by a symbol and one or more risk phrases, or
— by categories (category 1, category 2 or category 3) also assigned risk phrases when substances and preparations are shown to be carcinogenic, mutagenic or toxic for reproduction. Therefore it is important to consider, in addition to the symbol, all the phrases denoting specific risks which are assigned to each substance under consideration.
The systematic assessment of all the dangerous health effects is expressed by means of concentration limits, expressed as a weight/weight percentage except for gaseous preparations where they are expressed as a volume/volume percentage and in conjunction with the classification of the substance.
Where they are not given in Annex I to Directive 67/548/EEC, the concentration limits to be taken into account for the application of this conventional method are those set out in Part B of this Annex.
PART A
Procedure for evaluation of health hazards
The evaluation proceeds stepwise as follows:
1. The following preparations are to be classified as very toxic:
1.1. owing to their acute lethal effects and assigned the symbol ‘T+’, the indication of danger ‘very toxic’ and the risk phrases R26, R27 or R28;
1.1.1. preparations containing one or more substances classified as very toxic that produce such effects, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 1 in Part B of this Annex (Table I and I A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
1.1.2. preparations containing more than one substance classified as very toxic in lower individual concentrations than the limits specified under 1.1.1(a) or (b) if:
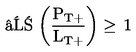
where:
|
PT+ |
= |
is the percentage by weight or by volume of each very toxic substance in the preparation, |
|
LT+ |
= |
is the very toxic limit specified for each very toxic substance, expressed as a percentage by weight or by volume; |
1.2. owing to their non-lethal irreversible effects after a single exposure and assigned the symbol ‘T+’, the indication of danger ‘very toxic’ and the risk phrase R39/route of exposure.
Preparations containing at least one dangerous substance that produces such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 2 in Part B of this Annex (Table II and II A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
2. The following preparations shall be classified as toxic:
2.1. owing to their acute lethal effects and assigned the symbol ‘T’, the indication of danger ‘toxic’ and the risk phrases R23, R24 or R25;
2.1.1. preparations containing one or more substances classified as very toxic or toxic that produce such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 1 in Part B of this Annex (Table I and I A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
2.1.2. preparations containing more than one substance classified as very toxic or toxic in lower individual concentrations than the limits specified under 2.1.1(a) or (b) if:

Where:
|
PT+ |
= |
is the percentage by weight or by volume of each very toxic substance in the preparation, |
|
PT |
= |
is the percentage by weight or by volume of each toxic substance in the preparation, |
|
LT |
= |
is the respective toxic limit specified for each very toxic or toxic substance, expressed as a percentage by weight or by volume; |
2.2. owing to their non-lethal irreversible effects after a single exposure and assigned the symbol ‘T’, the indication of danger ‘toxic’ and the risk phrase R39/route of exposure.
Preparations containing at least one dangerous substance classified as very toxic or toxic that produce such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 2 in Part B of this Annex (Table II and II A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
2.3. owing to their long-term effects and assigned the symbol ‘T’, the indication of danger ‘toxic’ and the risk phrase R48/route of exposure.
Preparations containing at least one dangerous substance that produces such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 3 in Part B of this Annex (Table III and III A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
3. The following preparations shall be classified as harmful:
3.1. owing to their acute lethal effects and assigned the symbol ‘Xn’ and the indication of danger ‘harmful’ and the risk phrases R20, R21 or R22;
3.1.1. preparations containing one or more substances classified as very toxic, toxic or harmful and that produce such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 1 in Part B of this Annex (Table I and I A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
3.1.2. preparations containing more than one substance classified as very toxic, toxic or harmful in lower individual concentrations than the limits specified under 3.1.1(a) or (b) if:

Where:
|
PT+ |
= |
is the percentage by weight or by volume of each very toxic substance in the preparation, |
|
PT |
= |
is the percentage by weight or by volume of each toxic substance in the preparation, |
|
PXn |
= |
is the percentage by weight or by volume of each harmful substance in the preparation, |
|
LXn |
= |
is the respective harmful limit specified for each very toxic, toxic or harmful substance, expressed as percentage by weight or by volume; |
3.2. owing to their acute effects to the lungs if swallowed and assigned the symbol ‘Xn’, and the indication of danger ‘harmful’ and the risk phrase R65.
Preparations classified as harmful according to the criteria specified in paragraph 3.2.3 of Annex VI to Directive 67/548/EEC. In applying the conventional method according to the above paragraph 3.1 no account shall be taken of the classification of a substance as R65;
3.3. owing to their non-lethal irreversible effects after a single exposure and assigned the symbol ‘Xn’, the indication of danger ‘harmful’ and the risk phrase ►M1 R68 ◄ /route of exposure.
Preparations containing at least one dangerous substance classified as very toxic, toxic or harmful that produces such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 2 in Part B of this Annex (Table II and II A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
3.4. owing to their long-term effects and assigned the symbol ‘Xn’, the indication of danger ‘harmful’ and the risk phrase R48/route of exposure.
Preparations containing at least one dangerous substance classified as toxic or harmful that produces such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 3 in Part B of this Annex (Table III and III A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
4. The following preparations are to be classified as corrosive
4.1. and assigned the symbol ‘C’, the indication of danger ‘corrosive’ and the risk phrase R35;
4.1.1. preparations containing one or more substances classified as corrosive to which is assigned the phrase R35 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 4 in Part B of this Annex (Table IV and IV A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
4.1.2. preparations containing more than one substance classified as corrosive to which is assigned phrase R35 in lower individual concentrations than the limits specified under 4.1.1(a) or (b) if:

where:
|
PC, R35 |
= |
is the percentage by weight or by volume of each corrosive substance which is assigned phrase R35 in the preparation, |
|
LC, R35 |
= |
is the corrosive limit R35 specified for each corrosive substance to which is assigned phrase R35, expressed as a percentage by weight or by volume; |
4.2. and assigned the symbol ‘C’, the indication of danger ‘corrosive’ and the risk phrase R34;
4.2.1. preparations containing one or more substances classified as corrosive to which is assigned the phrase R35 or R34 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 4 in Part B of this Annex (Table IV and IV A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
4.2.2. preparations containing more than one of the substances classified as corrosive to which is assigned the phrase R35 or R34 in lower individual concentrations than the limits specified under 4.2.1(a) or (b) if:
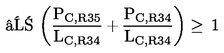
where:
|
PC, R35 |
= |
is the percentage by weight or by volume of each corrosive substance to which is assigned phrase R35 in the preparation, |
|
PC, R34 |
= |
is the percentage by weight or by volume of each corrosive substance to which is assigned phrase R34 in the preparation, |
|
LC, R34 |
= |
is the respective corrosive limit R34 specified for each corrosive substance to which is assigned phrase R35 or R34, expressed as a percentage by weight or by volume. |
5. The following preparations are to be classified as irritants:
5.1. liable to cause serious eye damage and assigned the symbol ‘Xi’, the indication of danger ‘irritant’ and the risk phrase R41;
5.1.1. preparations containing one or more substances classified as irritant to which is assigned phrase R41 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 4 in Part B of this Annex (Table IV and IV A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
5.1.2. preparations containing more than one of the substances classified as irritant and to which is assigned phrase R41, or classified as corrosive and to which is assigned phrase R35 or R34, in lower individual concentrations than the limits specified under 5.1.1(a) or (b) if:
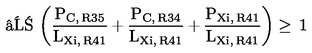
where:
|
PC, R35 |
= |
is the percentage by weight or by volume of each corrosive substance to which is assigned phrase R35 in the preparation, |
|
PC, R34 |
= |
is the percentage by weight or by volume of each corrosive substance to which is assigned phrase R34 in the preparation, |
|
PXi, R41 |
= |
is the percentage by weight or by volume of each irritant substance to which is assigned phrase R41 in the preparation, |
|
LXi, R41 |
= |
is the respective irritant limit R41 specified for each corrosive substance to which is assigned phrase R35 or R34 or irritant substance to which is assigned phrase R41, expressed as percentage by weight or by volume; |
5.2. irritant to eyes and assigned the symbol ‘Xi’, the indication of danger ‘irritant’ and the risk phrase R36;
5.2.1. preparations containing one or more substances classified as corrosive to which is assigned phrase R35 or R34 or as irritant and to which is assigned phrase R41 or R36 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 4 in Part B of this Annex (Table IV and IV A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
5.2.2. preparations containing more than one substance classified as irritant to which is assigned phrase R41 or R36, or as corrosive and to which is assigned phrase R35 or R34, in lower individual concentrations than the limits specified under 5.2.1(a) or (b) if:

where:
|
PC, R35 |
= |
is the percentage by weight or by volume of each corrosive substance to which is assigned phrase R35 in the preparation, |
|
PC, R34 |
= |
is the percentage by weight or by volume of each corrosive substance to which is assigned phrase R34 in the preparation, |
|
PXi, R41 |
= |
is the percentage by weight or by volume of each irritant substance to which is assigned phrase R41 in the preparation, |
|
PXi, R36 |
= |
is the percentage by weight or by volume of each irritant substance to which is assigned phrase R36 in the preparation, |
|
LXi, R36 |
= |
is the respective irritant limit R36 specified for each corrosive substance to which is assigned phrase R35 or R34 or irritant substance to which is assigned phrase R41, or R36 expressed as percentage by weight or by volume; |
5.3. irritant to skin and assigned the symbol ‘Xi’, the indication of danger ‘irritant’ and the risk phrase R38;
5.3.1. preparations containing one or more substances classified as irritant and to which is assigned phrase R38 or as corrosive and to which is assigned phrase R35 or R34 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 4 in Part B of this Annex (Table IV and IV A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
5.3.2. preparations containing more than one of the substances classified as irritant and to which is assigned phrase R38, or as corrosive and to which is assigned phrase R35 or R34 in lower individual concentrations than the limits specified under 5.3.1(a) or (b) if:

where:
|
PC, R35 |
= |
is the percentage by weight or by volume of each corrosive substance to which is assigned phrase R35 in the preparation, |
|
PC, R35 |
= |
is the percentage by weight or by volume of each corrosive substance to which is assigned phrase R34 in the preparation, |
|
PXi, R38 |
= |
is the percentage by weight or by volume of each irritant substance to which is assigned phrase R38 in the preparation, |
|
LXi, R38 |
= |
is the respective irritant limit R38 specified for each corrosive substance to which is assigned phrase R35 or R34 or irritant substance to which is assigned phrase R38, expressed as percentage by weight or by volume; |
5.4. irritant to respiratory system and assigned the symbol ‘Xi’, the indication of danger ‘irritant’ and the risk phrase R37;
5.4.1. preparations containing one or more substances classified as irritant and to which is assigned phrase R37 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 4 in Part B of this Annex (Table IV and IV A) where the substance or the substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
5.4.2. preparations containing more than one substance classified as irritant and to which is assigned phrase R37 in lower individual concentrations than the limits specified under 5.4.1(a) or (b) if:
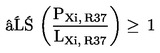
where:
|
PXi, R37 |
= |
is the percentage by weight or by volume of each irritant substance to which is assigned phrase R37 in the preparation, |
|
LXi, R37 |
= |
is the irritant limit R37 specified for each irritant substance to which is assigned phrase R37, expressed as percentage by weight or by volume; |
5.4.3. gaseous preparations containing more than one of the substances classified as irritant to which is assigned phrase R37 or as corrosive and to which is assigned phrase R35 or R34 in lower individual concentrations than the limits specified under 5.4.1(a) or (b) if:

where:
|
PC, R35 |
= |
is the percentage by volume of each corrosive substance to which is assigned phrase R35 in the preparation, |
|
PC, R34 |
= |
is the percentage by volume of each corrosive substance to which is assigned phrase R34 in the preparation, |
|
PXi, R37 |
= |
is the percentage by volume of each irritant substance to which is assigned phrase R37 in the preparation, |
|
LXi, R37 |
= |
is the respective irritant limit R37 specified for each gaseous corrosive substance to which is assigned phrase R35 or R34 or gaseous irritant substance to which is assigned phrase R37, expressed as percentage by weight or by volume. |
6. The following preparations are to be classified as sensitising:
6.1. by skin contact and assigned the symbol ‘Xi’, the indication of danger ‘irritant’ and the risk phrase R43.
Preparations containing at least one substance classified as sensitising and to which is assigned phrase R43 that produces such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 5 in Part B of this Annex (Table V and V A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
6.2. by inhalation and assigned the symbol ‘Xn’, the indication of danger ‘harmful’ and the risk phrase R42.
Preparations containing at least one substance classified as sensitising to which is assigned phrase R42 that produces such effects in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 5 in Part B of this Annex (Table V and V A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
7. The following preparations are to be classified as carcinogenic:
7.1. those of category 1 or 2 which are assigned the symbol ‘T’ and the phrase R45 or R49.
Preparations containing at least one substance producing such effects, classified as carcinogenic and to which is assigned phrase R45 or R49 which denotes carcinogenic substances in category 1 and category 2, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 6 in Part B of this Annex (Table VI and VI A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
7.2. those of category 3 which are assigned the symbol ‘Xn’ and the phrase R40.
Preparations containing at least one substance producing such effects classified as carcinogenic and to which is assigned phrase R40 which denotes carcinogenic substances in category 3, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 6 in Part B of this Annex (Table VI and VI A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
8. The following preparations are to be classified as mutagenic:
8.1. those of category 1 or 2 which are assigned the symbol ‘T’ and the phrase R46.
Preparations containing at least one substance producing such effects, classified as mutagenic and to which is assigned phrase R46 which denotes mutagenic substances in category 1 and category 2, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 6 in Part B of this Annex (Table VI and VI A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
8.2. those of category 3 which are assigned the symbol ‘Xn’ and the phrase ►M1 R68 ◄ .
Preparations containing at least one substance, producing such effects, classified as mutagenic and to which is assigned phrase ►M1 R68 ◄ which denotes mutagenic substances in category 3, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 6 in Part B of this Annex (Table VI and VI A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
9. The following preparations are to be classified as toxic for reproduction:
9.1. those of category 1 or 2 which are assigned the symbol ‘T’ and the phrase R60 (fertility).
Preparations containing at least one substance producing such effects, classified as toxic for reproduction and to which is assigned phrase R60 which denotes substances toxic for reproduction of category 1 and category 2, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 6 in Part B of this Annex (Table VI and VI A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
9.2. those of category 3 which are assigned the symbol ‘Xn’ and the phrase R62 (fertility).
Preparations containing at least one substance producing such effects, classified as toxic for reproduction and to which is assigned phrase R62 which denotes substances toxic for reproduction of category 3, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 6 in Part B of this Annex (Table VI and VI A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
9.3. those of category 1 or 2 which are assigned the symbol ‘T’ and the phrase R61 (development).
Preparations containing at least one substance producing such effects, classified as toxic for reproduction and to which is assigned phrase R61 which denotes substances toxic for reproduction of category 1 and category 2, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 6 in Part B of this Annex (Table VI and VI A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
9.4. those of category 3 which are assigned the symbol ‘Xn’ and the phrase R63 (development).
Preparations containing at least one substance producing such effects, classified as toxic for reproduction and to which is assigned phrase R63 which denotes substances toxic for reproduction of category 3, in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified at point 6 in Part B of this Annex (Table VI and VI A) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
PART B
Concentration limits to be used in evaluation of health hazards
For each health effect, the first table (Tables I to VI) sets out the concentration limits (expressed as a weight/weight percentage) to be used for non-gaseous preparations and the second table (Tables I A to VI A) sets out the concentration limits (expressed as a volume/volume percentage) to be used for gaseous preparations. These concentration limits are used in the absence of specific concentration limits for the substance under consideration in Annex I to Directive 67/548/EEC.
1. Acute lethal effects
1.1. Non-gaseous preparations
The concentration limits fixed in Table I, expressed as a weight/weight percentage, determine the classification of the preparation in relation to the individual concentration of the substance(s) present whose classification is also shown.
Table I
|
Classification of the substance |
Classification of the preparation |
||
|
T+ |
T |
Xn |
|
|
T+ with R26, R27, R28 |
concentration ≥ 7 % |
1 % ≤ concentration < 7 % |
0,1 % ≤ concentration < 1 % |
|
T with R23, R24, R25 |
concentration ≥ 25 % |
3 % ≤ concentration < 25 % |
|
|
Xn with R20, R21, R22 |
concentration ≥ 25 % |
||
The R phrases denoting risk are to be assigned to the preparation in accordance with the following criteria:
— the label shall include one or more of the abovementioned R phrases according to the classification used,
— in general, the R phrases selected should be those applicable to the substance(s) present in the concentration which gives rise to the most severe classification.
1.2. Gaseous preparations
The concentration limits expressed as a volume/volume percentage in Table I A below determine the classification of the gaseous preparations in relation to the individual concentration of the gas(es) present whose classification is also shown.
Table I A
|
Classification of the substance (gas) |
Classification of the gaseous preparation |
||
|
T+ |
T |
Xn |
|
|
T+ with R26, R27, R28 |
concentration ≥ 1 % |
0,2 % ≤ concentration < 1 % |
0,02 % ≤ concentration < 0,2 % |
|
T with R23, R24, R25 |
concentration ≥ 5 % |
0,5 % ≤ concentration < 5 % |
|
|
Xn with R20, R21, R22 |
concentration ≥ 5 % |
||
The R phrases denoting risk shall be assigned to the preparation in accordance with the following criteria:
— the label shall include one or more of the abovementioned R phrases according to the classification used,
— in general, the R phrases selected should be those applicable to the substance(s) present in the concentration which gives rise to the most severe classification.
2. Non-lethal irreversible effects after a single exposure
2.1. Non-gaseous preparations
For substances that produce non-lethal irreversible effects after a single exposure (R39/route of exposure, ►M1 R68 ◄ /route of exposure), the individual concentration limits specified in Table II, expressed as a weight/weight percentage, determine, when appropriate, the classification of the preparation.
Table II
|
Classification of the substance |
Classification of the preparation |
||
|
T+ |
T |
Xn |
|
|
T+ with R39/route of exposure |
concentration ≥ 10 % R39 (1) obligatory |
1 % ≤ concentration < 10 % R39 (1) obligatory |
0,1 % ≤ concentration < 1 % |
|
T with R39/route of exposure |
concentration ≥ 10 % R39 (1) obligatory |
1 % ≤ concentration < 10 % |
|
|
Xn with ►M1 R68 ◄ /route of exposure |
concentration ≥ 10 % |
||
|
(1) In order to indicate the route of administration/exposure (route of exposure) the combined R phrases listed under points 3.2.1, 3.2.2 and 3.2.3 of the labelling guide (Annex VI to Directive 67/548/EEC) are to be used. |
|||
2.2. Gaseous preparations
For gases that produce non-lethal irreversible effects after a single exposure (R39/route of exposure, ►M1 R68 ◄ /route of exposure), the individual concentration limits specified in Table II A, expressed as a volume/volume percentage, determine, when appropriate, the classification of the preparation.
Table II A
|
Classification of the substance (gas) |
Classification of the gaseous preparation |
||
|
T+ |
T |
Xn |
|
|
T+ with R39/route of exposure |
concentration ≥ 1 % R39 (1) obligatory |
0,2 % ≤ concentration < 1 % R39 (1) obligatory |
0,02 % ≤ concentration < 0,2 % |
|
T with R39/route of exposure |
concentration ≥ 5 % R39 (1) obligatory |
0,5 % ≤ concentration < 5 % |
|
|
Xn with ►M1 R68 ◄ /route of exposure |
concentration ≥ 5 % |
||
|
(1) In order to indicate the route of administration/exposure (route of exposure) the combined R phrases listed under points 3.2.1, 3.2.2 and 3.2.3 of the labelling guide (Annex VI to Directive 67/548/EEC) are to be used. |
|||
3. Severe effects after repeated or prolonged exposure
3.1. Non-gaseous preparations
For substances that produce severe effects after repeated or prolonged exposure (R 48/route of exposure), the individual concentration limits specified in Table III, expressed as a weight/weight percentage, determine, when appropriate, the classification of the preparation.
Table III
|
Classification of the substance |
Classification of the preparation |
|
|
T |
Xn |
|
|
T with R48/route of exposure |
concentration ≥ 10 % R48 (1) obligatory |
1 % ≤ concentration < 10 % R48 (1) obligatory |
|
Xn with R48/route of exposure |
concentration ≥ 10 % R48 (1) obligatory |
|
|
(1) In order to indicate the route of administration/exposure (route of exposure) the combined R phrases listed under points 3.2.1, 3.2.2 and 3.2.3 of the labelling guide (Annex VI to Directive 67/548/EEC) are to be used. |
||
3.2. Gaseous preparations
For gases that produce severe effects after repeated or prolonged exposure (R48/route of exposure), the individual concentration limits specified in Table III A below, expressed as a volume/volume percentage, determine, when appropriate, the classification of the preparation.
Table III A
|
Classification of the substance (gas) |
Classification of the gaseous preparation |
|
|
T |
Xn |
|
|
T with R48/route of exposure |
concentration ≥ 5 % R48 (1) obligatory |
0,5 % ≤ concentration < 5 % R48 (1) obligatory |
|
Xn with R48/route of exposure |
concentration ≥ 5 % R48 (1) obligatory |
|
|
(1) In order to indicate the route of administration/exposure (route of exposure) the combined R phrases listed under points 3.2.1, 3.2.2 and 3.2.3 of the labelling guide (Annex VI to Directive 67/548/EEC) are to be used. |
||
4. Corrosive and irritant effects including serious damage to the eye
4.1. Non-gaseous preparations
For substances that produce corrosive effects (R34, R35) or irritant effects (R36, R37, R38, R41), the individual concentration limits specified in Table IV, expressed as a weight/weight percentage, determine, when appropriate, the classification of the preparation.
Table IV
|
Classification of the substance |
Classification of the preparation |
|||
|
C with R35 |
C with R34 |
Xi with R41 |
Xi with R36, R37, R38 |
|
|
C with R35 |
concentration ≥ 10 % R35 obligatory |
5 % ≤ concentration < 10 % R34 obligatory |
5 % (1) |
1 % ≤ concentration < 5 % R36/38 obligatory |
|
C with R34 |
concentration ≥ 10 % R34 obligatory |
10 % (1) |
5 % ≤ concentration < 10 % R36/38 obligatory |
|
|
Xi with R41 |
concentration ≥ 10 % R41 obligatory |
5 % ≤ concentration < 10 % R36 obligatory |
||
|
Xi with R36, R37, R38 |
concentration ≥ 20 % R36, R37, R38 are obligatory in the light of the concentration present if they apply to the substances under consideration |
|||
|
(1) According to the labelling guide (Annex VI to Directive 67/548/EEC), corrosive substances assigned risk phrases R35 or R34 must also be considered as being assigned phrase R41. Consequently, if the preparation contains corrosive substances with R35 or R34 below the concentration limits for a classification of the preparation as corrosive, such substances can contribute to a classification of the preparation as irritant with R41 or irritant with R36. NB: Simple application of the conventional method to preparations containing substances classified as corrosive or irritant may result in under-classification or over-classification of the hazard, if other relevant factors (e.g. pH of the preparation) are not taken into account. Therefore, in classifying for corrosivity, consider the advice given in paragraph 3.2.5 of Annex VI to Directive 67/548/EEC and in the second and third indents of Article 6(3), of this Directive. |
||||
4.2. Gaseous preparations
For gases that produce such effects (R34, R35 or R36, R37, R38, R41), the individual concentration limits specified in Table IV A below, expressed as a volume/volume percentage determine, when appropriate, the classification of the preparation.
Table IV A
|
Classification of the substance (gas) |
Classification of the gaseous preparation |
|||
|
C with R35 |
C with R34 |
Xi with R41 |
Xi with R36, R37, R38 |
|
|
C with R35 |
concentration ≥ 1 % R35 obligatory |
0,2 % ≤ concentration < 1 % R34 obligatory |
0,2% (1) |
0,02 % ≤ concentration < 0,2 % R36/37/38 obligatory |
|
C with R34 |
concentration ≥ 5 % R34 obligatory |
5 % (1) |
0,5 % ≤ concentration < 5 % R36/37/38 obligatory |
|
|
Xi with R41 |
concentration ≥ 5 % R41 obligatory |
0,5 % ≤ concentration < 5 % R36 obligatory |
||
|
Xi with R36, R37, R38 |
concentration ≥ 5 % R36, R37, R38 obligatory as appropriate |
|||
|
(1) According to the labelling guide (Annex VI to Directive 67/548/EEC), corrosive substances assigned risk phrases R35 or R34 must also be considered as being assigned phrase R41. Consequently, if the preparation contains corrosive substances with R35 or R34 below the concentration limits for a classification of the preparation as corrosive, such substances can contribute to a classification of the preparation as irritant with R41 or irritant with R36. NB: Simple application of the conventional method to preparations containing substances classified as corrosive or irritant may result in under-classification or over-classification of the hazard, if other relevant factors (e.g. pH of the preparation) are not taken into account. Therefore, in classifying for corrosivity, consider the advice given in paragraph 3.2.5 of Annex VI to Directive 67/548/EEC and in the second and third indents of Article 6(3), of this Directive. |
||||
5. Sensitising effects
5.1. Non-gaseous preparations
Preparations that produce such effects are classified as sensitising and assigned:
— the symbol Xn and phrase R42 if this effect can be produced by inhalation,
— the symbol Xi and phrase R43 if this effect can be produced through contact with the skin.
The individual concentration limits specified in Table V, expressed as a weight/weight percentage, determine, when appropriate, the classification of the preparation.
Table V
|
Classification of the substance |
Classification of the preparation |
|
|
Sensitising with R42 |
Sensitising with R43 |
|
|
Sensitising with R42 |
concentration ≥ 1 % R42 obligatory |
|
|
Sensitising with R43 |
concentration ≥ 1 % R43 obligatory |
|
5.2. Gaseous preparations
Gaseous preparations that produce such effects are classified as sensitising and assigned:
— the symbol Xn and phrase R42 if this effect can be produced by inhalation,
— the symbol Xi and phrase R43 if this effect can be produced through contact with the skin.
The individual concentration limits specified in Table V A below, expressed as a volume/volume percentage, determine, when appropriate, the classification of the preparation.
Table V A
|
Classification of the substance (gas) |
Classification of the gaseous preparation |
|
|
Sensitising with R42 |
Sensitising with R43 |
|
|
Sensitising with R42 |
concentration ≥ 0,2 % R42 obligatory |
|
|
Sensitising with R43 |
concentration ≥ 0,2 % R43 obligatory |
|
6. Carcinogenic/mutagenic/toxic effects for reproduction
6.1. Non-gaseous preparations
For substances which produce such effects, the concentration limits laid down in Table VI, expressed as a weight/weight percentage, shall determine, where appropriate, the classification of the preparation. The following symbol and risk phrases are assigned:
|
Carcinogenic categories 1 and 2: |
T; R45 or R49 |
|
Carcinogenic category 3: |
Xn; R40 |
|
Mutagenic categories 1 and 2: |
T; R46 |
|
Mutagenic category 3: |
Xn; ►M1 R68 ◄ |
|
Toxic for reproduction fertility categories 1 and 2: |
T; R60 |
|
Toxic for reproduction development categories 1 and 2: |
T; R61 |
|
Toxic for reproduction fertility category 3: |
Xn; R62 |
|
Toxic for reproduction development category 3: |
Xn; R63 |
Table VI
|
Classification of the substance |
Classification of the preparation |
|
|
Categories 1 and 2 |
Category 3 |
|
|
Carcinogenic substances of category 1 or 2 with R45 or R49 |
Concentration ≥ 0,1 % carcinogenic R45, R49 obligatory as appropriate |
|
|
Carcinogenic substances of category 3 with R40 |
Concentration ≥ 1 % carcinogenic R40 obligatory (unless already assigned R45 (1)) |
|
|
Mutagenic substances of category 1 or 2 with R46 |
Concentration ≥ 0,1 % mutagenic R46 obligatory |
|
|
Mutagenic substances of category 3 with R68 |
Concentration ≥ 1 % mutagenic R68 obligatory (unless already assigned R46) |
|
|
Substances ‘toxic for reproduction’ of category 1 or 2 with R60 (fertility) |
Concentration ≥ 0,5 % toxic for reproduction (fertility) R60 obligatory |
|
|
Substances ‘toxic for reproduction’ of category 3 with R62 (fertility) |
Concentration ≥ 5 % toxic for reproduction (fertility) R62 obligatory (unless already assigned R60) |
|
|
Substances ‘toxic for reproduction’ of category 1 or 2 with R61 (development) |
Concentration ≥ 0,5 % toxic for reproduction (development) R61 obligatory |
|
|
Substances ‘toxic for reproduction’ of category 3 with R63 (development) |
Concentration ≥ 5 % toxic for reproduction (development) R63 obligatory (unless already assigned R61) |
|
|
(1) In cases where the preparation is assigned R49 and R40, both R phrases shall be kept, because R40 does not distinguish between the exposure routes, whereas R49 is only assigned for the inhalation route. |
||
6.2. Gaseous preparations
For gases which produce such effects, the concentration limits laid down in Table VI A, expressed as a volume/volume percentage, shall determine, where appropriate, the classification of the preparation. The following symbol and risk phrases are assigned:
|
Carcinogenic categories 1 and 2: |
T; R45 or R49 |
|
Carcinogenic category 3: |
Xn; R40 |
|
Mutagenic categories 1 and 2: |
T; R46 |
|
Mutagenic category 3: |
Xn; ►M1 R68 ◄ |
|
Toxic for reproduction fertility categories 1 and 2: |
T; R60 |
|
Toxic for reproduction development categories 1 and 2: |
T; R61 |
|
Toxic for reproduction fertility category 3: |
Xn; R62 |
|
Toxic for reproduction development category 3: |
Xn; R63 |
Table VI A
|
Classification of the substance |
Classification of the preparation |
|
|
Categories 1 and 2 |
Category 3 |
|
|
Carcinogenic substances of category 1 or 2 with R45 or R49 |
Concentration ≥ 0,1 % carcinogenic R45, R49 obligatory as appropriate |
|
|
Carcinogenic substances of category 3 with R40 |
Concentration ≥ 1 % carcinogenic R40 obligatory (unless already assigned R45 (1)) |
|
|
Mutagenic substances of category 1 or 2 with R46 |
Concentration ≥ 0,1 % mutagenic R46 obligatory |
|
|
Mutagenic substances of category 3 with R68 |
Concentration ≥ 1 % mutagenic R68 obligatory (unless already assigned R46) |
|
|
Substances ‘toxic for reproduction’ of category 1 or 2 with R60 (fertility) |
Concentration ≥ 0,2 % toxic for reproduction (fertility) R60 obligatory |
|
|
Substances ‘toxic for reproduction’ of category 3 with R62 (fertility) |
Concentration ≥ 1 % toxic for reproduction (fertility) R62 obligatory (unless already assigned R60) |
|
|
Substances ‘toxic for reproduction’ of category 1 or 2 with R61 (development) |
Concentration ≥ 0,2 % toxic for reproduction (development) R61 obligatory |
|
|
Substances ‘toxic for reproduction’ of category 3 with R63 (development) |
Concentration ≥ 1 % toxic for reproduction (development) R63 obligatory (unless already assigned R61) |
|
|
(1) In cases where the preparation is assigned R49 and R40, both R phrases shall be kept, because R40 does not distinguish between the exposure routes, whereas R49 is only assigned for the inhalation route. |
||
ANNEX III
METHODS FOR THE EVALUATION OF THE ENVIRONMENTAL HAZARDS OF PREPARATIONS IN ACCORDANCE WITH ARTICLE 7
Introduction
The systematic assessment of all the dangerous properties for the environment is expressed by means of concentration limits, expressed as a weight/weight percentage except for gaseous preparations where they are expressed as a volume/volume percentage and in conjunction with the classification of a substance.
Part A gives the calculation procedure according to Article 7(1)(a) and gives the R phrases to be assigned to the classification of the preparation.
Part B gives the concentration limits to be used when applying the conventional method and relevant symbols and R phrases for classification.
In accordance with Article 7(1)(a) the environmental hazards of a preparation shall be assessed by the conventional method described in parts A and B of this Annex, using individual concentration limits.
(a) Where the dangerous substances listed in Annex 1 to Directive 67/548/EEC are assigned concentration limits necessary for the application of the method of assessment described in Part A of this Annex, these concentration limits must be used.
(b) Where the dangerous substances do not appear in Annex I to Directive 67/548/EEC or appear there without the concentration limits necessary for the application of the method of evaluation described in Part A of this Annex, the concentration limits shall be assigned in accordance with the specification in Part B of this Annex.
Part C gives the test methods for the evaluation of the hazards for the aquatic environment.
PART A
Procedure for the evaluation of environmental hazards
(a) Aquatic environment
I. Conventional method for the evaluation of hazards to the aquatic environment
The conventional method for the evaluation of hazards to the aquatic environment ►C1 takes into account all the hazards that a preparation may entail ◄ for this medium according to the following specifications.
The following preparations are to be classified as dangerous for the environment:
1. and assigned the symbol ‘N’, the indication of danger ‘dangerous for the environment’ and the risk phrases R50 and R53 (R50-53):
1.1. preparations containing one or more substances classified as dangerous to the environment and to which is assigned phrases R50-53 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified in Part B of this Annex (Table 1) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
1.2. preparations containing more than one substance classified as dangerous for the environment and to which is assigned phrases R50-53 in lower individual concentrations than the limits specified under I.1.1(a) or (b) if:

where:
|
PN, R50—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrases R50-53 in the preparation, |
|
LN, R50—53 |
= |
is the limit R50-53 for each substance dangerous for the environment to which is assigned the phrases R50-53, expressed as percentage by weight |
2. and assigned the symbol ‘N’, the indication of danger ‘dangerous for the environment’ and the risk phrases R51 and R53 (R51-53) unless the preparation is already classified according to I.1 above;
2.1. preparations containing one or more than one substance classified as dangerous to the environment and to which is assigned phrases R50-53 or R51-53 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified in Part B of this Annex (Table 1) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
2.2. preparations containing more than one of the substances classified as dangerous for the environment and to which is assigned phrases R50-53 or R51-53 in lower individual concentrations than the limits specified under I.2. (a) or (b) if:

where:
|
PN, R50—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrases R50-53 in the preparation, |
|
PN, R51—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrases R51-53 in the preparation, |
|
LN, R51—53 |
= |
is the respective limit R51-53 for each substance dangerous for the environment to which is assigned phrases R50-53 or R51-53, expressed as percentage by weight |
3. and assigned the risk phrases R52 and R53 (R52-53) unless the preparation is already classified according to I.1 or I.2 above;
3.1. preparations containing one or more than one substance classified as dangerous to the environment and to which is assigned phrases R50-53 or R51-53 or R52-53 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified in Part B of this Annex (Table 1) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
3.2. preparations containing more than one of the substances classified as dangerous for the environment and to which is assigned phrases R51-53 or R50-53 or R52-53 in lower individual concentrations than the limits specified under I.3.1(a) or (b) if:
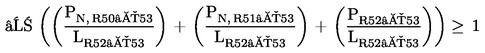
where:
|
PN, R50—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrases R50-53 in the preparation, |
|
PN, R51—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrases R51-53 in the preparation, |
|
PR52—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrases R52-53 in the preparation, |
|
LR52—53 |
= |
is the respective limit R52-53 for each substance dangerous for the environment to which is assigned phrases R50-53 or R51-53 or R52-53, expressed as percentage by weight; |
4. and assigned the symbol ‘N’, the indication of danger ‘dangerous for the environment’ and the risk phrase R50 unless the preparation is already classified according to I.1 above:
4.1. preparations containing one or more than one substance classified as dangerous to the environment and to which is assigned phrase R50 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified in Part B of this Annex (Table 2) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
4.2. preparations containing more than one substance classified as dangerous for the environment and to which is assigned phrase R50 in lower individual concentrations than the limits specified under I.4.1(a) or (b) if:

where:
|
PN, R50 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrase R50 in the preparation, |
|
LN, R50 |
= |
is the limit R50 for each substance dangerous for the environment to which is assigned phrase R50, expressed as percentage by weight. |
4.3. preparations containing one or more than one of the substances classified as dangerous for the environment and to which is assigned phrase R50 not meeting the criteria under I.4.1 or I.4.2 and containing one or more than one substance classified as dangerous for the environment and to which is assigned phrases R50-53 if:
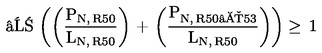
where:
|
PN, R50 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrase R50 in the preparation, |
|
PN, R50—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrases R50-53 in the preparation, |
|
LN, R50 |
= |
is the perspective limit R50 for each substance dangerous for the environment to which is assigned phrases R50 or R50-53, expressed as percentage by weight; |
5. and assigned the risk phrase R52 unless the preparation is already classified according to I.1, I.2, I.3, or I.4 above:
5.1. preparations containing one or more than one substance classified as dangerous to the environment and to which is assigned phrase R52 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified in Part B of this Annex (Table 3) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
5.2. preparations containing more than one substance classified as dangerous for the environment and to which is assigned phrase R52 in lower individual concentrations than the limits specified under I.5.1 (a) or (b) if:

where:
|
PR52 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrase R52 in the preparation, |
|
LR52 |
= |
is the limit R52 for each substance dangerous for the environment to which is assigned phrase R52, expressed as percentage by weight; |
6. and assigned the risk phrase R53 unless the preparation is already classified according to I.1, I.2, or I.3 above:
6.1. preparations containing one or more than one substance classified as dangerous to the environment and to which is assigned phrase R53 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified in Part B of this Annex (Table 4) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits;
6.2. preparations containing more than one substance classified as dangerous for the environment and to which is assigned phrase R 53 in lower individual concentrations than the limits specified under I.6.1(a) or (b) if:
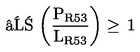
where:
|
PR53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrase R53 in the preparation, |
|
LR53 |
= |
is the limit R53 for each substance dangerous for the environment to which is assigned phrase R53, expressed as percentage by weight; |
6.3. preparations containing one or more than one of the substances classified as dangerous for the environment and to which is assigned phrase R53 not meeting the criteria under I.6.2 and containing one or more than one substance classified as dangerous for the environment and to which is assigned phrases R50-53 or R51-53 or R52-53 if:

where:
|
PR53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrase R53 in the preparation, |
|
PN, R50—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrase R50-53 in the preparation, |
|
PN, R51—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrase R51-53 in the preparation, |
|
PR52—53 |
= |
is the percentage by weight of each substance dangerous for the environment to which is assigned phrase R52-53 in the preparation, |
|
LR53 |
= |
is the respective limit R53 for each substance dangerous for the environment to which is assigned phrase R53 or R50-53 or R51-53 or R52-53, expressed as percentage by weight. |
(b) Non-aquatic environment
(1) OZONE LAYER
I. Conventional method for the evaluation of preparations dangerous for the ozone layer
1. and assigned the symbol ‘N’, the indication of danger ‘dangerous for the environment’ and the risk phrase R59;
1.1. preparations containing one or more substances classified as dangerous to the environment and to which is assigned the symbol ‘N’ and the risk phrase R59 in individual concentrations equal to or greater than:
(a) either the concentration specified in Annex I to Directive 67/548/EEC for the substance or substances under consideration, or
(b) the concentration specified in Part B of this Annex (Table 5) where the substance or substances do not appear in Annex I to Directive 67/548/EEC or appear in it without concentration limits.
▼M4 —————
(2) TERRESTRIAL ENVIRONMENT
I. Evaluation of preparations dangerous for the terrestrial environment
Classification of preparations using the risk phrases below will follow after the detailed criteria for use of the phrases have been incorporated in Annex VI to Directive 67/548/EEC.
|
R54 |
Toxic to flora |
|
R55 |
Toxic to fauna |
|
R56 |
Toxic to soil organisms |
|
R57 |
Toxic to bees |
|
R58 |
May cause long-term adverse effects in the environment. |
PART B
Concentration limits to be used for the evaluation of environmental hazards
I. For the aquatic environment
The concentration limits fixed in the following tables, expressed as a weight/weight percentage, determine the classification of the preparation in relation to the individual concentration of the substance(s) present whose classification is also shown.
Table 1a
Acute aquatic toxicity and long-term adverse effects
|
Classification of the substance |
Classification of the preparation |
||
|
N, R50-53 |
N, R51-53 |
R52-53 |
|
|
N, R50-53 |
see Table 1b |
see Table 1b |
see Table 1b |
|
N, R51-53 |
Cn ≥ 25 % |
2,5 % ≤ Cn< 25 % |
|
|
R52-53 |
Cn ≥ 25 % |
||
Preparations containing a substance classified with N, R50-53, the concentration limits and the resulting classification given in table 1b are applicable.
Table 1b
Acute aquatic toxicity and long-term adverse effects of substances very toxic to the aquatic environment
|
LC50 or EC50 value (‘L(E)C50’) of substance classified as N, R50-53 (mg/l) |
Classification of the preparation |
||
|
N, R50-53 |
N, R51-53 |
R52-53 |
|
|
0,1 < L(E)C50 ≤ 1 |
Cn ≥ 25 % |
2,5 % ≤ Cn< 25 % |
0,25 % ≤ Cn< 2,5 % |
|
0,01 < L(E)C50 ≤ 0,1 |
Cn ≥ 2,5 % |
0,25 % ≤ Cn< 2,5 % |
0,025 % ≤ Cn< 0,25 % |
|
0,001 < L(E)C50 ≤ 0,01 |
Cn ≥ 0,25 % |
0,025 % ≤ Cn< 0,25 % |
0,0025 % ≤ Cn< 0,025 % |
|
0,0001 < L(E)C50 ≤ 0,001 |
Cn ≥ 0,025 % |
0,0025 % ≤ Cn< 0,025 % |
0,00025 % ≤ Cn< 0,0025 % |
|
0,00001 < L(E)C50 ≤ 0,0001 |
Cn ≥ 0,0025 % |
0,00025 % ≤ Cn< 0,0025 % |
0,000025 % ≤ Cn< 0,00025 % |
|
For preparations containing substances with a lower LC50 or EC50 value than 0,00001 mg/l, the corresponding concentration limits are calculated accordingly (in factor 10 intervals). |
|||
Table 2
Acute aquatic toxicity
|
LC50 or EC50 value (‘L(E)C50’) of substance classified either as N, R50 or as N, R50-53 (mg/l) |
Classification of the preparation N, R50 |
|
0,1 < L(E)C50 ≤ 1 |
Cn ≥ 25 % |
|
0,01 < L(E)C50 ≤ 0,1 |
Cn ≥ 2,5 % |
|
0,001 < L(E)C50 ≤ 0,01 |
Cn ≥ 0,25 % |
|
0,0001 < L(E)C50 ≤ 0,001 |
Cn ≥ 0,025 % |
|
0,00001 < L(E)C50 ≤ 0,0001 |
Cn ≥ 0,0025 % |
|
For preparations containing substances with a lower LC50 or EC50 value than 0,00001 mg/l, the corresponding concentration limits are calculated accordingly (in factor 10 intervals). |
|
Table 3
Aquatic toxicity
|
Classification of the substance |
Classification of the preparation R52 R52 |
|
R52 |
Cn ≥ 25 % |
Table 4
Long-term adverse effects
|
Classification of the substance |
Classification of the preparation R53 R53 |
|
R53 |
Cn ≥ 25 % |
|
N, R50—53 |
Cn ≥ 25 % |
|
N, R51—53 |
Cn ≥ 25 % |
|
R52—53 |
Cn ≥ 25 % |
II. For the non-aquatic environment
The concentration limits fixed in the following tables, expressed as weight/weight percentage or, for gaseous preparations as a volume/volume percentage, determine the classification of the preparation in relation to the individual concentration of the substance(s) present whose classification is also shown.
Table 5
Dangerous for the ozone layer
|
Classification of the substance |
Classification of the preparation N, R59 |
|
N with R59 |
Cn ≥ 0,1 % |
PART C
Test methods for the evaluation of the hazards for the aquatic environment
Normally, the classification of a preparation is made on the basis of the conventional method. However, for the determination of the acute aquatic toxicity, there may be cases for which it is appropriate to carry out tests on the preparation.
The result of these tests on the preparation may only modify the classification concerning acute aquatic toxicity which would have been obtained by the application of the conventional method.
If such tests are chosen by the person responsible for the placing on the market, it must be ensured that the quality criteria of the test methods in Part C of Annex V to Directive 67/548/EEC have been complied with.
Furthermore, the tests are to be carried out on all three species in conformity with the criteria of Annex VI to Directive 67/548/EEC (algae, daphnia and fish), unless the highest hazard classification relating to acute aquatic toxicity has been assigned to the preparation after testing on one of the species or a test result was already available before this Directive entered into force.
ANNEX IV
SPECIAL PROVISIONS FOR CONTAINERS CONTAINING PREPARATIONS OFFERED OR SOLD TO THE GENERAL PUBLIC
PART A
Containers to be fitted with child-resistant fastenings
1. Containers of whatever capacity, containing preparations offered or sold to the general public and labelled as very toxic, toxic or corrosive in accordance with Article 10 and under the conditions laid down in Article 6 of this Directive, are to fitted with child-resistant fastenings.
2. Containers of whatever capacity containing preparations presenting an aspiration hazard (Xn, R65) and classified and labelled according to paragraph 3.2.3 of Annex VI to Directive 67/548/EEC with the exception of preparations placed on the market in the form of aerosols or in a container fitted with a sealed spray attachment.
3. Containers of wathever capacity, having at least one of the substances mentioned below present in a concentration equal to or greater then the maximum individual concentration specified,
|
No |
Identification of the substance |
Concentration limit |
||
|
CAS-Reg No |
Name |
Einecs No |
||
|
1 |
67-56-1 |
Methanol |
2006596 |
≥ 3 % |
|
2 |
75-09-2 |
Dichloromethane |
2008389 |
≥ 1 % |
which are offered or sold to the general public are to be fitted with child-resistant fastenings.
PART B
Containers to be fitted with a tactile warning of danger
Containers of whatever capacity, containing preparations offered or sold to the general public and labelled as very toxic, toxic, corrosive, harmful, extremely flammable or highly flammable in accordance with Article 10 and under the conditions laid down in Articles 5 and 6 of this Directive, are to carry a tactile warning of danger.
This provision does not apply to aerosols classified and labelled only as extremely flammable or highly flammable.
ANNEX V
SPECIAL PROVISIONS CONCERNING THE LABELLING OF CERTAIN PREPARATIONS
A. For preparations classified as dangerous within the meaning of Articles 5, 6 and 7
1. Preparations sold to the general public
1.1. The label on the packaging containing such preparations, in addition to the specific safety advice, must bear the relevant safety advice S1, S2, S45 or S46 in accordance with the criteria laid down in Annex VI to Directive 67/548/EEC.
1.2. When such preparations are classified as very toxic (T+), toxic (T) or corrosive (C) and where it is physically impossible to give such information on the package itself, packages containing such preparations must be accompanied by precise and easily understandable instructions for use including, where appropriate, instructions for the destruction of the empty package.
2. Preparations intended for use by spraying
The label on the packaging containing such preparations must compulsorily bear the safety advice S23 accompanied by safety advice S38 or S51 assigned to it in accordance with the criteria laid down in Annex VI to Directive 67/548/EEC.
3. Preparations containing a substance assigned phrase R33: Danger of cumulative effects
When a preparation contains at least one substance assigned the phrase R33, the label on the packaging of the preparation must carry the wording of this phrase as set out in Annex III to Directive 67/548/EEC, when the concentration of this substance present in the preparation is equal to or higher than 1 %, unless different values are set in Annex I to Directive 67/548/EEC.
4. Preparations containing a substance assigned phrase R64: May cause harm to breastfed babies
When a preparation contains at least one substance assigned phrase R64, the label on the packaging of the preparation must carry the wording of this phrase as set out in Annex III to Directive 67/548/EEC, when the concentration of this substance present in the preparation is equal to or higher than 1 %, unless different values are set in Annex I to Directive 67/548/EEC.
B. For preparations irrespective of their classification within the meaning of Articles 5, 6 and 7
1. Preparations containing lead
1.1. Paint and varnishes
The label on the packaging of paints and varnishes containing lead in quantities exceeding 0,15 % (expressed as weight of metal) of the total weight of the preparation, as determined in accordance with ISO standard 6503/1984, must show the following particulars:
‘Contains lead. Should not be used on surfaces liable to be chewed or sucked by children’.
In the case of packages the contents of which are less than 125 millilitres, the particulars may be as follows:
‘Warning! Contains lead’.
2. Preparations containing cyanoacrylates
2.1. Adhesives
The label on the immediate packaging of adhesives based on cyanoacrylate must bear the following inscriptions:
‘Cyanoacrylate
Danger
Bonds skin and eyes in seconds
Keep out of the reach of children’.
Appropriate advice on safety must accompany the package.
3. Preparations containing isocyanates
The label on the packaging of preparations containing isocyanates (as monomers, oligomers, prepolymers, etc., or as mixtures thereof) must bear the following inscriptions:
‘Contains isocyanates.
See information supplied by the manufacturer’.
4. Preparations containing epoxy constituents with an average molecular weight ≤ 700
The label on the packaging of preparations containing epoxy constituents with an average molecular weight ≤ 700 must bear the following inscriptions:
‘Contains epoxy constituents.
See information supplied by the manufacturer’.
5. Preparations sold to the general public which contain active chlorine
The label on the packaging of preparations containing more than 1 % of active chlorine must bear the following particular inscriptions:
‘Warning! Do not use together with other products. May release dangerous gases (chlorine)’.
6. Preparations containing cadmium (alloys) and intended to be used for brazing or soldering
The label on the packaging of the above mentioned preparations must bear the following inscription printed in clearly legible and indelible characters:
‘Warning! Contains cadmium.
Dangerous fumes are formed during use.
See information supplied by the manufacturer.
Comply with the safety instructions’.
7. Preparations available as aerosols
Without prejudice to the provisions of this Directive, preparations available as aerosols are also subject to the labelling provisions in accordance with points 2.2 and 2.3 of the Annex to Directive 75/324/EEC as last amended by Directive 94/1/EC.
8. Preparations containing substances not yet tested completely
Where a preparation contains at least one substance which, in accordance with Article 13.3 of Directive 67/548/EEC, bears the inscription ‘Caution — substance not yet tested completely’, the label on the packaging of the preparation must bear the inscription ‘Warning — this preparation contains a substance not yet tested completely’ if this substance is present in a concentration ≥ 1 %.
9. Preparations not classified as sensitising but containing at least one sensitising substance
The label on the packaging of preparations containing at least one substance classified as sensitising and being present in a concentration equal to or greater than 0,1 % or in a concentration equal to or greater than that specified under a specific note for the substance in Annex I to Directive 67/548/EEC must bear the inscription:
‘Contains (name of sensitising substance). May produce an allergic reaction’.
10. Liquid preparations containing halogenated hydrocarbons
For liquid preparations which show no flashpoint or a flashpoint higher than 55 °C and contain a halogenated hydrocarbon and more than 5 % flammable or highly flammable substances, the label on the packaging must bear the following inscription as appropriate:
‘Can become highly flammable in use’ or ‘Can become flammable in use’.
11. Preparations containing a substance assigned phrase R67: vapours may cause drowsiness and dizziness
When a preparation contains one or more substances assigned the phrase R67, the label on the packaging of the preparation must carry the wording of this phrase as set out in Annex III to Directive 67/548/EEC, when the total concentration of these substances present in the preparation is equal to or higher than 15 %, unless:
— the preparation is already classified with phrases R20, R23, R26, R68/20, R39/23 or R39/26,
— or the preparation is in a package not exceeding 125 ml.
12. Cements and cement preparations
The label on the packaging of cements and cement preparations containing more than 0,0002 % soluble chromium (VI) of the total dry weight of the cement must bear the inscription:
‘Contains chromium (VI). May produce an allergic reaction’
unless the preparation is already classified and labelled as a sensitiser with phrase R43.
C. For preparations not classified within the meaning of Articles 5, 6 and 7 but containing at least one dangerous substance
1. Preparations not intended for the general public
The label on the packaging of preparations referred to in Article 14.2.1(b) must bear the following inscription:
‘Safety data sheet available for professional user on request’.
ANNEX VI
CONFIDENTIALITY FOR THE CHEMICAL IDENTITY OF A SUBSTANCE
PART A
Information to be communicated in the request for confidentiality
Introductory notes
A. Article 15 indicates the conditions in which the person responsible for placing a preparation on the market may avail himself of the confidentiality.
B. To avoid multiple requests for confidentiality relating to the same substance used in different preparations, a single request for confidentiality may suffice if a certain number of preparations have:
— the same dangerous constituents present in the same concentration range,
— the same classification and labelling,
— the same expected uses.
A single alternative denomination must be used to mask the chemical identity of the same substance in the preparations concerned. Furthermore, the request for confidentiality must contain all information indicated in the following request, without forgetting the name or the trade name of each preparation.
C. The alternative designation used on the label must be the same as that given under heading 2 ‘Composition/information on ingredients’ of the Annex to Directive 91/155/EEC as last amended by Directive 93/112/EEC.
This implies that the alternative designation used will contain enough information about the substance to ensure risk-free handling.
D. In making the request to use an alternative designation the person responsible for placing on the market must take into account the need to provide enough information for necessary health and safety precautions to be taken in the workplace and to ensure that risks from handling the preparation can be minimised.
Request for confidentiality
In accordance with Article 15 the request for confidentiality must obligatorily contain the following information:
1. Name and full address (including telephone number) of the person established in the Community who is responsible for placing the preparation on the market (manufacturer, importer or distributor).
2. Precise identification of the substance(s) for which confidentiality is proposed and the alternative designation.
|
CAS No |
Einecs No |
Chemical name according to international nomenclature and classification (Annex I to Council Directive 67/548/EEC or provisional classification) |
Alternative designation |
|
(a) |
|||
|
(b) |
|||
|
(c) |
|||
|
NB: Where substances are classified provisionally, accompanying information (bibliographical references) should be provided as evidence that the provisional classification takes account of all existing pertinent information available on the properties of the substance. |
|||
3. Justification for confidentiality (probability — plausibility).
4. Designation(s) or commercial name(s) of the preparation(s).
5. Is the designation or commercial name the same for all the Community?
|
YES ☐ |
NO ☐ |
If no, specify the designation(s) or commercial name(s) used in the different Member States:
Belgium:
Czech Republic:
Denmark:
Germany:
Estonia:
Greece:
Spain:
France:
Ireland:
Italy:
Cyprus:
Latvia:
Lithuania:
Luxembourg:
Hungary:
Malta:
Netherlands:
Austria:
Poland:
Portugal:
Slovenia:
Slovakia:
Finland:
Sweden:
United Kingdom:
6. Composition of the preparation(s) defined in point 2 of the Annex to Directive 91/155/EEC as last amended by Directive 93/112/EEC.
7. Classification of the preparation(s) according to Article 6 of this Directive.
8. Labelling of the preparation(s) according to Article 10 of this Directive.
9. Intended uses for the preparation(s).
10. Safety data sheet(s) conforming to Directive 91/155/EEC as last amended by Directive 93/112/EEC.
PART B
Lexicon guide for establishing the alternative designations (generic names)
1. Introductory note
The lexicon guide is based on the procedure for the classification of dangerous substances (division of substances into families) which appears in Annex I to Directive 67/548/EEC.
Alternative designations to those based on this guide may be used. However, in all cases the names chosen must provide enough information to ensure the preparation can be handled without risk and that necessary health and safety precautions can be taken in the workplace.
The families are defined in the following manner:
— inorganic or organic substances whose properties are identified by having a common chemical element as their chief characteristic. The family name is derived from the name of the chemical element. These families are identified as in Annex I by the atomic number of the chemical element (001 to 103),
— organic substances whose properties are identified by having a common functional group as their chief characteristics.
The family name is derived from the functional group name.
These families are identified by the conventional number found in Annex I (601—650).
Sub-families bringing together substances with a common specific character have been added in certain cases.
2. Establishing the generic name
General principles
For the purposes of establishing the generic name, the following general approach, involving two successive stages, is adopted:
(i) identification of the functional groups and chemical elements present in the molecule;
(ii) determination of the extent to which account should be taken of the most important functional groups and chemical elements.
The identified functional groups and elements taken into account are the names of the families and sub-families set out in point 3 in the form of a non-restrictive list.
3. Division of substances into families and sub-families
|
Family No Annex I to Directive 67/548/EEC |
Families Sub-families |
|
001 |
Hydrogen compounds Hydrides |
|
002 |
Helium compounds |
|
003 |
Lithium compounds |
|
004 |
Beryllium compounds |
|
005 |
Boron compounds Boranes Borates |
|
006 |
Carbon compounds Carbamates Inorganic carbon compounds Salts of hydrogen cyanide Urea and derivatives |
|
007 |
Nitrogen compounds Quaternary ammonium compounds Acid nitrogen compounds Nitrates Nitrites |
|
008 |
Oxygen compounds |
|
009 |
Fluorine compounds Inorganic fluorides |
|
010 |
Neon compounds |
|
011 |
Sodium compounds |
|
012 |
Magnesium compounds Organometallic magnesium derivatives |
|
013 |
Aluminium compounds Organometallic aluminium derivatives |
|
014 |
Silicon compounds Silicones Silicates |
|
015 |
Phosphorus compounds Acid phosphorus compounds Phosphonium compounds Phosphoric esters Phosphates Phosphites Phosphoramides and derivatives |
|
016 |
Sulphur compounds Acid sulphur compounds Mercaptans Sulphates Sulphites |
|
017 |
Chlorine compounds Chlorates Perchlorates |
|
018 |
Argon compounds |
|
019 |
Potassium compounds |
|
020 |
Calcium compounds |
|
021 |
Scandium compounds |
|
022 |
Titanium compounds |
|
023 |
Vanadium compounds |
|
024 |
Chromium compounds Chromium VI compounds |
|
025 |
Manganese compounds |
|
026 |
Iron compounds |
|
027 |
Cobalt compounds |
|
028 |
Nickel compounds |
|
029 |
Copper compounds |
|
030 |
Zinc compounds Organometallic zinc derivatives |
|
031 |
Gallium compounds |
|
032 |
Germanium compounds |
|
033 |
Arsenic compounds |
|
034 |
Selenium compounds |
|
035 |
Bromine compounds |
|
036 |
Krypton compounds |
|
037 |
Rubidium compounds |
|
038 |
Strontium compounds |
|
039 |
Yttrium compounds |
|
040 |
Zirconium compounds |
|
041 |
Niobium compounds |
|
042 |
Molybdenum compounds |
|
043 |
Technetium compounds |
|
044 |
Ruthenium compounds |
|
045 |
Rhodium compounds |
|
046 |
Palladium compounds |
|
047 |
Silver compounds |
|
048 |
Cadmium compounds |
|
049 |
Indium compounds |
|
050 |
Tin compounds Organometallic tin derivatives |
|
051 |
Antimony compounds |
|
052 |
Tellurium compounds |
|
053 |
Iodine compounds |
|
054 |
Xenon compounds |
|
055 |
Caesium compounds |
|
056 |
Barium compounds |
|
057 |
Lanthanum compounds |
|
058 |
Cerium compounds |
|
059 |
Praseodymium compounds |
|
060 |
Neodymium compounds |
|
061 |
Promethium compounds |
|
062 |
Samarium compounds |
|
063 |
Europium compounds |
|
064 |
Gandolinium compounds |
|
065 |
Terbium compounds |
|
066 |
Dysprosium compounds |
|
067 |
Holmium compounds |
|
068 |
Erbium compounds |
|
069 |
Thulium compounds |
|
070 |
Ytterbium compounds |
|
071 |
Lutetium compounds |
|
072 |
Hafnium compounds |
|
073 |
Tantalum compounds |
|
074 |
Tungsten compounds |
|
075 |
Rhenium compounds |
|
076 |
Osmium compounds |
|
077 |
Iridium compounds |
|
078 |
Platinum compounds |
|
079 |
Gold compounds |
|
080 |
Mercury compounds Organometallic mercury derivatives |
|
081 |
Thallium compounds |
|
082 |
Lead compounds Organometallic lead derivatives |
|
083 |
Bismuth compounds |
|
084 |
Polonium compounds |
|
085 |
Astate compounds |
|
086 |
Radon compounds |
|
087 |
Francium compounds |
|
088 |
Radium compounds |
|
089 |
Actinium compounds |
|
090 |
Thorium compounds |
|
091 |
Protactinium compounds |
|
092 |
Uranium compounds |
|
093 |
Neptunium compounds |
|
094 |
Plutonium compounds |
|
095 |
Americium compounds |
|
096 |
Curium compounds |
|
097 |
Berkelium compounds |
|
098 |
Californium compounds |
|
099 |
Einsteinium compounds |
|
100 |
Fermium compounds |
|
101 |
Mendelevium compounds |
|
102 |
Nobelium compounds |
|
103 |
Lawrencium compounds |
|
601 |
Hydrocarbons Aliphatic hydrocarbons Aromatic hydrocarbons Alicyclic hydrocarbons Polycyclic aromatic hydrocarbons (PAH) |
|
602 |
Halogenated hydrocarbons (1) Halogenated aliphatic hydrocarbons (1) Halogenated aromatic hydrocarbons (1) Halogenated alicyclic hydrocarbons (1) |
|
603 |
Alcohols and derivatives Aliphatic alcohols Aromatic alcohols Alicyclic alcohols Alcanolamines Epoxy derivatives Ethers Glycolethers Glycols and polyols |
|
604 |
Phenols and derivatives Halogenated phenol derivatives (1) |
|
605 |
Aldehydes and derivatives Aliphatic aldehydes Aromatic aldehydes Alicyclic aldehydes Aliphatic acetals Aromatic acetals Alicyclic acetals |
|
606 |
Ketones and derivatives Aliphatic ketones Aromatic ketones (2) Alicyclic ketones |
|
607 |
Organic acids and derivatives Aliphatic acids Halogenated aliphatic acids (1) Aromatic acids Halogenated aromatic acids (1) Alicyclic acids Halogenated alicyclic acids (1) Aliphatic acid anhydrides Halogenated aliphatic acid anhydrides (1) Aromatic acid anhydrides Halogenated aromatic acid anhydrides (1) Alicyclic acid anhydrides Halogenated alicyclic acid anhydrides (1) Salts of aliphatic acid Salts of halogenated aliphatic acid (1) Salts of aromatic acid Salts of halogenated aromatic acid (1) Salts of alicyclic acid Salts of halogenated alicyclic acid (1) Esters of aliphatic acid Esters of halogenated alicyclic acid (1) Esters of aromatic acid Esters of halogenated aromatic acid (1) Esters of alicyclic acid Esters of halogenated alicyclic acid (1) Esters of glycol ether Acrylates Methacrylates Lactones Acyl halogenides |
|
608 |
Nitriles and derivatives |
|
609 |
Nitro compounds |
|
610 |
Chlornitrated compounds |
|
611 |
Azoxy and azo compounds |
|
612 |
Amine compounds Aliphatic amines and derivatives Alicyclic amines and derivatives Aromatic amines and derivatives Aniline and derivatives Benzidine and derivatives |
|
613 |
Heterocyclic bases and derivatives Benzimidazole and derivatives Imidazol and derivatives Pyrethrinoids Quinoline and derivatives Triazine and derivatives Triazole and derivatives |
|
614 |
Glycosides and alkaloids Alkaloid and derivatives Glycosides and derivatives |
|
615 |
Cyanates and isocyanates Cyanates Isocyanates |
|
616 |
Amides and derivatives Acetamide and derivatives Anilides |
|
617 |
Organic peroxides |
|
647 |
Enzymes |
|
648 |
Complex coal derivatives Acid extract Alkaline extract Anthracene oil Anthracene oil extract residue Anthracene oil fraction Carbolic oil Carbolic oil extract residue Coal liquids, liquid solvent extraction Coal liquids, liquid solvent extraction solvents Coal oil Coal tar Coal tar extract Coal tar solids residue Coke (coal tar) low temperature, high temperature pitch Coke (coal tar), high temperature pitch Coke (coal tar), mixed coal high temperature pitch Crude benzole Crude phenols Crude tar bases Distillate bases Distillate phenols Distillates Distillates (coal), liquid solvent extraction, primary Distillates (coal), solvent extraction, hydrocracked Distillates (coal), solvent extraction, hydrocracked hydrogenated middle Distillates (coal), solvent extraction, hydrocracked middle Extract residues (coal), low temperature coal tar alkaline Fresh oil Fuels, diesel, coal solvent extraction, hydrocracked, hydrogenated Fuels, jet aircraft, coal solvent extraction, hydrocracked, hydrogenated Gasoline, coal solvent extraction, hydrocracked naphtha Heat treatment products Heavy anthracene oil Heavy anthracene oil redistillate Light oil Light oil extract residues, high boiling Light oil extract residues, intermediate boiling Light oil extract residues, low boiling Light oil redistillate, high boiling Light oil redistillate, intermediate boiling Light oil redistillate, low boiling Methylnaphthalene oil Methylnaphthalene oil extract residue Naphtha (coal), solvent extraction, hydrocracked Naphthalene oil Naphthalene oil extract residue Naphthalene oil redistillate Pitch Pitch redistillate Pitch residue Pitch residue, heat treated Pitch residue, oxidised Pyrolysis products Redistillates Residues (coal), liquid solvent extractions Tar brown coal Tar brown coal, low temperature Tar oil, high boiling Tar oil, intermediate boiling Wash oil Wash oil extract residue Wash oil redistillate |
|
649 |
Complex oil derivatives Crude oil Petroleum gas Low boiling point naphtha Low boiling point modified naphtha Low boiling point cat-cracked naphtha Low boiling point cat-reformed naphtha Low boiling point thermally cracked naphtha Low boiling point hydrogen treated naphtha Low boiling point naphtha — unspecified Straight-run kerosine Kerosine — unspecified Cracked gas oil Gas oil — unspecified Heavy fuel oil Grease Unrefined or mildly refined base oil Base oil — unspecified Distillate aromatic extract Distillate aromatic extract (treated) Foots oil Slack wax Petrolatum |
|
650 |
Various substances Do not use this family. Instead, use the families or sub-families mentioned above. |
|
(1) Specify according to the family corresponding to halogen. (2) Quinones included. |
|
4. Practical application:
After having conducted a search to see if the substance belongs to one or more families or sub-families on the list, the generic name can be established in the following way:
4.1. If the name of a family or sub-family is sufficient to characterise the chemical elements or important functional groups, this name will be chosen as the generic name.
Examples:
— 1,4 dihydroxybenzen
—
|
family 604 |
: |
phenols and derivatives |
|
generic name |
: |
phenol derivatives |
— butanol
—
|
family 603 |
: |
alcohols and derivatives |
|
sub-family |
: |
aliphatic alcohols |
|
generic name |
: |
aliphatic alcohol |
— 2-Isopropoxyethanol
—
|
family 603 |
: |
alcohols and derivatives |
|
sub-family |
: |
glycolethers |
|
generic name |
: |
glycolether |
— methacrylate
—
|
family 607 |
: |
organic acids and derivatives |
|
sub-family |
: |
acrylates |
|
generic name |
: |
acrylate |
4.2. If the name of a family or sub-family is not sufficient to characterise the chemical elements of important functional groups, the generic name will be a combination of the corresponding different family or sub-family names:
Examples:
— chlorobenzene
—
|
family 602 |
: |
halogenated hydrocarbons |
|
sub-family |
: |
halogenated aromatic hydrocarbons |
|
family 017 |
: |
chlorine compounds |
|
generic name |
: |
chlorinated aromatic hydrocarbon |
— 2,3,6-trichlorophenylacetic acid
—
|
family 607 |
: |
organic acids |
|
sub-family |
: |
halogenated aromatic acids |
|
family 017 |
: |
chlorine compounds |
|
generic name |
: |
chlorinated aromatic acid |
— 1-chloro-1-nitropropane
—
|
family 610 |
: |
chloronitrated derivatives |
|
family 601 |
: |
hydrocarbons |
|
sub-family |
: |
aliphatic hydrocarbons |
|
generic name |
: |
chlorinated aliphatic hydrocarbon |
— tetrapropyl dithiopyrophosphate
—
|
family 015 |
: |
phosphorus compounds |
|
sub-family |
: |
phosphoric esters |
|
family 016 |
: |
sulphur compounds |
|
generic name |
: |
thiophosphoric ester |
NB:
In the case of certain elements, notably metals, the name of the family or sub-family may be indicated by the words‘organic’ or ‘inorganic’.
Examples:
— dimercury chloride
—
|
family 080 |
: |
mercury compounds |
|
generic name |
: |
inorganic mercury compound |
— barium acetate
—
|
family 056 |
: |
barium compounds |
|
generic name |
: |
organic barium compound |
— ethyl nitrite
—
|
family 007 |
: |
nitrogen compounds |
|
sub-family |
: |
nitrites |
|
generic name |
: |
organic nitrite |
— sodium hydrosulphite
—
|
family 016 |
: |
sulphur compounds |
|
generic name |
: |
inorganic sulphur compound |
(The examples cited are substances taken from Annex I to Directive 67/548/EEC (19th adaptation) in respect of which requests for confidentiality may be submitted).
ANNEX VII
PREPARATIONS COVERED BY ARTICLE 12(2)
Preparations as specified by paragraph 9.3 of Annex VI to Directive 67/548/EEC.
ANNEX VIII
PART A
Directives repealed in accordance with Article 21
— Directive 78/631/EEC on the approximation of the laws of the Member States relating to the classification, packaging and labelling of dangerous preparations (pesticides)
— Directive 88/379/EEC on the approximation of the laws of the Member States relating to the classification, packaging and labelling of dangerous preparations and its following adaptations to technical progress:
—— Directive 89/178/EEC
— Directive 90/492/EEC
— Directive 93/18/EEC
— Directive 96/65/EC
— Directive 90/35/EEC defining in accordance with Article 6 of Directive 88/379/EEC the category of preparations the packaging of which must be fitted with child-resistant fastenings and/or carry a tactile warning of danger
— Directive 91/442/EEC on dangerous preparations the packaging of which must be fitted with child-resistant fastenings
PART B
Deadlines for transposition and for application in accordance with Article 22
|
Directive |
Deadline for transposition |
Deadline for application |
|
78/631/EEC (OJ L 206, 29.7.1978, p. 13) |
1 January 1981 |
1 January 1981 |
|
88/379/EEC (OJ L 187, 16.7.1988, p. 14) |
7 June 1991 |
7 June 1991 |
|
89/178/EEC (OJ L 64, 8.3.1989, p. 18) |
1 December 1990 |
1 June 1991 |
|
90/492/EEC (OJ L 275, 5.10.1990, p. 35) |
1 June 1991 |
8 June 1991 |
|
93/18/EEC (OJ L 104, 29.4.1993, p. 46) |
1 July 1994 |
1 July 1994 |
|
90/35/EEC (OJ L 19, 24.1.1990, p. 14) |
1 August 1992 |
1 November 1992 |
|
91/442/EEC (OJ L 238, 27.8.1991, p. 25) |
1 August 1992 |
1 November 1992 |
|
96/65/EC (OJ L 265, 18.10.1996, p. 15) |
31 May 1998 |
31 May 1998 |
PART C
Special provisions for Austria, Finland and Sweden concerning the application of the following Directives in accordance with Article 21
1. Austria, Finland and Sweden do not transpose or apply Council Directive 78/631/EEC of 26 June 1978 on the approximation of the laws of the Member States relating to the classification, packaging and labelling of dangerous preparations (pesticides), as last amended by Council Directive 92/32/EEC of 30 April 1992.
2. Austria is to apply Council Directive 88/379/EEC of 7 June 1988 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous preparations, as last amended by Directive 96/65/EC of 11 October 1996 under the following conditions:
The following provisions of Directive 88/379/EEC will not apply to Austria
(a) Article 13 in conjunction with Articles 3 and 7 with respect to preparations containing substances listed in Appendix 1;
(b) Article 13 in conjunction with Article 7 with respect to labelling respecting the Austrian provisions on:
— safety advice for waste disposal,
— pictogram for waste disposal until two years after the entry into force of this Directive,
— safety advice for countermeasures in case of accidents;
(c) Article 13 in conjunction with Article 7(1)(c) concerning the chemical names of dangerous substances present in dangerous preparations, until two years after the entry into force of this Directive.
3. Sweden is to apply Council Directive 88/379/EEC of 7 June 1988 on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous preparations, as last amended by Directive 96/65/EC of 11 October 1996 under the following conditions:
The following provisions of Directive 88/379/EEC will not apply to Sweden:
(a) Article 13 in conjunction with Articles 3 and 7 with respect to preparations
— containing substances listed in Appendix 2,
— containing substances presenting neurotoxic effects and defatting effects on the skin not covered by criteria for classification of Annex VI to Directive 67/548/EEC, and by risk phrases of Annex III to Directive 67/548/EEC,
— containing substances presenting acutely toxic effects not covered by criteria for classification of Annex VI to Directive 67/548/EEC, and by risk phrases of Annex III to Directive 67/548/EEC, until two years after the entry into force of this Directive,
— which are not classified as dangerous according to the ‘måttligt skadliga’ (Swedish: ‘moderately harmful’) criteria of Directive 88/379/EEC.
(b) Article 13 in conjunction with Articles 3 and 7 with respect to
— the criteria for classification and labelling of preparations containing carcinogenic substances classified on the basis of criteria in point 4.2.1 of Annex VI to Directive 67/548/EEC,
— labelling of preparations classified as carcinogenic, category 3, with a special R-phrase instead of R-phrase 40.
Appendix 1
Substances referred to in Annex VIII, Part C, paragraph 2 (Austria)
|
Name of the substance |
Index number in Annex I to Directive 67/548/EEC |
|
Linuron |
006-021-00-1 |
|
Trichlorosilan |
014-001-00-9 |
|
Phosphorus trichloride |
015-007-00-4 |
|
Phosphorus pentachloride |
015-008-00-X |
|
Phosphorus oxychloride |
015-009-00-5 |
|
Sodium polysulphides |
016-010-00-3 |
|
Disulphur dichloride |
016-012-00-4 |
|
Thionyl chloride |
016-015-00-0 |
|
Calcium hypochlorite |
017-012-00-7 |
|
Potassium hydroxide |
019-002-00-8 |
|
2-Dimethylaminoethanol |
603-047-00-0 |
|
2-Diethylaminoethanol |
603-048-00-6 |
|
Diethanolamine |
603-071-00-1 |
|
N-Methyl-2-ethanolamine |
603-080-00-0 |
|
2-Ethylhexan-1,3-diol |
603-087-00-9 |
|
Isophorone |
606-012-00-8 |
|
6-Methyl-1,3-dithiolo(4,5-b)chinoxalin-2-one |
606-036-00-9 |
|
Acetic anhydride |
607-008-00-9 |
|
Methyl formate |
607-014-00-1 |
|
Ethyl formate |
607-015-00-7 |
|
Acrylic acid |
607-061-00-8 |
|
Chloroacetyl chloride |
607-080-00-1 |
|
Nitrofen |
609-040-00-9 |
|
Quintozen; Pentachloronitrobenzol |
609-043-00-5 |
|
Dichlofluanid |
616-006-00-7 |
|
Cumene hydroperoxide |
617-002-00-8 |
|
Monocrotophos |
015-072-00-9 |
|
Edifenphos |
015-121-00-4 |
|
Triazophos |
015-140-00-8 |
|
Methanol |
603-001-00-X |
|
Trifenmorph; 4-Tritylmorpholin |
613-052-00-X |
|
Diuron |
006-015-00-9 |
|
Fenbutanin oxide |
050-017-00-2 |
|
1-Butanol, 2-Butanol, iso-Butanol |
603-004-00-6 |
Appendix 2
Substances referred to in Annex VIII, Part C, paragraph 3 (Sweden)
|
Name of the substance |
Index number in Annex I to Directive 67/548/EEC |
|
Acetone |
606-001-00-8 |
|
Butanone |
606-002-00-3 |
|
Amyl formate |
607-018-00-3 |
|
Ethyl acetate |
607-022-00-5 |
|
n-Butylacetate |
607-025-00-1 |
|
sec-Butylacetate |
607-026-00-7 |
|
tert-Butylacetate |
607-026-00-7 |
|
iso-Butylacetate |
607-026-00-7 |
|
Butylformate |
607-017-00-8 |
|
Cyclohexane |
601-017-00-1 |
|
1,4-Dimethylcyclohexane |
601-019-00-2 |
|
Diethyl ether |
603-022-00-4 |
|
Ethyl methyl ether |
603-020-00-3 |
|
Amyl acetate |
607-130-00-2 |
|
Ethyl lactate |
607-129-00-7 |
|
Amyl propionate |
607-131-00-8 |
|
2,4-Dimethylpentan-3-one |
606-028-00-5 |
|
Di-n-propylether |
603-045-00-X |
|
Di-n-propyl ketone |
606-027-00-X |
|
Ethyl propionate |
607-028-00-8 |
|
Heptane |
601-008-00-2 |
|
Hexane (mixture of isomers) containing less than 5 % n-hexane |
601-007-00-7 |
|
Isopropyl acetate |
607-024-00-6 |
|
Isopropyl alcohol |
603-003-00-0 |
|
4-Methoxy-4-methylpentane-2-one |
606-023-00-8 |
|
Methyl acetate |
607-021-00-X |
|
Methyl cyclohexane |
601-018-00-7 |
|
5-Methylhexane-2-one |
606-026-00-4 |
|
Methyllactate |
607-092-00-7 |
|
4-Methylpentan-2-one |
606-004-00-4 |
|
Methyl propionate |
607-027-00-2 |
|
Octane |
601-009-00-8 |
|
Pentane |
601-006-00-1 |
|
Pentan-3-one |
606-006-00-5 |
|
Propan-1-ol |
603-003-00-0 |
|
Propyl acetate |
607-024-00-6 |
|
Propyl formate |
607-016-00-2 |
|
Propyl propionate |
607-030-00-9 |
|
Sodium bisulphite = polysulphite |
016-010-00-3 |
|
Toluene-2,4-diisocyanate |
615-006-00-4 |
|
Toluene-2,6-diisocyanate |
615-006-00-4 |
|
Cadmiumfluoride |
048-006-00-2 |
|
1,2-Epoxy-3(tolyloxy)-propane |
603-056-00-X |
|
Diphenylmethane-2,2′-diisocyanate |
615-005-00-9 |
|
Diphenylmethane-2,4′-diisocyanate |
615-005-00-9 |
|
Diphenylmethane-4,4′-diisocyanate |
615-005-00-9 |
|
Hydroquinone |
604-005-00-4 |
|
Hydroxypropyl acrylate |
607-108-00-2 |
|
Turpentine |
650-002-00-6 |
|
Butyl methyl ketone (2-Hexanone) |
606-030-00-6 |
|
Hexane |
601-007-00-7 |
|
Vanadium pentoxide |
023-001-00-8 |
|
Sodium nitrate |
|
|
Zinc oxide |
ANNEX IX
CORRELATION TABLE
|
This Directive |
88/379/EEC |
|
Article 1 |
Article 1 |
|
1.1 |
1.1 |
|
1.2 |
1.2 |
|
1.3 |
|
|
1.4 |
|
|
1.5 |
1.3 |
|
Article 2 |
Article 2 |
|
Article 3 |
Article 3.6 |
|
Article 4 |
Article 3.1 |
|
Article 4 |
|
|
Article 5 |
Article 3.2 |
|
5.1 |
3.2 |
|
5.1, third indent |
3.2, paragraph 3(b) |
|
5.2—5.3 |
|
|
5.4 |
|
|
Article 6 |
Article 3.3 |
|
6.1 |
3.3, paragraphs (a) and (b) |
|
6.2 |
|
|
6.3 |
3.3, paragraphs 3 and 4 |
|
6.4 |
3.4 |
|
6.5 |
3.5, paragraphs 1 to 3 |
|
Article 7 |
|
|
Article 8 |
Article 5 |
|
8.1 |
5.1 |
|
8.2 |
5.2 |
|
8.3 |
5.3 |
|
8.4 |
|
|
Article 9 |
Article 6 |
|
9.1 |
6.1(a) |
|
9.2 |
6.1(b) |
|
9.3 |
6.2 and 6.3, second paragraph |
|
Article 10 |
Article 7 |
|
10.1.1—1.2 |
|
|
10.2 |
7.1 |
|
10.2.3. |
7.1(c) |
|
10.2.4. |
7.1(d) |
|
10.2.5. |
7.4 |
|
Article 11 |
Article 8 |
|
Article 12 |
Article 9 |
|
Article 13 |
|
|
Article 14 |
Article 10 |
|
Article 15 |
Article 7 |
|
Article 16 |
Article 11 |
|
Article 17 |
Article 12 |
|
Article 18 |
Article 13 |
|
Article 19 |
Article 14 |
|
Article 20 |
Article 15 |
|
Article 21 |
|
|
Article 22 |
Article 16 |
|
Article 23 |
Article 16(3) |
|
Article 24 |
Article 17 |
CORRELATION TABLE
|
This Directive |
88/379/EEC |
90/35/EEC |
91/442/EEC |
93/18/EEC |
|
Annex I.A |
Article 3.2(2) |
|||
|
Annex I.B |
||||
|
Annex II.A. introduction (1—3) |
Annex I introduction |
|||
|
Annex II.A. introduction (4) |
||||
|
Annex II.A.1 |
Article 3.5(a) |
|||
|
Annex II.A.1.1.1 |
Article 3.5(a)(i) |
|||
|
Annex II.A.1.1.2 |
Article 3.5(a)(ii) |
|||
|
Annex II.A.1.2 |
Article 3.5(a)(iii) |
|||
|
Annex II.A.2 |
Article 3.5(b) |
|||
|
Annex II.A.2.1.1 |
Article 3.5(b)(i) |
|||
|
Annex II.A.2.1.2 |
Article 3.5(b)(ii) |
|||
|
Annex II.A.2.2 |
Article 3.5(b)(iii) |
|||
|
Annex II.A.2.3 |
Article 3.5(b)(iv) |
|||
|
Annex II.A.3 |
Article 3.5(c) |
|||
|
Annex II.A.3.1.1 |
Article 3.5(c)(i) |
|||
|
Annex II.A.3.1.2 |
Article 3.5(c)(ii) |
|||
|
Annex II.A.3.2 |
Article 3.5(c)(iii) |
|||
|
Annex II.A.3.3 |
Article 3.5(c)(iv) |
|||
|
Annex II.A.4 |
Article 3.5(d) |
|||
|
Annex II.A.4.1.1 |
Article 3.5(d)(i) |
|||
|
Annex II.A.4.1.2 |
Article 3.5(d)(ii) |
|||
|
Annex II.A.4.2.1 |
Article 3.5(e)(i) |
|||
|
Annex II.A.4.2.2 |
Article 3.5(e)(ii) |
|||
|
Annex II.A.5 |
Article 3.5(f) |
|||
|
Annex II.A.5.1.1 |
Article 3.5(f)(i) |
|||
|
Annex II.A.5.1.2 |
Article 3.5(f)(ii) |
|||
|
Annex II.A.5.2.1 |
Article 3.5(h)(i) |
|||
|
Annex II.A.5.2.2 |
Article 3.5(h)(ii) |
|||
|
Annex II.A.5.3.1 |
Article 3.5(g)(i) |
|||
|
Annex II.A.5.3.2 |
Article 3.5(g)(ii) |
|||
|
Annex II.A.5.4.1 |
Article 3.5(i)(i) |
|||
|
Annex II.A.5.4.2 |
Article 3.5(i)(ii) |
|||
|
Annex II.A.6 |
||||
|
Annex II.A.6.1 |
Article 3.5(g)(iii) |
|||
|
Annex II.A.6.2 |
Article 3.5(c)(v) |
|||
|
Annex II.A.7.1 |
Article 3.5(j) |
Annex I.6 |
||
|
Annex II.A.7.2 |
Article 3.5(k) |
|||
|
Annex II.A.8.1 |
Article 3.5(l), (m) |
|||
|
Annex II.A.8.2 |
Article 3.5(n) Article 3.5(o), (p) |
|||
|
Annex II.A.9.1—9.4 |
||||
|
Annex II.B. introduction |
Annex I. introduction |
|||
|
Annex II.B.1 |
Annex I.1 |
|||
|
Annex II.B.1.1 |
Annex I.1.1 |
|||
|
Annex II.B.1.2 |
Annex I.1.2 |
|||
|
Annex II.B.2 |
Annex I.2 |
|||
|
Annex II.B.2.1 |
Annex I.2.1 |
|||
|
Annex II.B.2.2 |
Annex I.2.2 |
|||
|
Annex II.B.3 |
Annex I.3 |
|||
|
Annex II.B.3.1 |
Annex I.3.1 |
|||
|
Annex II.B.3.2 |
Annex I.3.2 |
|||
|
Annex II.B.4 |
Annex I.4 |
|||
|
Annex II.B.4.1 |
Annex I.4.1 |
|||
|
Annex II.B.4.2 |
Annex I.4.2 |
|||
|
Annex II.B.5 |
Annex I.5 |
|||
|
Annex II.B.5.1 |
Annex I.5.1 |
|||
|
Annex II.B.5.2 |
Annex I.5.2 |
|||
|
Annex II.B.6 |
Annex I.6 |
|||
|
Annex II.B.6.1 |
Annex I.6.1 |
|||
|
Annex II.B.6.2 |
Annex I.6.2 |
|||
|
Annex III.A |
||||
|
Annex III.B |
||||
|
Annex III.C |
||||
|
Annex IV.B |
Articles 1 and 2 |
|||
|
Annex IV.A.1 |
Article 1(1) |
|||
|
Annex IV.A.2 |
Article 2; Annex (a) |
|||
|
Annex IV.A.3 |
Article 1; Annex (b) |
|||
|
Annex V.A.1 |
Annex II.A.1 |
|||
|
Annex V.A.2 |
Annex II.A.2 |
|||
|
Annex V.A.3 |
Annex II.A.3 |
|||
|
Annex V.A.4 |
Annex II.A.4 |
|||
|
Annex V.B.1 |
Annex II.B.1 |
|||
|
Annex V.B.2 |
Annex II.B.2 |
|||
|
Annex V.B.3 |
Annex II.B.3 |
|||
|
Annex V.B.4 |
Annex II.B.4 |
|||
|
Annex V.B.5 |
Annex II.B.5 |
|||
|
Annex V.B.6 |
Annex II.B.6 |
|||
|
Annex V.B.7 |
Article 3.2(3)(b) |
|||
|
Annex V.B.8 |
Article 3.5(4) |
|||
|
Annex V.C |
||||
|
Annex VI |
||||
|
Annex VII |
||||
|
Annex VIII |
||||
|
Annex IX |
( 1 ) OJ C 283, 26.9.1996, p. 1, and OJ C 337, 7.11.1997, p. 45.
( 2 ) OJ C 158, 26.5.1997, p. 76.
( 3 ) Opinion of the European Parliament of 26 June 1997 (OJ C 222, 21.7.1997, p. 26), Council common position of 24 September 1998 (OJ C 360, 23.11.1998, p. 1) and Decision of the European Parliament of 10 February 1999(OJ C 150, 28.5.1999). Council Decision of 11 May 1999.
( 4 ) OJ L 187, 16.7.1988, p. 14. Directive as last amended by Commission Directive 96/65/EC (OJ L 265, 18.10.1996, p. 15).
( 5 ) OJ L 154, 5. 6. 1992, p. 1.
( 6 ) OJ L 110, 4.5.1993, p. 20.
( 7 ) OJ L 358, 18.12.1986, p. 1.
( 8 ) OJ L 206, 29.7.1978, p. 13. Directive as last amended by Council Directive 92/32/EEC.
( 9 ) OJ L 230, 19.8.1991, p. 1. Directive as last amended by Commission Directive 96/68/EC (OJ L 277, 30.10.1996, p. 25).
( 10 ) OJ L 123, 24.4.1998, p. 1.
( 11 ) OJ L 76, 22.3.1991, p. 35. Directive as last amended by Commission Directive 93/112/EEC (OJ L 314, 16.12.1993, p. 38).
( 12 ) OJ L 22, 9.2.1965, p. 369. Directive as last amended by Directive 93/39/EEC (OJ L 214, 24.8.1993, p. 22).
( 13 ) OJ L 262, 27.9.1976, p. 169. Directive as last amended by Directive 97/18/EC (OJ L 114, 1.5.1997, p. 43).
( 14 ) OJ L 194, 25.7.1975, p. 39. Directive as last amended by Commission Decision 96/350/EC (OJ L 135, 6.6.1996, p. 32).
( 15 ) OJ L 84, 31.3.1978, p. 43.
( 16 ) OJ L 246, 17.9.1980, p. 1. Directive as amended by Directive 84/467/Euratom (OJ L 265, 5.10.1984, p. 4).
( 17 ) OJ L 147, 9.6.1975, p. 40. Directive as last amended by Directive 94/1/EC (OJ L 23, 28.1.1994, p. 28).
( 18 ) OJ L 15, 17.1.1987, p. 29.
( 19 ) OJ L 144, 4.6.1997, p. 19.
( 20 ) Council Decision 1999/468/EC of 28 June 1999 laying down the procedures for the exercise of implementing powers conferred on the Commission (OJ L 184, 17.7.1999, p. 23).







