

This document is an excerpt from the EUR-Lex website
Document 02008R1235-20150701
Commission Regulation (EC) No 1235/2008 of 8 December 2008 laying down detailed rules for implementation of Council Regulation (EC) No 834/2007 as regards the arrangements for imports of organic products from third countries
Consolidated text: Commission Regulation (EC) No 1235/2008 of 8 December 2008 laying down detailed rules for implementation of Council Regulation (EC) No 834/2007 as regards the arrangements for imports of organic products from third countries
Commission Regulation (EC) No 1235/2008 of 8 December 2008 laying down detailed rules for implementation of Council Regulation (EC) No 834/2007 as regards the arrangements for imports of organic products from third countries
2008R1235 — EN — 01.07.2015 — 015.001
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
COMMISSION REGULATION (EC) No 1235/2008 of 8 December 2008 (OJ L 334 12.12.2008, p. 25) |
Amended by:
Corrected by:
COMMISSION REGULATION (EC) No 1235/2008
of 8 December 2008
laying down detailed rules for implementation of Council Regulation (EC) No 834/2007 as regards the arrangements for imports of organic products from third countries
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Regulation (EC) No 834/2007 of 28 June 2007 on organic production and labelling of organic products and repealing Regulation (EEC) No 2092/91 ( 1 ), and in particular Article 33(2), Article 38(d) and Article 40 thereof,
Whereas:|
(1) |
Articles 32 and 33 of Regulation (EC) No 834/2007 lay down general provisions for import of organic products. With a view to guarantee that these provisions will be applied in a correct and uniform way, detailed rules and procedures for the application of those provisions should be laid down. |
|
(2) |
As substantial experience has been built up since 1992 with the import of products providing equivalent guarantees, a relatively short period should be given to control bodies and control authorities to request their inclusion in the list for the purpose of equivalence in accordance with Article 33 of Regulation (EC) No 834/2007. However, as there is no experience with the direct application of Community rules on organic production and labelling of organic products outside the territory of the Community, more time should be given to control bodies and control authorities wishing to request their inclusion in the list for the purpose of compliance in accordance with Article 32 of Regulation (EC) No 834/2007. Therefore a longer period should be provided for sending in the requests and for examining them. |
|
(3) |
For products imported according to Article 32 of Regulation (EC) No 834/2007, the operators concerned should be able to provide documentary evidence. It is necessary to establish a model for this documentary evidence. Products imported according to Article 33 of Regulation (EC) No 834/2007 should be covered by a certificate of inspection. It is necessary to lay down detailed rules with regard to the issuing of this certificate. Moreover, a procedure in order to coordinate at Community level certain controls on products imported from third countries which are intended to be marketed in the Community as organic should be laid down. |
|
(4) |
Argentina, Australia, Costa Rica, India, Israel, New Zealand and Switzerland were previously listed as third countries from which imported products could be marketed in the Community as organic, under Commission Regulation (EC) No 345/2008 of 17 April 2008 laying down detailed rules for implementing the arrangements for imports from third countries provided for in Council Regulation (EEC) No 2092/91 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs ( 2 ). The Commission has re-examined the situation of those countries according to the criteria set out in Regulation (EC) No 834/2007, taking into consideration the production rules applied and the experience gained with the import of organic products from these third countries as previously listed under Article 11(1) of Council Regulation (EEC) No 2092/2091. On this basis it is concluded that the conditions for inclusion of Argentina, Australia, Costa Rica, India, Israel, and New Zealand in the list of third countries for equivalency according to Article 33(1) of Regulation (EC) No 834/2007 are fulfilled. |
|
(5) |
The European Community and the Swiss Confederation have concluded an Agreement on trade in agricultural products ( 3 ) approved by Decision 2002/309/EC of the Council and of the Commission ( 4 ). Annex 9 to that Agreement covers organically produced agricultural products and foodstuffs and sets out that the Parties must take the necessary measures so that organic products complying with each other’s laws and regulations can be imported and placed on the market. For the sake of clarity, Switzerland should also be listed in the list of third countries for equivalency according to Article 33(1) of Regulation (EC) No 834/2007. |
|
(6) |
Member States’ authorities have acquired substantial experience and expertise in the field of granting access for organic imported goods into the territory of the Community. To establish and maintain the lists of third countries and control bodies and control authorities, this experience should be used and the Commission should be able to take account of reports from Member States and other experts. The tasks involved should be divided in a just and proportionate way. |
|
(7) |
Provision should also be made for transitional measures applicable to third country applications received by the Commission before 1 January 2009, the date from which Regulation (EC) No 834/2007 applies. |
|
(8) |
In order not to disrupt international trade, and to facilitate the transition between the rules established by Regulation (EEC) No 2092/2091 and those established by Regulation (EC) No 834/2007, it is necessary to extend the possibility of Member States to continue to grant authorisations to importers on a case by case basis for placing on the Community market of products until the measures necessary for the functioning of the new import rules have been put in place, in particular as regards the recognition of control bodies and control authorities referred to in Article 33(3) of Regulation (EC) No 834/2007. This possibility should be gradually phased out as the list of control bodies referred to in that Article is being established. |
|
(9) |
In order to improve transparency and guarantee the application of this Regulation, an electronic system for exchange of information between the Commission, the Member States, the third countries, and the control bodies and control authorities should be foreseen. |
|
(10) |
The detailed rules laid down in this Regulation replace those laid down in Commission Regulation (EC) No 345/2008 and in Commission Regulation (EC) No 605/2008 of 20 June 2008 laying down detailed rules for implementing the provisions concerning the certificate of inspection for imports from third countries under Article 11 of Council Regulation (EEC) No 2092/91 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs ( 5 ). Those Regulations should therefore be repealed and replaced by a new Regulation. |
|
(11) |
The measures provided for in this Regulation are in accordance with the opinion of the regulatory Committee on organic production, |
HAS ADOPTED THIS REGULATION:
TITLE I
INTRODUCTORY PROVISIONS
Article 1
Subject matter
This Regulation lays down the detailed rules for the import of compliant products and the import of products providing equivalent guarantees as provided for in Articles 32 and 33 of Regulation (EC) No 834/2007.
Article 2
Definitions
For the purposes of this Regulation:
1. ‘certificate of inspection’: means the certificate of inspection referred to in Article 33(1)(d) of Regulation (EC) No 834/2007 covering one consignment;
2. ‘documentary evidence’: means the document referred to in Article 68 of Commission Regulation (EC) No 889/2008 ( 6 ) and in Article 6 of this Regulation, for which the model is set out in Annex II to this Regulation;
3. ‘consignment’: means a quantity of products under one or more Combined Nomenclature codes, covered by a single certificate of inspection, conveyed by the same means of transport and imported from the same third country;
4. ‘first consignee’: means the natural or legal person as defined in Article 2(d) of Regulation (EC) No 889/2008;
5. ‘verification of the consignment’: means the verification by the relevant Member States’ authorities of the certificate of inspection to satisfy Article 13 of this Regulation, and, where these authorities consider appropriate, of the products, in relation to the requirements of Regulation (EC) No 834/2007, of Regulation (EC) No 889/2008 and of this Regulation;
6. ‘relevant Member States authorities’: means the customs authorities or other authorities, designated by the Member States;
7. ‘assessment report’: means the assessment report referred to in Articles 32(2) and 33(3) of Regulation (EC) No 834/2007 drawn up by an independent third party fulfilling the requirements of ISO Standard 17011 or by a relevant competent authority, which includes information on document reviews, including the descriptions referred to in Articles 4(3)(b) and 11(3)(b) of this Regulation, on office audits, including critical locations and on risk-oriented witness audits conducted in representative third countries.
TITLE II
IMPORT OF COMPLIANT PRODUCTS
CHAPTER 1
List of recognised control bodies and control authorities for the purpose of compliance
Article 3
Compilation and content of the list of recognised control bodies and control authorities for the purpose of compliance
1. The Commission shall draw up a list of control bodies and control authorities, recognised for the purpose of compliance in accordance with Article 32(2) of Regulation (EC) No 834/2007. The list shall be published in Annex I to this Regulation. The procedures for drawing up and amending the list are defined in Articles 4, 16 and 17 of this Regulation. The list shall be made available to the public on the Internet in accordance with Articles 16(4) and 17 of this Regulation.
2. The list shall contain all the information necessary in respect of each control body or control authority to allow verifying whether products placed on the Community market have been controlled by a control body or authority recognised in accordance with Article 32(2) of Regulation (EC) No 834/2007 and in particular:
(a) the name and address of the control body or control authority, including e-mail and Internet address and their code number;
(b) the third countries concerned and in which the products have their origin;
(c) the product categories concerned for each third country;
(d) the duration of the inclusion in the list;
(e) the Internet address where the list of operators subject to the control system can be found, including their certification status and the product categories concerned, as well as suspended and decertified operators and products.
Article 4
Procedure for requesting inclusion in the list of recognised control bodies and control authorities for the purpose of compliance
1. The Commission shall consider whether to recognise and include a control body or control authority in the list provided for in Article 3 upon receipt of a request for inclusion in this list from the representative of the control body or control authority concerned. Only complete requests that have been received before ►M17 31 October 2015 ◄ shall be considered, on the basis of the model of application made available by the Commission in accordance with Article 17(2), for the drawing up of the first list. For the following calendar years, only complete requests that have been received before 31 October of each year shall be considered.
2. The request can be introduced by control bodies and control authorities established in the Community or in a third country.
3. The request shall consist of a technical dossier, which shall comprise all the information needed for the Commission to ensure that the conditions set out in Article 32(1) and (2) of Regulation (EC) No 834/2007 are met for all organic products intended for export to the Community, namely:
(a) an overview of the activities of the control body or control authority in the third country or third countries concerned, including an estimate of the number of operators involved and an indication of the expected nature and quantities of agricultural products and foodstuffs originated from the third country or third countries concerned and intended for export to the Community under the rules set out in Article 32(1) and (2) of Regulation (EC) No 834/2007;
(b) a detailed description of how Titles II, III and IV of Regulation (EC) No 834/2007 as well as the provisions of Regulation (EC) No 889/2008 have been implemented in the third country or in each of the third countries concerned;
(c) a copy of the assessment report as set out in the fourth subparagraph of Article 32(2) of Regulation (EC) No 834/2007:
(i) proving that the control body or control authority has been satisfactorily assessed on its ability to meet the conditions set out in Article 32(1) and (2) of Regulation (EC) No 834/2007;
(ii) giving guarantees on the elements referred to in Article 27(2), (3), (5), (6) and (12) of Regulation (EC) No 834/2007;
(iii) ensuring that the control body or control authority meets the control requirements and precautionary measures set out in Title IV of Regulation (EC) No 889/2008; and
(iv) confirming that it has effectively implemented its control activities according to these conditions and requirements;
(d) proof that the control body or authority has notified its activities to the authorities of the third country concerned and its undertaking to respect the legal requirements imposed on it by the authorities of the third country concerned;
(e) the website address where the list of operators subject to the control system can be found, as well as a contact point where information is readily available on their certification status, the product categories concerned, as well as suspended and decertified operators and products;
(f) an undertaking to comply with the provisions of Article 5 of this Regulation;
(g) any other information deemed relevant by the control body or control authority or by the Commission.
4. When examining a request for inclusion in the list of control body or control authority, and also any time after its inclusion, the Commission may request any further information, including the presentation of one or more on-the-spot examination reports established by independent experts. Furthermore, the Commission may, based on risk-assessment and in case of suspected irregularities, organise an on-the-spot examination by experts it designates.
5. The Commission shall assess whether the technical dossier referred to in paragraph 3 and the information referred to in paragraph 4 are satisfactory and may subsequently decide to recognise and include a control body or control authority in the list. The decision shall be taken in accordance with the procedure referred to in Article 37(2) of Regulation (EC) No 834/2007.
Article 5
Management and review of the list of recognised control bodies and control authorities for the purpose of compliance
1. A control body or control authority may only be included in the list referred to in Article 3 when it fulfils the following obligations:
(a) if, after the control body or control authority has been included in the list, any changes are made to the measures applied by the control body or control authority, that control body or control authority shall notify the Commission thereof; requests to amend the information in respect of a control body or control authority referred to in Article 3(2) shall also be notified to the Commission;
(b) a control body or control authority included in the list shall keep available and communicate at first request all information related to its control activities in the third country; it shall give access to its offices and facilities to experts designated by the Commission;
(c) by 31 March every year, the control body or control authority shall send a concise annual report to the Commission; the annual report shall update the information of the technical dossier referred to in Article 4(3); it shall describe in particular the control activities carried out by the control body or control authority in the third countries during the previous year, the results obtained, the irregularities and infringements observed and the corrective measures taken; it shall furthermore contain the most recent assessment report or update of such report, which shall contain the results of the regular on-the-spot evaluation, surveillance and multiannual reassessment as referred to in Article 32(2) of Regulation (EC) No 834/2007; the Commission may request any other information deemed necessary;
(d) in the light of any information received, the Commission may at any time amend the specifications relating to the control body or control authority and may suspend the entry of that body or authority in the list referred to in Article 3; a similar decision may also be made where a control body or authority has not supplied information required or where it has not agreed to an on-the-spot examination;
(e) the control body or control authority shall make available to interested parties, on an Internet website, a continuously updated list of operators and products certified as organic.
2. If a control body or a control authority does not send the annual report, referred to in paragraph 1(c), does not keep available or does not communicate all information related to its technical dossier, control system or updated list of operators and products certified as organic, or does not agree to an on-the-spot examination, after request by the Commission within a period which the Commission shall determine according to the severity of the problem and which generally may not be less than 30 days, that control body or control authority may be withdrawn from the list of control bodies and control authorities, in accordance with the procedure referred to in Article 37(2) of Regulation (EC) No 834/2007.
If a control body or a control authority fails to take appropriate and timely remedial action, the Commission shall withdraw it from the list without delay.
CHAPTER 2
Documentary evidence required for import of compliant products
Article 6
Documentary evidence
1. The documentary evidence required for import of compliant products referred to in Article 32(1)(c) of Regulation (EC) No 834/2007, shall, in accordance with Article 17(2) of this Regulation, be established on the basis of the model set out in Annex II to this Regulation and contain at least all the elements that are part of that model.
2. The original documentary evidence shall be established by a control authority or the control body which has been recognised for issuing that documentary by a decision as referred to in Article 4.
3. The authority or body issuing the documentary evidence shall follow the rules established in accordance with Article 17(2) and in the model, notes and guidelines made available by the Commission via the computer system enabling electronic exchange of documents referred to in Article 17(1).
TITLE III
IMPORT OF PRODUCTS PROVIDING EQUIVALENT GUARANTEES
CHAPTER 1
List of recognised third countries
Article 7
Compilation and content of the list of third countries
1. The Commission shall establish a list of recognised third countries in accordance with Article 33(2) of Regulation (EC) No 834/2007. The list of recognised countries is set out in Annex III to this Regulation. The procedures for drawing up and amending the list are defined in Articles 8 and 16 of this Regulation. Amendments to the list shall be made available to the public on the Internet in accordance with Articles 16(4) and 17 of this Regulation.
2. The list shall contain all the information necessary in respect of each third country to allow verifying whether products placed on the Community market have been subject to the control system of the third country recognised in accordance with Article 33(2) of Regulation (EC) No 834/2007 and in particular:
(a) the product categories concerned;
(b) the origin of the products;
(c) a reference to the production standards applied in the third country;
(d) the competent authority in the third country responsible for the control system, its address, including e-mail and Internet addresses;
(e) the names and internet addresses of the control authority or authorities or the control body or bodies recognised by the competent authority referred to in point (d) to carry out controls;
(f) the names, internet addresses and code numbers of the authority or authorities or the control body or bodies responsible in the third country for issuing certificates with a view to importing into the Union;
(g) the duration of the inclusion in the list.
Article 8
Procedure for requesting inclusion in the list of third countries
1. The Commission shall consider whether to include a third country in the list provided for in Article 7 upon receipt of a request for inclusion from the representative of the third country concerned, provided that such request is submitted before 1 July 2014.
2. The Commission shall only be required to consider a request for inclusion which meets the following preconditions.
The request for inclusion shall be completed by a technical dossier, which shall comprise all the information needed for the Commission to ensure that the conditions set out in Article 33(1) of Regulation (EC) No 834/2007 are met for products intended for export to the Community, namely:
(a) general information on the development of organic production in the third country, the products produced, the area in cultivation, the production regions, the number of producers, the food processing taking place;
(b) an indication of the expected nature and quantities of organic agricultural products and foodstuffs intended for export to the Community;
(c) the production standards applied in the third country as well as an assessment of their equivalence to the standards applied in the Community;
(d) the control system applied in the third country, including the monitoring and supervisory activities carried out by the competent authorities in the third country, as well as an assessment of its equivalent effectiveness when compared to the control system applied in the Community;
(e) the Internet or other address where the list of operators subject to the control system can be found, as well as a contact point where information is readily available on their certification status and the product categories concerned;
(f) the information the third country proposes to include in the list as referred to in Article 7;
(g) an undertaking to comply with the provisions of Article 9;
(h) any other information deemed relevant by the third country or by the Commission.
3. When examining a request for inclusion in the list of recognised third countries, and also any time after its inclusion, the Commission may request any further information, including the presentation of one or more on-the-spot examination reports established by independent experts. Furthermore, the Commission may, based on risk-assessment and in case of suspected irregularities organise an on-the-spot examination by experts it designates.
Experts from other third countries recognised in accordance with Article 33(2) of Regulation (EC) No 834/2007 may be invited by the Commission to attend on-the-spot-examination as observers.
4. The Commission shall assess whether the technical dossier referred to in paragraph 2 and the information referred to in paragraph 3 are satisfactory and may subsequently decide to recognise and include a third country in the list for a three-year period. Where the Commission considers that the conditions laid down in Regulation (EC) No 834/2007 and this Regulation continue to be met, it may decide to extend the inclusion of the third country after that three-year period.
The decisions referred to in the first subparagraph shall be taken in accordance with the procedure referred to in Article 37(2) of Regulation (EC) No 834/2007.
Article 9
Management and review of the list of third countries
1. The Commission shall only be required to consider a request for inclusion when the third country undertakes to accept the following conditions:
(a) if, after a third country has been included in the list, any changes are made to the measures in force in the third country or their implementation and in particular to its control system, that third country shall notify the Commission thereof; requests to amend the information in respect of a third country referred to in Article 7(2) shall also be notified to the Commission;
(b) the annual report referred to in Article 33(2) of Regulation (EC) No 834/2007 shall update the information of the technical dossier referred to in Article 8(2) of this Regulation; it shall describe in particular the monitoring and supervisory activities carried out by the competent authority of the third country, the results obtained and the corrective measures taken;
(c) in the light of any information received, the Commission may at any time amend the specifications relating to the third country and may suspend the entry of that country from the list referred to in Article 7; a similar decision may also be made where a third country has not supplied information required or where it has not agreed to an on-the-spot examination.
2. If a third country does not send the annual report, referred to Article 33(2) of Regulation (EC) No 834/2007, does not keep available or does not communicate all information related to its technical dossier or control system or does not agree to an on-the-spot examination, after request by the Commission within a period which the Commission shall determine according to the severity of the problem and which generally may not be less than 30 days, that third country may be withdrawn from the list, in accordance with the procedure referred to in Article 37(2) of Regulation (EC) No 834/2007.
CHAPTER 2
List of recognised control bodies and control authorities for the purpose of equivalence
Article 10
Compilation and content of the list of recognised control bodies and control authorities for the purpose of equivalence
1. The Commission shall draw up a list of control bodies and control authorities, recognised for the purpose of equivalence in accordance with Article 33(3) of Regulation (EC) No 834/2007. The list shall be published in Annex IV to this Regulation. The procedures for drawing up and amending the list are defined in Articles 11, 16 and 17 of this Regulation. The list shall be made available to the public on the Internet in accordance with Articles 16(4) and 17 of this Regulation.
2. The list shall contain all the information necessary in respect of each control body or authority to allow verifying whether products placed on the Community market have been controlled by a control body or authority recognised in accordance with Article 33(3) of Regulation (EC) No 834/2007 and in particular:
(a) the name, address and code number of the control body or authority, and, when appropriate, its e-mail and Internet address;
(b) the third countries not listed in the list provided for in Article 7 where the products have their origin;
(c) the product categories concerned for each third country;
(d) the duration of the inclusion in the list; and
(e) the internet website where an updated list of operators subject to the control system can be found, indicating their certification status and the product categories concerned as well as a contact point where information is available on suspended and decertified operators and products; and
(f) the internet website where a complete presentation of the production standard and control measures applied by the control body or control authority in a third country can be found.
3. By way of derogation from paragraph 2(b), those products originating from third countries listed in the list of recognised third countries as referred to in Article 7 which belong to a category which is not referred to in that list may be listed in the list provided for in this Article.
Article 11
Procedure for requesting inclusion in the list of recognised control bodies and control authorities for the purpose of equivalence
1. The Commission shall consider whether to include a control body or control authority in the list provided for in Article 10 upon receipt of a request for inclusion from the representative of the control body or control authority concerned on the basis of the model of application made available by the Commission in accordance with Article 17(2). Only complete requests that have been received by 30 September of each year shall be considered for updating the list. The Commission shall undertake regular updates of the list as appropriate on the basis of complete requests that have been received before 30 September of each year.
2. The request can be introduced by control bodies and control authorities established in the Community or in a third country.
3. The request for inclusion shall consist of a technical dossier, which shall comprise all the information needed for the Commission to ensure that the conditions set out in Article 33(3) of Regulation (EC) No 834/2007 are met for products intended for export to the Community, namely:
(a) an overview of the activities of the control body or control authority in the third country or third countries, including an estimate of the number of operators involved and the expected nature and quantities of agricultural products and foodstuffs intended for export to the Community under the rules set out in Article 33(1) and (3) of Regulation (EC) No 834/2007;
(b) a description of the production standards and control measures applied in the third countries, including an assessment of the equivalence of these standards and measures with Titles III, IV and V of Regulation (EC) No 834/2007 as well as with the associated implementing rules laid down in Regulation (EC) No 889/2008;
(c) a copy of the assessment report as set out in the fourth subparagraph of Article 33(3) of Regulation (EC) No 834/2007:
(i) proving that the control body or control authority has been satisfactorily assessed on its ability to meet the conditions set out in Article 33(1) and (3) of Regulation (EC) No 834/2007;
(ii) confirming that it has effectively implemented its activities according to those conditions; and
(iii) demonstrating and confirming the equivalence of the production standards and control measures referred to in subparagraph (b) of this paragraph;
(d) proof that the control body or control authority has notified its activities to the authorities of each of the third countries concerned and its undertaking to respect the legal requirements imposed on it by the authorities of each of the third countries concerned;
(e) the Internet website where the list of operators subject to the control system can be found, as well as a contact point where information is readily available on their certification status, the product categories concerned, as well as suspended and decertified operators and products;
(f) an undertaking to comply with the provisions of Article 12;
(g) any other information deemed relevant by the control body or control authority or by the Commission.
4. When examining a request for inclusion in the list of control body or control authority, and also any time after its inclusion, the Commission may request any further information, including the presentation of one or more on-the-spot examination reports established by independent expert. Furthermore, the Commission may organise an on-the-spot examination by experts it designates on a risk-based approach and in case of suspected irregularities.
5. The Commission shall assess whether the technical dossier referred to in paragraph 2 and the information referred to in paragraph 3 are satisfactory and may subsequently decide to recognise and include a control body or control authority in the list. The decision shall be taken in accordance with the procedure referred to in Article 37(2) of Regulation (EC) No 834/2007.
Article 12
Management and review of the list of control bodies and control authorities for the purpose of equivalence
1. A control body or control authority may only be included in the list referred to in Article 10 when it fulfils the following obligations:
(a) if, after a control body or control authority has been included in the list, any changes are made to the measures applied by the control body or control authority, that control body or control authority shall notify the Commission thereof; requests to amend the information in respect of a control body or authority referred to in Article 10(2), shall also be notified to the Commission;
(b) by ►M12 28 February ◄ every year, the control body or control authority shall send a concise annual report to the Commission. The annual report shall update the information of the technical dossier referred to in Article 11(3); it shall describe in particular the control activities carried out by the control body or control authority in the third countries in the previous year, the results obtained, the irregularities and infringements observed and the corrective measures taken; It shall furthermore contain the most recent assessment report or update of such report, which shall contain the results of the regular on-the-spot evaluation, surveillance and multiannual reassessment as referred to in Article 33(3) of Regulation (EC) No 834/2007; the Commission may request any other information deemed necessary;
(c) in the light of any information received, the Commission may at any time amend the specifications relating to the control body or control authority and may suspend the entry of that body or authority from the list referred to in Article 10; a similar decision may also be made where a control body or control authority has not supplied information required or where it has not agreed to an on-the-spot examination;
(d) the control body or control authority shall make available to interested parties, by electronic means, a continuously updated list of operators, and of products certified as organic.
2. In accordance with the procedure referred to in Article 37(2) of Regulation (EC) No 834/2007 a control body or a control authority, or a reference to a specific product category or to a specific third country in relation to that control body or control authority, may be withdrawn from the list referred to in Article 10 of this Regulation in the following cases:
(a) if its annual report referred to paragraph 1(b) has not been received by the Commission by ►M12 28 February ◄ ;
(b) if it does not notify the Commission in due time of changes to its technical dossier;
(c) if it does not provide information to the Commission during the investigations of an irregularity case;
(d) if it fails to take adequate corrective measures in response to the irregularities and infringements observed;
(e) if it does not agree to an on-the-spot examination required by the Commission, or if an on-the-spot examination comes up with a negative result due to systematic malfunctioning of control measures;
(f) in any other situation presenting the risk for the consumer to be misled about the true nature of the products certified by the control body or the control authority.
If a control body or a control authority fails to take appropriate and timely remedial action after request by the Commission within a period which the Commission shall determine according to the severity of the problem and which generally may not be less than 30 days, the Commission shall withdraw it from the list without delay in accordance with the procedure referred to in Article 37(2) of Regulation (EC) No 834/2007. That withdrawal decision shall be published in the Official Journal of the European Union. The Commission shall make the amended list available as soon as possible to the public by any appropriate technical means, including publication on the Internet.
CHAPTER 3
Release for free circulation of products imported in accordance with Article 33 of Regulation (EC) No 834/2007
Article 13
Certificate of inspection
1. The release for free circulation in the Community of a consignment of products referred to in Article 1(2) of Regulation (EC) No 834/2007 and imported in accordance with Article 33 of that Regulation shall be conditional on:
(a) the submission of an original certificate of inspection to the relevant Member State’s authority; and
(b) on the verification of the consignment by the relevant Member State’s authority and the endorsement of the certificate of inspection in accordance with paragraph 8 of this Article.
2. The original certificate of inspection shall be established in accordance with Article 17(2) and paragraphs 3 to 7 of this Article, on the basis of the model and the notes set out in Annex V. The model notes, together with guidelines referred to in Article 17(2), shall be made available by the Commission via the computer system enabling electronic exchange of documents referred to in Article 17.
3. To be accepted, the certificate of inspection must have been issued by:
(a) the control authority or control body which has been accepted for issuing the certificate of inspection, as referred to in Article 7(2), from a third country recognised under Article 8(4); or
(b) the control authority or control body in the third country listed for the third country concerned recognised under Article 11(5).
4. The authority or body issuing the certificate of inspection shall only issue the certificate of inspection and endorse the declaration in box 15 of the certificate, after:
(a) it has carried out a documentary check on the basis of all relevant inspection documents, including in particular the production plan for the products concerned, transport documents and commercial documents;
(b) it has either made a physical check of the consignment, or it has received an explicit declaration of the exporter declaring that the consignment concerned has been produced and/or prepared in accordance with Article 33 of Regulation (EC) No 834/2007; it shall carry out a risk-oriented verification of the credibility of this declaration; and
(c) it has verified, for control bodies recognised in accordance with Article 33(3) of Regulation (EC) No 834/2007, that the products covered by the certificate and in the case of processed agricultural products for use as food and feed, all organic ingredients of such products, have been certified by a control authority or control body of a third country recognised in accordance with Article 33(2) of that Regulation or by a control authority or control body recognised in accordance with Article 33(3) of that Regulation or produced and certified in the Union in accordance with that Regulation. At the request of the Commission or of the competent authority of a Member State, it shall make available without delay the list of all operators in the organic production chain and the competent authorities or control bodies under whose control those operators have placed their operations.
It shall furthermore give a serial number to each issued certificate and keep a register of the delivered certificates in chronological order.
5. The certificate of inspection shall be drawn up in one of the official languages of the Community and filled in, except for the stamps and signatures, either entirely in capital letters or entirely in typescript.
The certificate of inspection shall be in one of the official languages of the Member State of destination. Where necessary, the relevant Member State’s authorities may request a translation of the certificate of inspection in one of its official languages.
Uncertified alterations or erasures shall invalidate the certificate.
6. The certificate of inspection shall be made in one single original.
The first consignee or, where relevant, the importer may make a copy for the purpose of informing the control authorities and control bodies in accordance with Article 83 of Regulation (EC) No 889/2008. Any such copy shall carry the indication ‘COPY’ or ‘DUPLICATE’ printed or stamped thereon.
7. For products imported under the transitional rules stipulated in Article 19 of this Regulation, the following shall apply:
(a) the certificate of inspection referred to in paragraph 3(b) shall, at the time it is submitted in accordance with paragraph 1, include in box 16 the declaration of the competent authority in the Member State which granted the authorisation according to the procedure provided for in Article 19;
(b) the competent authority in the Member State which granted the authorisation may delegate the competence for the declaration in box 16 to the control authority or control body inspecting the importer in accordance with the control measures set out in Title V of Regulation (EC) No 834/2007, or to the authorities defined as the Member State’s relevant authorities;
(c) the declaration in box 16 is not required:
(i) when the importer presents an original document, issued by the competent authority of the Member State which granted the authorisation in accordance with Article 19 of this Regulation, demonstrating that the consignment is covered by that authorisation; or
(ii) when the Member State’s authority, which granted the authorisation referred to in Article 19, has given satisfactory evidence that the consignment is covered by that authorisation, directly to the authority in charge of the verification of the consignment; this procedure of direct information is optional for the Member State which granted the authorisation;
(d) the document giving the evidence required in points c(i) and (ii), shall include:
(i) the reference number of the import authorisation and the date of expiration of the authorisation;
(ii) the name and address of the importer;
(iii) the third country of origin;
(iv) the details of the issuing body or authority, and, where different, the details of the inspection body or authority in the third country;
(v) the names of the products concerned.
8. At the verification of a consignment, the original certificate of inspection shall be endorsed by the relevant Member State’s authorities in box 17 and returned to the person who submitted the certificate.
9. The first consignee shall, at the reception of the consignment, complete box 18 of the original of the certificate of inspection, to certify that the reception of the consignment has been carried out in accordance with Article 34 of Regulation (EC) No 889/2008.
The first consignee shall then send the original of the certificate to the importer mentioned in box 11 of the certificate, for the purpose of the requirement laid down in the second subparagraph of Article 33(1) of Regulation (EC) No 834/2007, unless the certificate has to further accompany the consignment referred to in paragraph 1 of this Article.
10. The certificate of inspection may be established by electronic means, using the method made available to the control authorities or control bodies by the Member State concerned. The competent authorities of the Member States may require that the electronic certificate of inspection be accompanied by an advance electronic signature within the meaning of Article 2(2) of Directive 1999/93/EC of the European Parliament and of the Council ( 7 ). In all other cases, the competent authorities shall require an electronic signature offering equivalent assurances with regard to the functionalities attributed to a signature by applying the same rules and conditions as these defined in the Commission’s provisions on electronic and digitised documents, set out by Commission Decision 2004/563/EC, Euratom ( 8 ).
Article 14
Special customs procedures
1. Where a consignment coming from a third country is assigned to customs warehousing or inward processing in the form of a system of suspension as provided for in Council Regulation (EEC) No 2913/92 ( 9 ), and subject to one or more preparations as defined in Article 2(i) of Regulation (EC) No 834/2007, the consignment shall be subject, before the first preparation is carried out, to the measures referred to in Article 13(1) of this Regulation.
The preparation may include operations such as:
(a) packaging or repackaging; or
(b) labelling concerning the presentation of the organic production method.
After this preparation, the endorsed original of the certificate of inspection shall accompany the consignment, and shall be presented to the relevant Member State’s authority, which shall verify the consignment for the purpose of its release for free circulation.
After this procedure, the original of the certificate of inspection shall, where relevant, be returned to the importer of the consignment, referred to in box 11 of the certificate to fulfil the requirement laid down in the second subparagraph of Article 33(1) of Regulation (EC) No 834/2007.
2. Where, under a suspensive customs procedure pursuant to Regulation (EEC) No 2913/92, a consignment coming from a third country is intended to be submitted in a Member State, before its release for free circulation in the Community, to a splitting into different batches, the consignment shall be subject, before this splitting is carried out, to the measures referred to in Article 13(1) of this Regulation.
For each of the batches which results from the splitting, an extract of the certificate of inspection shall be submitted to the relevant Member State’s authority, in accordance with the model and the notes set out in Annex VI. The extract from the certificate of inspection shall be endorsed by the relevant Member State’s authorities in box 14.
A copy of each endorsed extract from the certificate of inspection shall be kept together with the original certificate of inspection by the person identified as the original importer of the consignment and mentioned in box 11 of the certificate of inspection. This copy shall carry the indication ‘COPY’ or ‘DUPLICATE’ printed or stamped thereon.
After the splitting, the endorsed original of each extract of the certificate of inspection shall accompany the batch concerned, and shall be presented to the relevant Member State’s authority, which shall verify the batch concerned for the purpose of its release for free circulation.
The consignee of a batch shall, at the reception thereof complete the original of the extract of the certificate of inspection in box 15, in order to certify that the reception of the batch has been carried out in accordance with Article 34 of Regulation (EC) No 889/2008.
The consignee of a batch shall keep the extract of the certificate of inspection at the disposal of the control authorities and/or control bodies for not less than two years.
3. The preparation and splitting operations referred to in paragraphs 1 and 2 shall be carried out in accordance with the relevant provisions set out in Title V of Regulation (EC) No 834/2007 and in Title IV of Regulation (EC) No 889/2008.
Article 15
Non-compliant products
1. Without prejudice to any measures or actions taken in accordance with Article 30 of Regulation (EC) No 834/2007 and/or Regulation (EC) No 889/2008, the release for free circulation in the Union of products not in conformity with the requirements of Regulation (EC) No 834/2007 shall be conditional on the removal of references to organic production from the labelling, advertising and accompanying documents.
2. ►M9 Without prejudice to any measures or actions to be taken in accordance with Article 30 of Regulation (EC) No 834/2007, in case of suspicion of infringements and irregularities as regards compliance of imported organic products from third countries recognised in accordance with Article 33(2) of Regulation (EC) No 834/2007 or imported organic products controlled by control authorities or control bodies recognised in accordance with Article 33(3) of that Regulation with the requirements laid down in that Regulation, the importer shall take all necessary measures in accordance with Article 91(1) of Regulation (EC) No 889/2008. ◄
The importer and the control authority or control body which issued the certificate of inspection as referred to in Article 13 of this Regulation shall immediately inform the control bodies, control authorities and competent authorities of the Member States concerned and of the third countries involved in the organic production of the products in question and, where appropriate, the Commission. The control authority or control body may require that the product cannot be placed on the market with indications referring to the organic production method until it is satisfied, by the information received from the operator or from other sources, that the doubt has been eliminated.
3. Without prejudice to any measures or actions to be taken in accordance with Article 30 of Regulation (EC) No 834/2007, where a control authority or control body of a Member State or a third country has a substantiated suspicion of an infringement or irregularity as regards compliance of imported organic products from third countries recognised in accordance with Article 33(2) of Regulation (EC) No 834/2007 or imported organic products controlled by control authorities or control bodies recognised in accordance with Article 33(3) of that Regulation with the requirements laid down in that Regulation, it shall take all necessary measures in accordance with Article 91(2) of Regulation (EC) No 889/2008 and shall immediately inform the control bodies, control authorities and competent authorities of the Member States concerned and of the third countries involved in the organic production of the products in question and the Commission.
4. Where a competent authority of a third country recognised in accordance with Article 33(2) of Regulation (EC) No 834/2007 or a control authority or control body recognised in accordance with Article 33(3) of that Regulation is notified by the Commission after having received a communication from a Member State informing it of a substantiated suspicion of an infringement or irregularity as regards compliance of imported organic products with the requirements laid down in that Regulation or this Regulation, it shall investigate the origin of the suspected irregularity or infringement and shall inform the Commission and the Member State which sent the initial communication of the result of the investigation and of the action taken. That information shall be sent within 30 calendar days from the date of sending of the original notification by the Commission.
The Member State which sent the initial communication may ask the Commission to request additional information, if needed, which shall be sent to the Commission and to the Member State concerned. In any case, after receiving a reply or additional information, the Member State which sent the initial communication shall make the necessary entries and updates in the computer system referred to in Article 94(1) of Regulation (EC) No 889/2008.
TITLE IV
COMMON RULES
Article 16
Assessment of the requests and publication of the lists
1. The Commission shall examine the requests received in accordance with Articles 4, 8 and 11 with the assistance of the Committee on organic production, referred to in Article 37(1) of Regulation (EC) No 834/2007 (hereafter called ‘the Committee’). For this purposes the Committee shall adopt specific internal rules of procedure.
In order to assist the Commission with the examination of the requests and with the management and review of the lists, the Commission shall set up an expert group consisting of governmental and private experts.
2. For each request received, and after appropriate consultation with Member States in accordance with the specific internal rules of procedure, the Commission shall nominate two Member States to act as co-reporters. The Commission shall divide the requests between the Member States proportionally with the number of votes of each Member State in the Committee on organic production. The co-reporting Member States shall examine the documentation and information as set out in Articles 4, 8 and 11 related to the request and shall draw up a report. For the management and review of the lists, they shall also examine the annual reports and any other information referred to in Articles 5, 9 and 12 related to the entries on the lists.
3. Taking into account the result of the examination by the co-reporting Member States, the Commission shall decide, in accordance with the procedure referred to in Article 37(2) of Regulation (EC) No 834/2007, on the recognition of third countries, control bodies or control authorities, their inclusion on the lists or any modification of the lists, including the attribution of a code number to those bodies and authorities. The decisions shall be published in the Official Journal of the European Union.
4. The Commission shall make the lists available to the public by any appropriate technical means, including publication on the Internet.
Article 17
Communication
1. When transmitting documents or other information referred to in Articles 32 and 33 of Regulation (EC) No 834/2007 and in this Regulation to the Commission or the Member States, the competent authorities of third countries, the control authorities or the control bodies shall use electronic transmission. When specific electronic transmission systems are made available by the Commission or the Member States, they shall use these systems. The Commission and the Member States shall also use these systems to provide each other with concerned documents.
2. For the form and content of documents and information referred to in Articles 32 and 33 of Regulation (EC) No 834/2007 and in this Regulation, the Commission shall set out guidelines, models and questionnaires where appropriate and make them available in the computer system referred to in paragraph 1 of this Article. These guidelines, models and questionnaires shall be adapted and updated by the Commission, after having informed the Member States and the competent authorities of third countries, as well as the control authorities and control bodies recognised in accordance with this Regulation.
3. The computer system provided for in paragraph 1 shall be able to collect the requests, documents and information referred to in this Regulation where appropriate, including the authorisations granted pursuant to Article 19.
4. The supporting documents referred to in Articles 32 and 33 of Regulation (EC) No 834/2007 and in this Regulation, in particular in Articles 4, 8 and 11, shall be kept by the competent authorities of third countries, the control authorities or the control bodies at the disposal of the Commission and the Member States for at least three years following the year in which the controls took place or the certificates of inspection and documentary evidence were delivered.
5. Where a document or procedure provided for in Articles 32 and 33 of Regulation (EC) No 834/2007 or in the detailed rules for its application requires the signature of an authorised person or the approval of a person at one or more of the stages of that procedure, the computer systems set up for the communication of those documents must make it possible to identify each person unambiguously and provide reasonable assurance that the contents of the documents, including as regards the stages of the procedure, cannot be altered, in accordance with Community legislation, and in particular with Commission Decision 2004/563/EC, Euratom.
TITLE V
FINAL AND TRANSITIONAL RULES
Article 18
Transitional rules on the list of third countries
Requests for inclusion from third countries submitted in accordance with Article 2 of Regulation (EC) No 345/2008 before the 1 January 2009 shall be treated as applications under Article 8 of this Regulation.
The first list of recognised countries shall include Argentina, Australia, Costa Rica, India, Israel, New Zealand and Switzerland. It shall not contain the code numbers referred to in Article 7(2)(f) of this Regulation. These code numbers shall be added before 1 July 2010 by updating the list in accordance with Article 17(2).
Article 19
Transitional rules on equivalent import of products not originating in listed third countries
1. In accordance with Article 40 of Regulation (EC) No 834/2007 the competent authority of a Member State may authorise importers in that Member State, where the importer has notified his activity in accordance with Article 28 of that Regulation, to place on the market products imported from third countries which are not included in the list referred to in Article 33(2) of that Regulation, provided that the importer provides sufficient evidence showing that the conditions referred to in Article 33(1)(a) and (b) of that Regulation are satisfied. ►M7 The competent authority of a Member State may also grant such authorisations under the same conditions to products imported from a third country included in the list referred to in Article 33(2) of Regulation (EC) No 834/2007 if the imported products in question are goods which are not covered by the categories and/or origin listed for that country. ◄
Where, having first allowed the importer or any other person concerned to comment, the Member State considers that those conditions are no longer satisfied, it shall withdraw the authorisation.
Authorisations shall expire at the latest 12 months after being granted except those which have already been granted for a longer period before 1 July 2012. ►M7 Authorisations granted before 1 July 2012 shall expire on 1 July 2014 at the latest. ◄
The imported product shall be covered by a certificate of inspection as set out in Article 13, issued by the control authority or the control body which has been accepted for issuing the certificate of inspection by the competent authority of the authorising Member State. The original of the certificate must accompany the goods to the premises of the first consignee. Thereafter the importer must keep the certificate at the disposal of the control body and, as appropriate the control authority, for not less than two years.
2. Each Member State shall inform the other Member States and the Commission of each authorisation granted pursuant to this Article, including information on the production standards and control arrangements concerned, within 15 days from the date of issue.
3. At the request of a Member State or at the Commission’s initiative, an authorisation granted pursuant to this Article shall be examined by the Committee on organic production. If this examination discloses that the conditions referred to in Article 33(1)(a) and (b) of Regulation (EC) No 834/2007 are not satisfied, the Commission shall require the Member State which granted the authorisation to withdraw it.
4. Member States shall no longer grant the authorisations referred to in paragraph 1 of this Article from 1 July 2013 unless:
— the imported products in question are goods for which the organic production in the third country was controlled by a control body or a control authority not on the list set up in accordance with Article 10, or
— the imported products in question are goods for which the organic production in the third country was controlled by a control body or a control authority on the list set up in accordance with Article 10 but the goods do not belong to any of the product categories listed in Annex IV in respect of the control body or control authority for that third country.
5. Member States shall no longer grant any authorisation referred to in paragraph 1 from ►M5 1 July 2014 ◄ .
▼M7 —————
Article 20
Repeal
Regulations (EC) No 345/2008 and (EC) No 605/2008 are repealed.
References to the repealed Regulations shall be construed as references to this Regulation and shall be read in accordance with the correlation table in Annex VII.
Article 21
Entry into force
This Regulation shall enter into force on the seventh day following its publication in the Official Journal of the European Union.
It shall apply as from 1 January 2009.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
ANNEX I
LIST OF CONTROL BODIES AND CONTROL AUTHORITIES FOR THE PURPOSE OF COMPLIANCE AND RELEVANT SPECIFICATIONS REFERRED TO IN ARTICLE 3
ANNEX II

ANNEX III
LIST OF THIRD COUNTRIES AND RELEVANT SPECIFICATIONS REFERRED TO IN ARTICLE 7
ARGENTINA
|
1. |
Product categories :
|
||||||||||||||||||
|
2. |
Origin : products of category A, B and F and organically grown ingredients in products of category D that have been produced in Argentina. |
|
3. |
Production standard : Ley 25 127 sobre ‘Producción ecológica, biológica y orgánica’. |
|
4. |
Competent authority : Servicio Nacional de Sanidad y Calidad Agroalimentaria SENASA, www.senasa.gov.ar |
|
5. |
Control bodies :
|
|
6. |
Certificate issuing bodies : as at point 5. |
|
7. |
Duration of the inclusion : unspecified. |
AUSTRALIA
|
1. |
Product categories :
|
|||||||||||||||
|
2. |
Origin : products of category A and F and organically grown ingredients in products of category D that have been grown in Australia. |
|
3. |
Production standard : national standard for organic and bio-dynamic produce. |
|
4. |
Competent authority : Australian Quarantine and Inspection Service, www.aqis.gov.au |
|
5. |
Control bodies :
|
|
6. |
Certificate issuing bodies : as at point 5. |
|
7. |
Duration of the inclusion : unspecified. |
CANADA
|
1. |
Product categories :
|
|||||||||||||||||||||
|
2. |
Origin : products of category A, B and F and organically grown ingredients in products of category D and E that have been grown in Canada. |
|
3. |
Production standard : Organic Products Regulation. |
|
4. |
Competent authority : Canadian Food Inspection Agency (CFIA), www.inspection.gc.ca |
|
5. |
Control bodies :
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
6. |
Certificate issuing bodies : as at point 5. |
|
7. |
Duration of the inclusion : unspecified. |
COSTA-RICA
|
1. |
Product categories :
|
|||||||||||||||
|
2. |
Origin : products of category A and F and organically grown ingredients in products of category D that have been produced in Costa-Rica. |
|
3. |
Production standard : Reglamento sobre la agricultura orgánica. |
|
4. |
Competent authority : Servicio Fitosanitario del Estado, Ministerio de Agricultura y Ganadería, www.sfe.go.cr |
|
5. |
Control bodies :
|
|
6. |
Certificate issuing bodies : Ministerio de Agricultura y Ganadería |
|
7. |
Duration of the inclusion : unspecified. |
INDIA
|
1. |
Product categories :
|
|
2. |
Origin : products of categories A and F that have been grown in India. |
|
3. |
Production standard : National Programme for Organic Production. |
|
4. |
Competent authority : Agricultural and Processed Food Export Development Authority APEDA, http://www.apeda.gov.in/apedawebsite/index.asp |
|
5. |
Control bodies :
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
6. |
Certificate issuing bodies : as at point 5. |
|
7. |
Duration of the inclusion : unspecified. |
ISRAEL
|
1. |
Product categories :
|
|||||||||||||||
|
2. |
Origin : products of category A and F and organically grown ingredients in products of category D that have been produced in Israel or that have been imported into Israel: — either from the Union, — or from a third country in the framework of a regime which is recognised as equivalent in accordance with the provisions of Article 33(2) of Regulation (EC) No 834/2007. |
|
3. |
Production standard : Law for the Regulation of Organic Produce, 5765-2005, and its relevant Regulations. |
|
4. |
Competent authority : Plant Protection and Inspection Services (PPIS), www.ppis.moag.gov.il. |
|
5. |
Control bodies :
|
|||||||||||||||||||
|
6. |
Certificate issuing bodies : as at point 5 |
|
7. |
Duration of the inclusion : unspecified. |
JAPAN
|
1. |
Product categories :
|
|||||||||||||||
|
2. |
Origin : products of categories A and F and organically grown ingredients in products of category D that have been grown in Japan or that have been imported into Japan: — either from the Union, — or from a third country for which Japan has recognised that the products have been produced and controlled in that third country in accordance with the rules equivalent to those laid down in the Japanese legislation. |
|
3. |
Production standards : Japanese Agricultural Standard for Organic Plants (Notification No 1605 of the MAFF of October 27, 2005), Japanese Agricultural Standard for Organic Processed Foods (Notification No 1606 of MAFF of October 27, 2005). |
|
4. |
Competent authorities : Labelling and Standards Division, Food Safety and Consumer Affairs Bureau, Ministry of Agriculture, Forestry and Fisheries, www.maff.go.jp/j/jas/index.html and Food and Agricultural Materials Inspection Center (FAMIC), www.famic.go.jp |
|
5. |
Control bodies :
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
6. |
Certificate issuing bodies : as at point 5. |
|
7. |
Duration of the inclusion : unspecified. |
SWITZERLAND
|
1. |
Product categories :
|
|||||||||||||||||||||
|
2. |
Origin : products of category A and F and organically produced ingredients in products of category D and E that have been produced in Switzerland or that have been imported into Switzerland: — either from the Union, — or from a third country for which Switzerland has recognised that the products have been produced and controlled in that third country to rules equivalent to those laid down in the Swiss legislation. |
|
3. |
Production standard : Ordinance on organic farming and the labelling of organically produced plant products and foodstuffs. |
|
4. |
Competent authority : Federal Office for Agriculture FOAG, Federal Office for Agriculture FOAG, http://www.blw.admin.ch/themen/00013/00085/00092/index.html?lang=en |
|
5. |
Control bodies :
|
|
6. |
Certificate issuing bodies : as at point 5. |
|
7. |
Duration of the inclusion : unspecified. |
TUNISIA
|
1. |
Product categories :
|
|||||||||||||||
|
2. |
Origin : products of category A and F and organically grown ingredients in products of category D that have been grown in Tunisia. |
|
3. |
Production standards : Law No 99-30 of 5 April 1999 relating to Organic farming; Decree of the Minister for Agriculture of 28 February 2001, approving the standard specifications of the crop production according to the organic method. |
|
4. |
Competent authority : Direction Générale de l’Agriculture Biologique (Ministère de l’Agriculture et de l’Environnement); www.agriportail.tn |
|
5. |
Control bodies :
|
|
6. |
Certificate issuing bodies : as at point 5. |
|
7. |
Duration of the inclusion : 30 June 2015. |
UNITED STATES
|
1. |
Product categories :
|
|||||||||||||||||||||
|
2. |
Origin : products of categories A, B and F and organically grown ingredients in products of categories D and E that: — have been grown in the United States, or — have been imported into the United States and processed or packaged in the United States in accordance with US legislation. |
|
3. |
Production standards : Organic Foods Production Act of 1990 (7 U.S.C. 6501 et seq.), National Organic Program (7 CFR 205). |
|
4. |
Competent authority : United States Department of Agriculture (USDA), Agricultural Marketing Service (AMS), www.usda.gov |
|
5. |
Control bodies :
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
6. |
Certificate issuing bodies : as at point 5. |
|
7. |
Duration of the inclusion : 30 June 2015. |
NEW ZEALAND
|
1. |
Product categories :
|
||||||||||||||||||
|
2. |
Origin : products of category A, B and F and organically grown ingredients in products of category D that have been produced in New Zealand or that have been imported into New Zealand: — either from the Union, — or from a third country in the framework of a regime which is recognised as equivalent in accordance with the provisions of Article 33(2) of Regulation (EC) No 834/2007, — or from a third country whose rules of production and inspection have been recognised as equivalent to the MAF Official Organic Assurance Programme on the basis of assurances and information provided by this country’s competent authority in accordance with the provisions established by MAF and provided that only organically produced ingredients intended to be incorporated, up to a maximum of 5 % of products of agricultural origin, in products of category D prepared in New Zealand are imported. |
|
3. |
Production standard : MAF Official Organic Assurance Programme Technical Rules for Organic Production. |
|
4. |
Competent authority : Ministry for Primary Industries (MPI) http://www.foodsafety.govt.nz/industry/sectors/organics/ |
|
5. |
Control bodies :
|
|
6. |
►M16 Certificate-issuing bodies : Ministry for Primary Industries (MPI) ◄ |
|
7. |
Duration of the inclusion : unspecified. |
REPUBLIC OF KOREA
|
1. |
Product categories
:
|
|
2. |
Origin
: organically grown ingredients in products of category D that have been grown in the Republic of Korea or that have been imported into the Republic of Korea: — either from the Union — or from a third country for which the Republic of Korea has recognised that the products have been produced and controlled in that third country in accordance with the rules equivalent to those laid down in the legislation of the Republic of Korea. |
|
3. |
Production standards : Act on Promotion of Environmentally-friendly Agriculture and Fisheries and Management and Support for Organic Food. |
|
4. |
Competent authorities : Ministry of Agriculture, Food and Rural Affairs. |
|
5. |
Control bodies
:
|
|
6. |
Certificate issuing bodies and authorities : as at point 5. |
|
7. |
Duration of the inclusion : 31 January 2018. |
ANNEX IV
LIST OF CONTROL BODIES AND CONTROL AUTHORITIES FOR THE PURPOSE OF EQUIVALENCE AND RELEVANT SPECIFICATIONS REFERRED TO IN ARTICLE 10
For the purpose of this Annex, the product categories are designated by the following codes:
A : Unprocessed plant products
B : Live animals or unprocessed animal products
C : Aquaculture products and seaweeds
D : Processed agricultural products for use as food ( 10 )
E : Processed agricultural products for use as feed (10)
F : Vegetative propagating material and seeds for cultivation
The internet website, in accordance with Article 10(2)(e), where the list of operators subject to the control system can be found, as well as a contact point where information is readily available on their certification status, the product categories concerned, as well as the suspended and decertified operators and products, can be found at the internet address referred to in point 2 for each control body or control authority, unless otherwise specified.
‘Abcert AG’
1. Address: Martinstraße 42-44, 73728 Esslingen am Neckar, Germany
2. Internet address: http://www.abcert.de
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Azerbaijan |
AZ-BIO-137 |
x |
— |
— |
x |
— |
— |
|
Belarus |
BY-BIO-137 |
x |
— |
— |
x |
— |
— |
|
Georgia |
GE-BIO-137 |
x |
— |
— |
x |
— |
— |
|
Iran |
IR-BIO-137 |
x |
— |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-137 |
x |
— |
— |
— |
— |
— |
|
Moldova |
MD-BIO-137 |
x |
— |
— |
— |
— |
— |
|
Russia |
RU-BIO-137 |
x |
x |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-137 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Afrisco Certified Organic, CC’
1. Address: 39A Idol Road, Lynnwood Glen, Pretoria 0081, South Africa
2. Internet address: http://www.afrisco.net
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Angola |
AO-BIO-155 |
x |
— |
— |
— |
— |
— |
|
Botswana |
BW-BIO-155 |
x |
— |
— |
— |
— |
— |
|
Lesotho |
LS-BIO-155 |
x |
|
— |
— |
— |
— |
|
Malawi |
MW-BIO-155 |
x |
|
— |
— |
— |
— |
|
Mozambique |
MZ-BIO-155 |
x |
— |
— |
x |
— |
— |
|
Namibia |
NA-BIO-155 |
x |
— |
— |
— |
— |
— |
|
South Africa |
ZA-BIO-155 |
x |
— |
— |
x |
— |
— |
|
Swaziland |
SZ-BIO-155 |
x |
|
|
x |
|
|
|
Zambia |
ZM-BIO-155 |
x |
— |
— |
— |
— |
— |
|
Zimbabwe |
ZW-BIO-155 |
x |
|
|
|
|
|
4. Exceptions: in-conversion products
5. Duration of inclusion in the list: until 30 June 2016.
‘Agreco R.F. Göderz GmbH’
1. Address: Mündener Straße 19, 37218 Witzenhausen
2. Internet address: http://agrecogmbh.de
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Azerbaijan |
AZ-BIO-151 |
x |
— |
— |
x |
— |
— |
|
Cameroon |
CM-BIO-151 |
x |
— |
— |
x |
— |
— |
|
Ghana |
GH-BIO-151 |
x |
— |
— |
x |
— |
— |
|
Moldova |
MD-BIO-151 |
x |
— |
— |
x |
— |
— |
|
Morocco |
MA-BIO-151 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-151 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Albinspekt’
1. Address: Rruga Ded Gjon Luli, Pall. 5, Shk.1, Ap.8, 1000 Tirana, Albania
2. Internet address: http://www.albinspekt.com
3. Third countries, code numbers and product categories concerned:
4. Exceptions: In-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘ARGENCERT SA’
1. Address: Bernardo de Irigoyen 972 4 piso ‘B’, C1072AAT Buenos Aires, Argentina
2. Internet address: www.argencert.com.ar
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Argentina |
AR-BIO-138 |
— |
— |
— |
x |
— |
— |
|
Chile |
CL-BIO-138 |
x |
— |
— |
x |
— |
— |
|
Paraguay |
PY-BIO-138 |
x |
— |
— |
x |
— |
— |
|
Uruguay |
UY-BIO-138 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘AsureQuality Limited’
1. Address: Level 4, 8 Pacific Rise, Mt Wellington, Auckland, New Zealand
2. Internet address: http://www.organiccertification.co.nz
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
New Zealand |
NZ-BIO-156 |
— |
— |
x |
x |
— |
— |
|
Cook Islands |
CK-BIO-156 |
x |
— |
— |
— |
— |
— |
4. Exceptions: in-conversion products, wine, products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2016.
‘Australian Certified Organic’
1. Address: PO Box 810 — 18 Eton St, Nundah, QLD 4012, Australia
2. Internet address: http://www.aco.net.au
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Australia |
AU-BIO-107 |
— |
x |
— |
x |
— |
x |
|
China |
CN-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Cook Islands |
CK-BIO-107 |
x |
— |
— |
— |
— |
— |
|
Fiji |
FJ-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Falkland Islands |
FK-BIO-107 |
— |
x |
— |
— |
— |
— |
|
Hong Kong |
HK-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Indonesia |
ID-BIO-107 |
x |
— |
— |
x |
— |
— |
|
▼M18 ————— |
|||||||
|
Madagascar |
MG-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Myanmar/Burma |
MM-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Malaysia |
MY-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Papua New Guinea |
PG-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Singapore |
SG-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Taiwan |
TW-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Tonga |
TO-BIO-107 |
x |
— |
— |
x |
— |
— |
|
Vanuatu |
VU-BIO-107 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Austria Bio Garantie GmbH’
1. Address: Ardaggerstr. 17/1, 3300 Amstetten, Austria
2. Internet address: http://www.abg.at
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Albania |
AL-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Armenia |
AM-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Afghanistan |
AF-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Azerbaijan |
AZ-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Belarus |
BY-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Bosnia and Herzegovina |
BA-BIO-131 |
x |
— |
— |
— |
— |
x |
|
▼M10 ————— |
|||||||
|
Cuba |
CU-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Georgia |
GE-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Iran |
IR-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Iraq |
IQ-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Jordan |
JO-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Kazakhstan |
KZ-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Kosovo (1) |
XK-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Kyrgyzstan |
KG-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Lebanon |
LB-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Former Yugoslav Republic of Macedonia |
MK-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Mexico |
MX-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Moldova |
MD-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Montenegro |
ME-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Russia |
RU-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Serbia |
RS-BIO-131 |
x |
x |
— |
— |
— |
— |
|
Tajikistan |
TJ-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Turkey |
TR-BIO-131 |
x |
x |
— |
— |
— |
— |
|
Turkmenistan |
TM-BIO-131 |
x |
— |
— |
— |
— |
— |
|
Ukraine |
UA-BIO-131 |
x |
x |
— |
— |
x |
x |
|
Uzbekistan |
UZ-BIO-131 |
x |
x |
— |
— |
— |
x |
|
(1) This designation is without prejudice to positions on status, and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo declaration of independence. |
|||||||
4. Exceptions: In-conversion products
5. Duration of inclusion in the list: until 30 June 2015.
‘Balkan Biocert Skopje’
1. Address: 2/9, Frederik Sopen Str., 1000 Skopje, the former Yugoslav Republic of Macedonia
2. Internet address: http://www.balkanbiocert.mk
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
former Yugoslav Republic of Macedonia |
MK-BIO-157 |
x |
x |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2016.
‘BCS Öko-Garantie GmbH’
1. Address: Marientorgraben 3-5, 90402 Nürnberg, Germany
2. Internet address: http://www.bcs-oeko.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Albania |
AL-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Algeria |
DZ-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Angola |
AO-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Armenia |
AM-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Azerbaijan |
AZ-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Belarus |
BY-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Bolivia |
BO-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Botswana |
BW-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Brazil |
BR-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Cambodia |
KH-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Chad |
TD-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Chile |
CL-BIO-141 |
x |
x |
x |
x |
— |
x |
|
China |
CN-BIO-141 |
x |
x |
x |
x |
x |
x |
|
Colombia |
CO-BIO-141 |
x |
x |
— |
x |
— |
— |
|
Costa Rica |
CR-BIO-141 |
— |
— |
x |
— |
— |
— |
|
Côte d'Ivoire |
CI-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Cuba |
CU-BIO-141 |
x |
x |
— |
x |
— |
— |
|
Dominican Republic |
DO-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Ecuador |
EC-BIO-141 |
x |
x |
x |
x |
x |
— |
|
Egypt |
EG-BIO-141 |
x |
— |
— |
x |
— |
— |
|
El Salvador |
SV-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Ethiopia |
ET-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Georgia |
GE-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Ghana |
GH-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Guatemala |
GT-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Guinea-Bissau |
GW-BIO-141 |
x |
— |
— |
x |
— |
x |
|
Haiti |
HT-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Honduras |
HN-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Hong Kong |
HK-BIO-141 |
x |
— |
— |
x |
— |
— |
|
India |
IN-BIO-141 |
— |
— |
— |
x |
— |
— |
|
Indonesia |
ID-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Iran |
IR-BIO-141 |
x |
x |
— |
x |
— |
— |
|
Japan |
JP-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Kenya |
KE-BIO-141 |
— |
— |
— |
x |
— |
— |
|
Kosovo (1) |
XK-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Kyrgyzstan |
KG-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Laos |
LA-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Lesotho |
LS-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Former Yugoslav Republic of Macedonia |
MK-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Malawi |
MW-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Malaysia |
MY-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Moldova |
MD-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Montenegro |
ME-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Mozambique |
MZ-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Myanmar/Burma |
MM-BIO-141 |
x |
— |
x |
x |
— |
— |
|
Namibia |
NA-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Nicaragua |
NI-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Oman |
OM-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Panama |
PA-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Paraguay |
PY-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Peru |
PE-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Philippines |
PH-BIO-141 |
x |
— |
x |
x |
— |
— |
|
Russia |
RU-BIO-141 |
x |
— |
— |
x |
x |
— |
|
Saudi Arabia |
SA-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Senegal |
SN-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Serbia |
RS-BIO-141 |
x |
— |
— |
x |
— |
— |
|
South Africa |
ZA-BIO-141 |
x |
x |
— |
x |
x |
— |
|
►M18 Re-public of Korea ◄ |
KR-BIO-141 |
x |
— |
x |
►M18 x ◄ |
x |
— |
|
Sri Lanka |
LK-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Sudan |
SD-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Swaziland |
SZ-BIO-141 |
x |
— |
— |
x |
— |
— |
|
French Polynesia |
PF-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Taiwan |
TW-BIO-141 |
x |
— |
x |
x |
— |
— |
|
Tanzania |
TZ-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-141 |
x |
— |
x |
x |
x |
— |
|
Turkey |
TR-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Uganda |
UG-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-141 |
x |
— |
— |
x |
x |
— |
|
United Arab Emirates |
AE-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Uruguay |
UY-BIO-141 |
x |
x |
— |
x |
x |
— |
|
Venezuela |
VE-BIO-141 |
x |
— |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-141 |
x |
— |
x |
x |
— |
— |
|
(1) This designation is without prejudice to positions on status, and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo declaration of independence. |
|||||||
4. Exceptions: in-conversion products, products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Bio Latina Certificadora’
1. Address: Jr. Domingo Millán 852, Jesús Maria, Lima 11, Lima- Peru
2. Internet address: http://www.biolatina.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Bolivia |
BO-BIO-118 |
x |
x |
— |
x |
— |
— |
|
Colombia |
CO-BIO-118 |
x |
— |
— |
x |
— |
— |
|
Guatemala |
GT-BIO-118 |
x |
— |
— |
x |
— |
— |
|
Honduras |
HN-BIO-118 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-118 |
x |
— |
— |
x |
— |
— |
|
Nicaragua |
NI-BIO-118 |
— |
x |
— |
x |
— |
— |
|
Panama |
PA-BIO-118 |
x |
— |
— |
x |
— |
— |
|
Peru |
PE-BIO-118 |
— |
x |
x |
x |
— |
— |
|
El Salvador |
SV-BIO-118 |
x |
— |
— |
x |
— |
— |
|
Venezuela |
VE-BIO-118 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Bioagricert S.r.l.’
1. Address: Via dei Macabraccia 8, Casalecchio di Reno, 40033 Bologna, Italy
2. Internet address: http://www.bioagricert.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Brazil |
BR-BIO-132 |
x |
— |
— |
x |
— |
— |
|
Cambodia |
KH-BIO-132 |
x |
— |
— |
x |
— |
— |
|
China |
CN-BIO-132 |
x |
— |
— |
x |
— |
— |
|
Ecuador |
EC-BIO-132 |
x |
— |
— |
x |
— |
— |
|
French Polynesia |
PF-BIO-132 |
x |
— |
— |
x |
— |
— |
|
India |
IN-BIO-132 |
— |
— |
— |
x |
— |
— |
|
Laos |
LA-BIO-132 |
x |
— |
— |
x |
— |
— |
|
Nepal |
NP-BIO-132 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-132 |
x |
x |
— |
x |
— |
— |
|
Morocco |
MA-BIO-132 |
x |
— |
— |
x |
— |
— |
|
Myanmar/Burma |
MM-BIO-132 |
x |
— |
— |
x |
— |
— |
|
San Marino |
SM-BIO-132 |
— |
— |
— |
x |
— |
— |
|
Serbia |
RS-BIO-132 |
x |
x |
— |
— |
— |
— |
|
►M18 Re-public of Korea ◄ |
KR-BIO-132 |
x |
— |
— |
►M18 x ◄ |
— |
— |
|
Thailand |
TH-BIO-132 |
x |
x |
— |
x |
— |
— |
|
Togo |
TG-BIO-132 |
x |
— |
— |
x |
— |
— |
|
Turkey |
TR-BIO-132 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-132 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘BioGro New Zealand Limited’
1. Address: P.O. Box 9693 Marion Square, Wellington 6141, New Zealand
2. Internet address: http://www.biogro.co.nz
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Fiji |
FJ-BIO-130 |
x |
— |
— |
x |
— |
— |
|
Malaysia |
MY-BIO-130 |
— |
— |
— |
x |
— |
— |
|
Niue |
NU-BIO-130 |
x |
— |
— |
x |
— |
— |
|
Samoa |
WS-BIO-130 |
x |
— |
— |
x |
— |
— |
|
Vanuatu |
VU-BIO-130 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Bio.inspecta AG’
1. Address: Ackerstrasse, 5070 Frick, Switzerland
2. Internet address: http://www.bio-inspecta.ch
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Armenia |
AM-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Albania |
AL-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Azerbaijan |
AZ-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Benin |
BJ-BIO-161 |
x |
— |
— |
— |
— |
— |
|
Brazil |
BR-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Burkina Faso |
BF-BIO-161 |
x |
— |
— |
— |
— |
— |
|
Cuba |
CU-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Dominican Republic |
DO-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Ethiopia |
ET-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Georgia |
GE-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Ghana |
GH-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Indonesia |
ID-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Iran |
IR-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Kenya |
KE-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Kosovo (1) |
XK-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Kyrgyzstan |
KZ-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Lebanon |
LB-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Moldova |
MD-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Philippines |
PH-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Russia |
RU-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Senegal |
SN-BIO-161 |
x |
— |
— |
x |
— |
— |
|
South Africa |
ZA-BIO-161 |
x |
— |
— |
x |
— |
— |
|
►M18 Re-public of Korea ◄ |
KR-BIO-161 |
x |
— |
— |
►M18 x ◄ |
— |
— |
|
Tanzania |
TZ-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Tajikistan |
TJ-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Turkey |
TR-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Uzbekistan |
UZ-BIO-161 |
x |
— |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-161 |
x |
— |
— |
x |
— |
— |
|
(1) This designation is without prejudice to positions on status, and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo declaration of independence. |
|||||||
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2016.
‘Bolicert Ltd’
1. Address: Street Colon 756, floor 2, office 2A, Edif. Valdivia Casilla 13030, La Paz, Bolivia
2. Internet address: http://www.bolicert.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Bolivia |
BO-BIO-126 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Caucacert Ltd’
1. Address: 2, Marshal Gelovani Street, 5th floor, Suite 410, Tbilisi 0159, Georgia
2. Internet address: http://www.caucascert.ge
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Georgia |
GE-BIO-117 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products
5. Duration of inclusion in the list: until 30 June 2015.
‘CCOF Certification Services’
1. Address: 2155 Delaware Avenue, Suite 150, Santa Cruz, CA 95060, USA
2. Internet address: http://www.ccof.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Canada |
CA-BIO-105 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-105 |
x |
— |
— |
x |
— |
x |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘CCPB Srl’
1. Address: Via Jacopo Barozzi N.8, 40126 Bologna, Italy
2. Internet address: http://www.ccpb.it
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
China |
CN-BIO-102 |
x |
— |
— |
x |
— |
— |
|
Egypt |
EG-BIO-102 |
x |
x |
— |
x |
— |
— |
|
Iraq |
IQ-BIO-102 |
x |
— |
— |
x |
— |
— |
|
Lebanon |
LB-BIO-102 |
x |
x |
— |
x |
— |
— |
|
Morocco |
MA-BIO-102 |
x |
x |
— |
x |
— |
— |
|
Philippines |
PH-BIO-102 |
x |
— |
— |
x |
— |
— |
|
San Marino |
SM-BIO-102 |
x |
x |
— |
x |
— |
— |
|
Syria |
SY-BIO-102 |
x |
— |
— |
x |
— |
— |
|
Tunisia |
TN-BIO-102 |
— |
x |
— |
— |
— |
— |
|
Turkey |
TR-BIO-102 |
x |
x |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
▼M13 —————
‘CERES Certification of Environmental Standards GmbH’
1. Address: Vorderhaslach 1, 91230 Happurg, Germany
2. Internet address: http://www.ceres-cert.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Albania |
AL-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Azerbaijan |
AZ-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Benin |
BJ-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Bolivia |
BO-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Burkina Faso |
BF-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Bhutan |
BT-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Brazil |
BR-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Chile |
CL-BIO-140 |
x |
x |
— |
x |
— |
— |
|
China |
CN-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Colombia |
CO-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Dominican Republic |
DO-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Ecuador |
EC-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Egypt |
EG-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Ethiopia |
ET-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Ghana |
GH-BIO-140 |
x |
|
|
|
|
|
|
Grenada |
GD-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Indonesia |
ID-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Iran |
IR-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Jamaica |
JM-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Kenya |
KE-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Kyrgyzstan |
KG-BIO-140 |
x |
— |
— |
x |
— |
— |
|
The former Yugoslav Republic of Macedonia |
MK-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Mali |
ML-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Moldova |
MD-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Morocco |
MA-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Papua New Guinea |
PG-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Paraguay |
PY-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Peru |
PE-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Philippines |
PH-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Russia |
RU-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Rwanda |
RW-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Saudi Arabia |
SA-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Senegal |
SN-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Serbia |
RS-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Singapore |
SG-BIO-140 |
x |
x |
— |
x |
— |
— |
|
South Africa |
ZA-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Saint Lucia |
LC-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Taiwan |
TW-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Tanzania |
TZ-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Thailand |
TH-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Turkey |
TR-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Togo |
TG-BIO-140 |
x |
— |
— |
x |
— |
— |
|
Uganda |
UG-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Uzbekistan |
UZ-BIO-140 |
x |
x |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-140 |
x |
x |
— |
x |
— |
— |
4. Exceptions: in-conversion products
5. Duration of inclusion in the list: until 30 June 2015.
‘Certificadora Mexicana de productos y procesos ecológicos S.C.’
1. Address: Calle 16 de septiembre No 204, Ejido Guadalupe Victoria, Oaxaca, Mexico, C.P. 68026
2. Internet address: http://www.certimexsc.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Dominican Republic |
DO-BIO-104 |
x |
— |
— |
— |
— |
— |
|
Guatemala |
GT-BIO-104 |
x |
— |
— |
— |
— |
— |
|
Mexico |
MX-BIO-104 |
x |
x |
— |
x |
— |
— |
|
El Salvador |
SV-BIO-104 |
x |
— |
— |
— |
— |
— |
4. Exceptions: In-conversion products; wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Certisys’
1. Address: Rue Joseph Bouché 57/3, 5310 Bolinne, Belgium
2. Internet address: http://www.certisys.eu
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Benin |
BJ-BIO-128 |
x |
— |
— |
x |
— |
— |
|
Burkina Faso |
BF-BIO-128 |
x |
— |
— |
x |
— |
— |
|
Cote d'Ivoire |
CI-BIO-128 |
x |
— |
— |
x |
— |
— |
|
Ghana |
GH-BIO-128 |
x |
— |
— |
x |
— |
— |
|
Mali |
ML-BIO-128 |
x |
— |
— |
x |
— |
— |
|
Senegal |
SN-BIO-128 |
x |
— |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-128 |
x |
— |
— |
x |
— |
— |
|
Togo |
TG-BIO-128 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Company of Organic Agriculture in Palestine’
1. Address: Alsafa building- first floor Al-Masaeif, Ramallah, Palestine
2. Internet address: http://coap.org.ps
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Occupied Palestinian territory |
PS-BIO-163 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2018.
‘Control Union Certifications’
1. Address: Meeuwenlaan 4-6, 8011 BZ, Zwolle, The Netherlands
2. Internet address: http://certification.controlunion.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Afghanistan |
AF-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Albania |
AL-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Bermuda |
BM-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Bhutan |
BT-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Brazil |
BR-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Burkina Faso |
BF-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Cambodia |
KH-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Canada |
CA-BIO-149 |
— |
— |
x |
— |
— |
— |
|
China |
CN-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Colombia |
CO-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Costa Rica |
CR-BIO-149 |
— |
x |
x |
— |
x |
— |
|
Côte d'Ivoire |
CI-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Dominican Republic |
DO-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Ecuador |
EC-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Egypt |
EG-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Ethiopia |
ET-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Ghana |
GH-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Guinea |
GN-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Honduras |
HN-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Hong Kong |
HK-BIO-149 |
x |
x |
x |
x |
x |
x |
|
India |
IN-BIO-149 |
— |
x |
x |
x |
x |
— |
|
Indonesia |
ID-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Iran |
IR-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Israel |
IL-BIO-149 |
— |
x |
x |
— |
x |
— |
|
Japan |
JP-BIO-149 |
— |
x |
x |
— |
x |
— |
|
►M18 Re-public of Korea ◄ |
KR-BIO-149 |
x |
x |
x |
►M18 x ◄ |
x |
x |
|
Kyrgyzstan |
KG-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Laos |
LA-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Former Yugoslav Republic of Macedonia |
MK-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Malaysia |
MY-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Mali |
ML-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Mauritius |
MU-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Mexico |
MX-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Moldova |
MD-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Mozambique |
MZ-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Myanmar/Burma |
MM-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Nepal |
NP-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Nigeria |
NG-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Pakistan |
PK-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Occupied Palestinian territory |
PS-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Panama |
PA-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Paraguay |
PY-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Peru |
PE-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Philippines |
PH-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Rwanda |
RW-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Serbia |
RS-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Sierra Leone |
SL-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Singapore |
SG-BIO-149 |
x |
x |
x |
x |
x |
x |
|
South Africa |
ZA-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Sri Lanka |
LK-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Switzerland |
CH-BIO-149 |
— |
— |
x |
— |
— |
— |
|
Syria |
SY-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Tanzania |
TZ-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Thailand |
TH-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Timor-Leste |
TL-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Turkey |
TR-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Uganda |
UG-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Ukraine |
UA-BIO-149 |
x |
x |
x |
x |
x |
x |
|
United Arab Emirates |
AE-BIO-149 |
x |
x |
x |
x |
x |
x |
|
United States |
US-BIO-149 |
— |
— |
x |
— |
— |
— |
|
Uruguay |
UY-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Uzbekistan |
UZ-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Vietnam |
VN-BIO-149 |
x |
x |
x |
x |
x |
x |
|
Zambia |
ZM-BIO-149 |
x |
x |
x |
x |
x |
x |
4. Exceptions: in-conversion products, products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Doalnara Certified Organic Korea, LLC’
1. Address: 192-3 Jangyang-ri, Socho-myeon, Wonju-si, Gangwon, South Korea
2. Internet address: http://dcok.systemdcok.or.kr
3. Third countries, code numbers and product categories concerned:
4. Exceptions: in-conversion products, ►M18 ————— ◄
5. Duration of inclusion in the list: until 30 June 2015.
‘Ecocert SA’
1. Address: BP 47, 32600 L’Isle-Jourdain, France
2. Internet address: http://www.ecocert.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Algeria |
DZ-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Andorra |
AD-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Azerbaijan |
AZ-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Bahrain |
BH-BIO-154 |
— |
— |
— |
x |
— |
— |
|
Benin |
BJ-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Bosnia and Herzegovina |
BA-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Brazil |
BR-BIO-154 |
x |
x |
— |
x |
x |
x |
|
Brunei |
BN-BIO-154 |
— |
— |
x |
— |
— |
— |
|
Burkina Faso |
BF-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Burundi |
BI-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Cambodia |
KH-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Cameroon |
CM-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Canada |
CA-BIO-154 |
— |
— |
— |
x |
— |
— |
|
Chad |
TD-BIO-154 |
x |
— |
— |
— |
— |
— |
|
China |
CN-BIO-154 |
x |
x |
x |
x |
x |
x |
|
Colombia |
CO-BIO-154 |
x |
— |
— |
x |
— |
x |
|
Comoros |
KM-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Côte d'Ivoire |
CI-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Cuba |
CU-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Dominican Republic |
DO-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Ecuador |
EC-BIO-154 |
x |
— |
x |
x |
x |
— |
|
Fiji |
FJ-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Ghana |
GH-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Guatemala |
GT-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Guinea |
GN-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Guyana |
GY-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Haiti |
HT-BIO-154 |
x |
— |
— |
x |
— |
— |
|
India |
IN-BIO-154 |
— |
— |
x |
x |
— |
— |
|
Indonesia |
ID-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Iran |
IR-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Japan |
JP-BIO-154 |
— |
— |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-154 |
x |
— |
— |
— |
— |
— |
|
Kenya |
KE-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Kuwait |
KW-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Kyrgyzstan |
KG-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Laos |
LA-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Former Yugoslav Republic of Macedonia |
MK-BIO-154 |
x |
— |
— |
x |
— |
x |
|
Madagascar |
MG-BIO-154 |
x |
x |
x |
x |
— |
— |
|
Malawi |
MW-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Malaysia |
MY-BIO-154 |
x |
x |
— |
x |
— |
— |
|
Mali |
ML-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Mauritius |
MU-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Moldova |
MD-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Monaco |
MC-BIO-154 |
x |
— |
— |
x |
x |
— |
|
Mongolia |
MN-BIO-154 |
x |
— |
— |
— |
— |
— |
|
Morocco |
MA-BIO-154 |
x |
x |
x |
x |
— |
x |
|
Mozambique |
MZ-BIO-154 |
x |
— |
x |
x |
— |
— |
|
Namibia |
NA-BIO-154 |
x |
— |
— |
— |
— |
— |
|
Nepal |
NP-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Niger |
NE-BIO-154 |
x |
— |
— |
— |
— |
— |
|
Nigeria |
NG-BIO-154 |
x |
— |
— |
— |
— |
— |
|
Pakistan |
PK-BIO-154 |
x |
— |
— |
— |
— |
x |
|
Paraguay |
PY-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Peru |
PE-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Philippines |
PH-BIO-154 |
x |
— |
— |
x |
x |
x |
|
Russia |
RU-BIO-154 |
x |
— |
— |
— |
— |
— |
|
Rwanda |
RW-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Sao Tome and Principe |
ST-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Saudi Arabia |
SA-BIO-154 |
x |
— |
— |
x |
x |
x |
|
Senegal |
SN-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Serbia |
RS-BIO-154 |
x |
— |
— |
x |
— |
x |
|
Somalia |
SO-BIO-154 |
x |
— |
— |
x |
— |
— |
|
South Africa |
ZA-BIO-154 |
x |
x |
— |
x |
x |
x |
|
►M18 Re-public of Korea ◄ |
KR-BIO-154 |
x |
— |
— |
►M18 x ◄ |
— |
— |
|
Sudan |
SD-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Swaziland |
SZ-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Syria |
SY-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Tanzania |
TZ-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-154 |
x |
x |
x |
x |
— |
x |
|
Togo |
TG-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Tunisia |
TN-BIO-154 |
— |
— |
x |
x |
— |
— |
|
Turkey |
TR-BIO-154 |
x |
x |
x |
x |
x |
x |
|
Uganda |
UG-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-154 |
x |
— |
— |
— |
— |
— |
|
United Arab Emirates |
AE-BIO-154 |
x |
— |
— |
x |
— |
— |
|
United States |
US-BIO-154 |
— |
— |
x |
— |
— |
— |
|
Uruguay |
UY-BIO-154 |
x |
x |
— |
x |
— |
— |
|
Uzbekistan |
UZ-BIO-154 |
x |
— |
— |
— |
— |
— |
|
Vanuatu |
VU-BIO-154 |
x |
— |
— |
— |
— |
x |
|
Vietnam |
VN-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Zambia |
ZM-BIO-154 |
x |
— |
— |
x |
— |
— |
|
Zimbabwe |
ZW-BIO-154 |
x |
— |
— |
x |
— |
x |
4. Exceptions: in-conversion products, products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Ecoglobe’
1. Address: 1, Aram Khachatryan Street, apt. 66, 0033 Yerevan, Armenia
2. Internet address: http://www.ecoglobe.am
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Afghanistan |
AF-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Armenia |
AM-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Belarus |
BY-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Iran |
IR-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Kyrgyzstan |
KG-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Pakistan |
PK-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Russia |
RU-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Tajikistan |
TJ-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Turkmenistan |
TM-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-112 |
x |
— |
— |
x |
— |
— |
|
Uzbekistan |
UZ-BIO-112 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products
5. Duration of inclusion in the list: until 30 June 2015.
‘Egyptian Center Of Organic Agriculture (ECOA)’
1. Address: 29 Yathreb St., Dokki 12311, Ciza Governorate, Egypt
2. Internet address: http://www.ecoa.com.eg/
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Egypt |
EG-BIO-164 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2018.
‘Ekolojik Tarim Kontrol Organizasyonu’
1. Address: 160 Sok. 13/7 Bornova, 35040 Izmir, Turkey
2. Internet address: http://www.etko.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Azerbaijan |
AZ-BIO-109 |
x |
— |
— |
x |
— |
— |
|
Cote d'Ivoire |
CI-BIO-109 |
x |
— |
— |
x |
— |
— |
|
Ethiopia |
ET-BIO-109 |
x |
— |
— |
x |
— |
— |
|
Georgia |
GE-BIO-109 |
x |
— |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-109 |
x |
— |
— |
x |
x |
— |
|
Kyrgyzstan |
KG-BIO-109 |
x |
— |
— |
x |
— |
— |
|
Russia |
RU-BIO-109 |
x |
— |
— |
x |
x |
— |
|
Serbia |
RS-BIO-109 |
x |
x |
— |
x |
x |
— |
|
Tadzhikistan |
TJ-BIO-109 |
x |
— |
— |
x |
— |
— |
|
Turkey |
TR-BIO-109 |
x |
x |
— |
x |
x |
— |
|
Ukraine |
UA-BIO-109 |
x |
x |
— |
x |
x |
— |
|
Uzbekistan |
UZ-BIO-109 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Florida Certified Organic Growers and Consumers, Inc. (FOG), DBA as Quality Certification Services (QCS)’
1. Address: P.O. Box 12311, Gainesville FL, 32604 United States
2. Internet address: http://www.qcsinfo.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Bahamas |
BS-BIO-144 |
x |
— |
— |
x |
— |
x |
|
China |
CN-BIO-144 |
x |
— |
x |
x |
— |
x |
|
Dominican Republic |
DO-BIO-144 |
x |
— |
x |
x |
— |
x |
|
Ecuador |
EC-BIO-144 |
x |
— |
x |
— |
x |
x |
|
Guatemala |
GT-BIO-144 |
x |
— |
— |
x |
— |
— |
|
Honduras |
HN-BIO-144 |
x |
— |
x |
x |
x |
— |
|
Malaysia |
MY-BIO-144 |
x |
— |
— |
x |
— |
x |
|
Mexico |
MX-BIO-144 |
x |
— |
— |
x |
— |
x |
|
Nicaragua |
NI-BIO-144 |
x |
— |
x |
x |
— |
x |
|
Peru |
PE-BIO-144 |
x |
— |
— |
x |
— |
x |
|
Philippines |
PH-BIO-144 |
x |
— |
x |
x |
— |
x |
|
El Salvador |
SV-BIO-144 |
x |
— |
x |
x |
— |
x |
|
South Africa |
ZA-BIO-144 |
x |
— |
— |
x |
— |
x |
|
Taiwan |
TW-BIO-144 |
x |
— |
x |
x |
— |
x |
|
Turkey |
TR-BIO-144 |
x |
— |
— |
x |
— |
x |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘IBD Certifications Ltd’
1. Address: Rua Amando de Barros 2275, Centro, CEP: 18.602.150, Botucatu SP, Brazil
2. Internet address: http://www.ibd.com.br
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Brazil |
BR-BIO-122 |
x |
x |
x |
x |
x |
— |
|
China |
CN-BIO-122 |
x |
— |
— |
x |
x |
— |
|
Mexico |
MX-BIO-122 |
— |
x |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘IMO Control Latinoamérica Ltda.’
1. Address: Calle Pasoskanki 2134, Cochabamba, Bolivia
2. Internet address: http://www.imo.ch
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Bolivia |
BO-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Colombia |
CO-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Dominican Republic |
DO-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Ecuador |
EC-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Guatemala |
GT-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Haiti |
HT-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Nicaragua |
NI-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Peru |
PE-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Paraguay |
PY-BIO-123 |
x |
— |
— |
x |
— |
— |
|
El Salvador |
SV-BIO-123 |
x |
— |
— |
x |
— |
— |
|
Venezuela |
VE-BIO-123 |
x |
— |
— |
x |
— |
— |
4. Exceptions: In-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘IMO Control Private Limited’
1. Address: No 3627, 1st Floor, 7th Cross, 13th ‘G’ Main, H.A.L. 2nd Stage, Bangalore 560008, India
2. Internet address: www.imocontrol.in
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Afghanistan |
AF-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Bangladesh |
BD-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Bhutan |
BT-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Indonesia |
ID-BIO-147 |
x |
— |
— |
x |
— |
— |
|
India |
IN-BIO-147 |
- |
— |
— |
x |
— |
— |
|
Iran |
IR-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Malaysia |
MY-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Nepal |
NP-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Pakistan |
PK-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Philippines |
PH-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Sri Lanka |
LK-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-147 |
x |
— |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-147 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘IMO-Control Sertifikasyon Tic. Ltd Ști’
1. Address: 225 Sok. No:29 D:7 Bornova, 35040 Izmir, Turkey
2. Internet address: http://www.imo.ch
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Afghanistan |
AF-BIO-158 |
x |
— |
— |
x |
— |
— |
|
Azerbaijan |
AZ-BIO-158 |
x |
— |
— |
x |
— |
— |
|
Georgia |
GE-BIO-158 |
x |
— |
— |
— |
— |
— |
|
Kazakhstan |
KZ-BIO-158 |
x |
— |
— |
— |
x |
— |
|
Kyrgyz Republic |
KG-BIO-158 |
x |
— |
— |
x |
— |
— |
|
Russia |
RU-BIO-158 |
x |
— |
— |
— |
x |
— |
|
Tajikistan |
TJ-BIO-158 |
x |
— |
— |
x |
— |
— |
|
Turkey |
TR-BIO-158 |
x |
— |
— |
x |
— |
— |
|
Turkmenistan |
TM-BIO-158 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-158 |
x |
— |
— |
x |
x |
— |
|
Uzbekistan |
UZ-BIO-158 |
x |
— |
— |
x |
— |
— |
|
United Arab Emirates |
AE-BIO-158 |
— |
— |
— |
— |
x |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2016.
‘IMO Institut für Marktökologie GmbH’
1. Address: Postfach 100934, 78409 Konstanz, Germany
2. Internet address: http://www.imo.ch
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Armenia |
AM-BIO-146 |
x |
— |
— |
— |
— |
— |
|
Azerbaijan |
AZ-BIO-146 |
x |
— |
— |
— |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Indocert’
1. Address: Thottumugham post, Aluva, Ernakulam, Kerala, India
2. Internet address: http://www.indocert.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
India |
IN-BIO-148 |
— |
— |
x |
x |
x |
— |
|
Sri Lanka |
LK-BIO-148 |
x |
— |
— |
— |
— |
— |
|
Cambodia |
KH-BIO-148 |
x |
— |
— |
— |
— |
— |
4. Exceptions: in-conversion products, products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘IMOswiss AG’
1. Address: Weststrasse 1, 8570 Weinfelden, Switzerland
2. Internet address: http://www.imo.ch
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Afghanistan |
AF-BIO-143 |
x |
x |
— |
x |
— |
— |
|
Albania |
AL-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Armenia |
AM-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Azerbaijan |
AZ-BIO -143 |
x |
— |
— |
x |
— |
— |
|
Bangladesh |
BD-BIO-143 |
x |
— |
x |
x |
— |
— |
|
Bolivia |
BO-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Bosnia and Herzegovina |
BA-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Burkina Faso |
BF-BIO-143 |
x |
— |
— |
— |
— |
— |
|
Cameroon |
CM-BIO-143 |
x |
— |
— |
— |
— |
— |
|
Canada |
CA-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Chile |
CL-BIO-143 |
x |
x |
x |
x |
— |
x |
|
Colombia |
CO-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Democratic Republic of Congo |
CD-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Côte d'Ivoire |
CI-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Dominican Republic |
DO-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Ecuador |
EC-BIO-143 |
x |
— |
x |
— |
— |
— |
|
El Salvador |
SV-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Ethiopia |
ET-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Georgia |
GE-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Ghana |
GH-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Guatemala |
GT-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Haiti |
HT-BIO-143 |
x |
— |
— |
x |
— |
— |
|
India |
IN-BIO-143 |
— |
— |
x |
x |
— |
— |
|
Indonesia |
ID-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Japan |
JP-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Jordan |
JO-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Kenya |
KE-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Kyrgyzstan |
KG-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Liechtenstein |
LI-BIO-143 |
x |
— |
— |
— |
— |
— |
|
Mali |
ML-BIO-143 |
x |
— |
— |
— |
— |
— |
|
Mexico |
MX-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Morocco |
MA-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Namibia |
NA-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Nepal |
NP-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Nicaragua |
NI-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Niger |
NE-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Nigeria |
NG-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Occupied Palestinian territory |
PS-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Pakistan |
PK-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Paraguay |
PY-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Peru |
PE-BIO-143 |
x |
— |
x |
x |
— |
— |
|
Philippines |
PH-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Russia |
RU-BIO-143 |
x |
— |
— |
x |
— |
x |
|
Rwanda |
RW-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Sierra Leone |
SL-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Singapore |
SG-BIO-143 |
— |
— |
— |
x |
— |
— |
|
South Africa |
ZA-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Sri Lanka |
LK-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Sudan |
SD-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Syria |
SY-BIO-143 |
x |
— |
— |
— |
— |
— |
|
Tajikistan |
TJ-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Taiwan |
TW-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Tanzania |
TZ-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Togo |
TG-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Uganda |
UG-BIO-143 |
x |
— |
— |
x |
— |
x |
|
Ukraine |
UA-BIO-143 |
x |
x |
— |
x |
— |
x |
|
United Arab Emirates |
AE-BIO-143 |
— |
— |
— |
x |
— |
— |
|
Uzbekistan |
UZ-BIO-143 |
x |
— |
— |
x |
— |
x |
|
Venezuela |
VE-BIO-143 |
x |
— |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-143 |
x |
— |
x |
x |
— |
— |
4. Exceptions: in-conversion products and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘International Certification Services, Inc.’
1. Address: 3015th Ave SE Medina, ND 58467, United States
2. Internet address: http://www.ics-intl.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Canada |
CA-BIO-111 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-111 |
— |
— |
— |
x |
— |
— |
|
French Polynesia |
PF-BIO-111 |
— |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Istituto Certificazione Etica e Ambientale’
1. Address: Via Nazario Sauro 2, 40121 Bologna, Italy
2. Internet address: http://www.icea.info
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Albania |
AL-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Armenia |
AM-BIO-115 |
— |
x |
— |
x |
— |
— |
|
Ecuador |
EC-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Ethiopia |
ET-BIO-115 |
x |
— |
— |
— |
— |
— |
|
Iran |
IR-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Japan |
JP-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-115 |
x |
— |
— |
— |
— |
— |
|
Lebanon |
LB-BIO-115 |
— |
— |
— |
x |
— |
— |
|
Madagascar |
MG-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Malaysia |
MY-BIO-115 |
— |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-115 |
x |
x |
— |
x |
— |
— |
|
Moldova |
MD-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Russia |
RU-BIO-115 |
x |
x |
— |
x |
— |
— |
|
San Marino |
SM-BIO-115 |
— |
— |
— |
x |
— |
— |
|
Senegal |
SN-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Sri Lanka |
LK-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Syria |
SY-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-115 |
— |
— |
— |
x |
— |
— |
|
Turkey |
TR-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-115 |
x |
— |
— |
x |
— |
— |
|
United Arab Emirates |
AE-BIO-115 |
x |
x |
— |
x |
— |
— |
|
Uruguay |
UY-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Uzbekistan |
UZ-BIO-115 |
x |
— |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-115 |
— |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
▼M17 —————
‘Japan Organic and Natural Foods Association’
1. Address: Takegashi Bldg. 3rd Fl., 3-5-3 Kyobashi, Chuo-ku, Tokyo, Japan
2. Internet address: http://jona-japan.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
China |
CN-BIO-145 |
x |
— |
— |
x |
— |
— |
|
Taiwan |
TW-BIO-145 |
x |
— |
— |
x |
— |
— |
|
Japan |
JP-BIO-145 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘LACON GmbH’
1. Address: Moltkestrasse 4, 77654 Offenburg, Germany
2. Internet address: http://www.lacon-institut.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Azerbaijan |
AZ-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Bangladesh |
BD-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Brazil |
BR-BIO-134 |
— |
x |
— |
— |
— |
— |
|
Burkina Faso |
BF-BIO-134 |
x |
x |
— |
x |
— |
— |
|
Ghana |
GH-BIO-134 |
x |
— |
— |
x |
— |
— |
|
India |
IN-BIO-134 |
— |
x |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-134 |
x |
— |
— |
— |
— |
— |
|
Madagascar |
MG-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Mali |
ML-BIO-134 |
x |
— |
— |
— |
— |
— |
|
Mexico |
MX-BIO-134 |
x |
x |
— |
— |
— |
— |
|
Morocco |
MA-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Namibia |
NA-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Nepal |
NP-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Russia |
RU-BIO-134 |
x |
— |
— |
— |
— |
— |
|
Serbia |
RS-BIO-134 |
x |
— |
— |
x |
— |
— |
|
South Africa |
ZA-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Tanzania |
TZ-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Togo |
TG-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Turkey |
TR-BIO-134 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-134 |
x |
— |
— |
— |
— |
— |
|
United Arab Emirates |
AE-BIO-134 |
— |
— |
— |
x |
— |
— |
4. Exceptions: In-conversion products, wine, products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Letis S.A.’
1. Address: Urquiza 1564, S2000ANR, Rosario, Santa Fe, Argentina
2. Internet address: http://www.letis.org
3. Third countries, code numbers and product categories concerned:
4. Exceptions: in-conversion products, products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
▼M16 —————
‘NASAA Certified Organic Pty Ltd’
1. Address: Unit 7/3 Mount Barker Road, Stirling SA 5152, Australia
2. Internet address: http://www.nasaa.com.au
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Australia |
AU-BIO-119 |
— |
— |
— |
x |
— |
— |
|
Indonesia |
ID-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Malaysia |
MY-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Nepal |
NP-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Papua New Guinea |
PG-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Samoa |
WS-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Singapore |
SG-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Solomon Islands |
SB-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Sri Lanka |
LK-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Timor-Leste |
TL-BIO-119 |
x |
— |
— |
x |
— |
— |
|
Tonga |
TO-BIO-119 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘ÖkoP Zertifizierungs GmbH’
1. Address: Schlesische Straße 17d, 94315 Straubing, Germany
2. Internet address: http://www.oekop.de
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Serbia |
RS-BIO-133 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Onecert, Inc.’
1. Address: 427 North 33rd Street, Lincoln, NE 68503-3217 USA
2. Internet address: http://www.onecert.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Nepal |
NE-BIO-152 |
x |
— |
— |
x |
— |
— |
|
Samoa |
WS-BIO-152 |
x |
— |
— |
x |
— |
— |
|
India |
IN-BIO-152 |
— |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-152 |
x |
|
|
x |
|
|
|
Uganda |
UG-BIO-152 |
x |
|
|
x |
|
|
|
United Arab Emirates |
AE-BIO-152 |
— |
— |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-152 |
x |
|
|
x |
|
|
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Oregon Tilth’
1. Address: 260 SW Madison Ave, Ste 106, Corvallis, OR 97333, United States
2. Internet address: http://tilth.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Bolivia |
BO-BIO-116 |
x |
— |
— |
— |
— |
— |
|
Canada |
CA-BIO-116 |
— |
— |
— |
x |
— |
— |
|
Chile |
CL-BIO-116 |
x |
— |
— |
x |
— |
— |
|
China |
CN-BIO-116 |
— |
— |
— |
x |
— |
— |
|
Honduras |
HN-BIO-116 |
— |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-116 |
x |
— |
— |
x |
— |
— |
|
Panama |
PN-BIO-116 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Organic agriculture certification Thailand’
1. Address: 619/43 Kiatngamwong Building, Ngamwongwan Rd., Tambon Bangkhen, Muang District, Nonthaburi 11000, Thailand
2. Internet address: http://www.actorganic-cert.or.th
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Indonesia |
ID-BIO-121 |
x |
— |
— |
x |
— |
— |
|
Laos |
LA-BIO-121 |
x |
— |
— |
x |
— |
— |
|
Malaysia |
MY-BIO-121 |
— |
— |
— |
x |
— |
— |
|
Myanmar/Burma |
MM-BIO-121 |
— |
— |
— |
x |
— |
— |
|
Nepal |
NP-BIO-121 |
— |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-121 |
x |
— |
— |
x |
— |
— |
|
Vietnam |
VN-BIO-121 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Organic Certifiers’
1. Address: 6500 Casitas Pass Road, Ventura, CA 93001, USA
2. Internet address: http://www.organiccertifiers.com
3. Third countries, code numbers and product categories concerned:
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Organic Control System’
1. Address: Trg cara Jovana Nenada 15, 24000 Subotica, Srbija
2. Internet address: www.organica.rs
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Serbia |
RS-BIO-162 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2016.
‘Organic crop improvement association’
1. Address: 1340 North Cotner Boulevard, Lincoln, NE 68505-1838, USA
2. Internet address: http://www.ocia.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Canada |
CA-BIO-120 |
x |
— |
— |
x |
— |
— |
|
Guatemala |
GT-BIO-120 |
x |
x |
— |
x |
— |
— |
|
Japan |
JP-BIO-120 |
x |
x |
— |
x |
— |
— |
|
Mexico |
MX-BIO-120 |
x |
x |
— |
x |
— |
— |
|
Nicaragua |
NI-BIO-120 |
x |
x |
— |
x |
— |
— |
|
Peru |
PE-BIO-120 |
x |
x |
— |
x |
— |
— |
|
El Salvador |
SV-BIO-120 |
x |
x |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
▼M17 —————
‘Organic Standard’
1. Address: 51-B, Bohdana Khmelnytskoho str., Kyiv, 010330, Ukraine
2. Internet address: http://www.organicstandard.com.ua
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Belarus |
BY-BIO-108 |
x |
x |
— |
— |
— |
— |
|
Ukraine |
UA-BIO-108 |
x |
x |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Organización Internacional Agropecuaria’
1. Address: Av. Santa Fe 830 - (B1641ABN) – Acassuso, Buenos Aires — Argentina
2. Internet address: http://www.oia.com.ar
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Argentina |
AR-BIO-110 |
— |
— |
x |
— |
— |
— |
|
Brazil |
BR-BIO-110 |
x |
— |
— |
— |
— |
— |
|
Mexico |
MX-BIO-110 |
x |
— |
— |
x |
— |
— |
|
Panama |
PA-BIO-110 |
x |
— |
— |
x |
— |
— |
|
Uruguay |
UY-BIO-110 |
x |
x |
— |
x |
— |
— |
4. Exceptions: in-conversion products and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘Organska Kontrola’
1. Address: Dzemala Bijedića br.2, 71000 Sarajevo, Bosnia and Herzegovina
2. Internet address: http://www.organskakontrola.ba
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Montenegro |
ME-BIO-101 |
x |
— |
— |
x |
— |
— |
|
Serbia |
RS-BIO-101 |
x |
— |
— |
x |
— |
— |
|
Bosnia and Herzegovina |
BA-BIO-101 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘QC&I GmbH’
1. Address: Tiergartenstraße 32, 54595 Prüm, Germany
2. Internet address: http://www.qci.de
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Azerbaijan |
AZ-BIO-153 |
x |
— |
— |
x |
— |
— |
|
Belize |
BZ-BIO-153 |
x |
— |
— |
x |
— |
— |
|
Morocco |
MA-BIO-153 |
x |
— |
x |
x |
— |
— |
|
Sri Lanka |
LK-BIO-153 |
x |
— |
— |
x |
— |
— |
|
Thailand |
TH-BIO-153 |
x |
— |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-153 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Quality Assurance International’
1. Address: 9191 Towne Centre Drive, Suite 200, San Diego, CA 92122, United States
2. Internet address: http://www.qai-inc.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Canada |
CA-BIO-113 |
x |
— |
— |
x |
— |
— |
|
Mexico |
MX-BIO-113 |
x |
— |
— |
x |
— |
— |
|
Paraguay |
PY-BIO-113 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine and products covered by Annex III
5. Duration of inclusion in the list: until 30 June 2015.
‘SGS Austria Controll-Co. GmbH’
1. Address: Diefenbachgasse 35, 1150 Wien, Austria
2. Internet address: http://www.sgs-kontrolle.at
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Armenia |
AM-BIO-159 |
x |
— |
— |
x |
— |
— |
|
Kazakhstan |
KZ-BIO-159 |
x |
— |
— |
x |
— |
— |
|
Republic of Moldova |
MD-BIO-159 |
x |
— |
— |
x |
— |
— |
|
Serbia |
RS-BIO-159 |
x |
— |
— |
x |
— |
— |
|
South Africa |
ZA-BIO-159 |
x |
x |
— |
x |
— |
— |
|
Ukraine |
UA-BIO-159 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products
5. Duration of inclusion in the list: until 30 June 2016.
‘Soil Association Certification Limited’
1. Address: South Plaza, Marlborough Street, Bristol, BS1 3NX, United Kingdom
2. Internet address: http://www.soilassociation.org/certification
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Belize |
BZ-BIO-142 |
x |
— |
— |
x |
— |
— |
|
Cameroon |
CM-BIO-142 |
— |
x |
— |
x |
— |
— |
|
Colombia |
CO-BIO-142 |
— |
— |
— |
x |
— |
— |
|
Egypt |
EG-BIO-142 |
x |
— |
— |
x |
— |
— |
|
Ghana |
GH-BIO-142 |
x |
— |
— |
x |
— |
— |
|
Iran |
IR-BIO-142 |
x |
— |
— |
x |
— |
— |
|
Kenya |
KE-BIO-142 |
x |
— |
— |
x |
— |
— |
|
South Africa |
ZA-BIO-142 |
x |
x |
— |
x |
— |
— |
|
Thailand |
TH-BIO-142 |
x |
— |
— |
x |
— |
— |
|
Uganda |
UG-BIO-142 |
x |
— |
— |
x |
— |
— |
|
Venezuela |
VE-BIO-142 |
x |
— |
— |
— |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
‘Suolo e Salute srl’
1. Address: Via Paolo Borsellino 12, 61032 Fano (PU) Italy
2. Internet address: http://www.suoloesalute.it
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
San Marino |
SM-BIO-150 |
x |
— |
— |
— |
— |
— |
|
Senegal |
SN-BIO-150 |
x |
— |
— |
— |
— |
— |
|
Serbia |
RS-BIO-150 |
x |
— |
— |
— |
— |
— |
|
Ukraine |
UA-BIO-150 |
x |
— |
— |
— |
— |
— |
4. Exceptions: in-conversion products
5. Duration of inclusion in the list: until 30 June 2015.
‘TÜV Nord Integra’
1. Address: Statiestraat 164, 2600 Berchem (Antwerp), Belgium
2. Internet address: http://www.tuv-nord-integra.com
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Burkina Faso |
BF-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Cameroon |
CM-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Egypt |
EG-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Côte d'Ivoire |
CI-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Jordan |
JO-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Madagascar |
MG-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Mali |
ML-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Morocco |
MA-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Curaçao |
CW-BIO-160 |
x |
— |
— |
x |
— |
— |
|
Senegal |
SN-BIO-160 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2016.
‘Uganda Organic Certification Ltd’
1. Address: P.O. Box 33743, Kampala, Uganda
2. Internet address: http://www.ugocert.org
3. Third countries, code numbers and product categories concerned:
|
Third country |
Code number |
Category of products |
|||||
|
|
|
A |
B |
C |
D |
E |
F |
|
Uganda |
UG-BIO-124 |
x |
— |
— |
x |
— |
— |
4. Exceptions: in-conversion products, wine
5. Duration of inclusion in the list: until 30 June 2015.
ANNEX V
MODEL OF THE CERTIFICATE OF INSPECTION
for import of products from organic production into the European Community referred to in Article 13
The model of the certificate is determined with regard to:
— the text,
— the format, on one single sheet,
— the layout and the dimensions of the boxes.



ANNEX VI
MODEL OF THE EXTRACT OF THE CERTIFICATE OF INSPECTION
referred to in Article 14
The model of the extract is determined with regard to:
— the text,
— the format,
— the layout and the dimensions of the boxes.
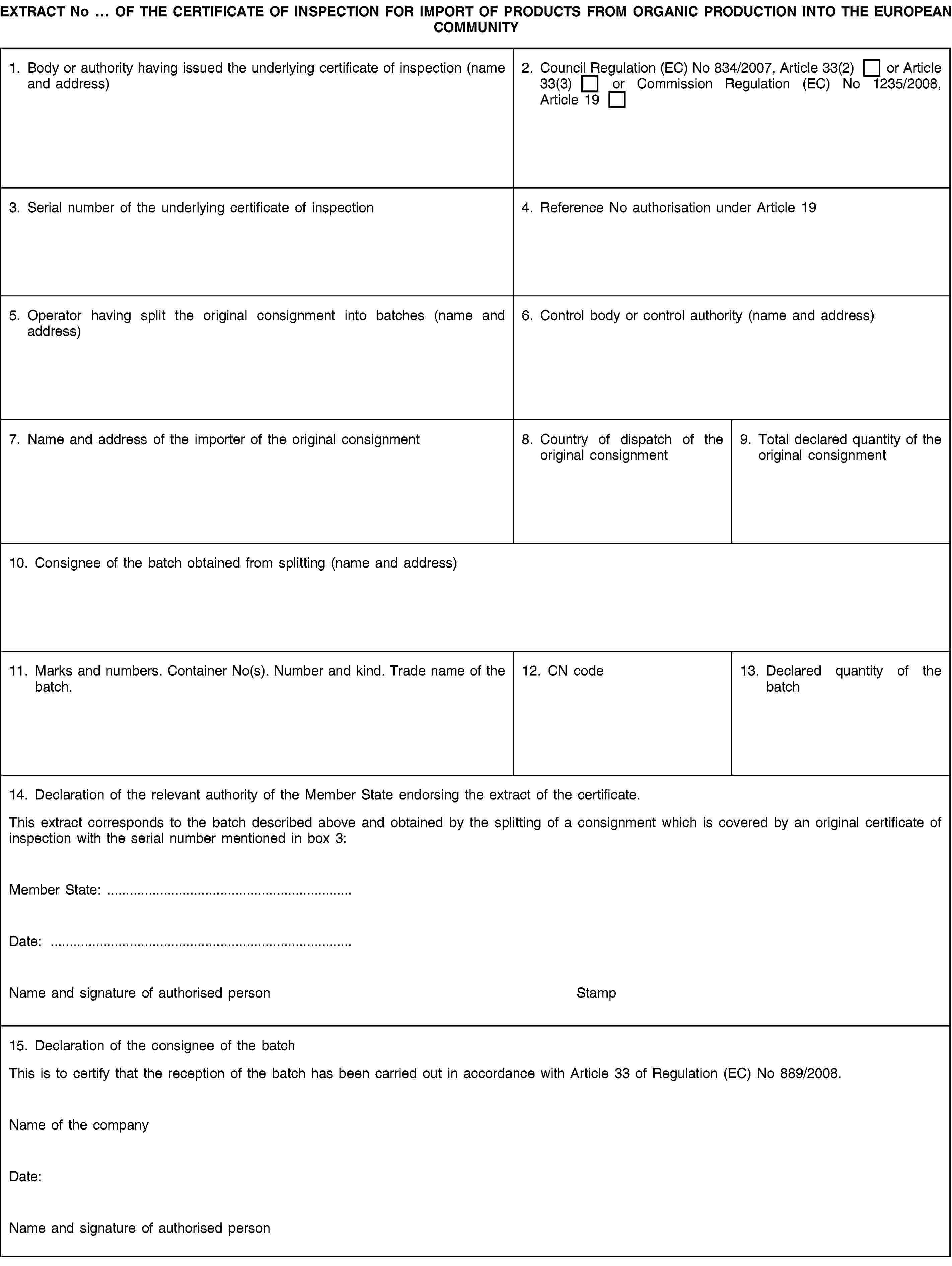

ANNEX VII
Correlation Table referred to in Article 20
|
Regulation (EC) No 345/2008 |
Regulation (EC) No 605/2008 |
This Regulation |
|
— |
Article 1(1) |
Article 1 |
|
— |
Article 1(2) |
— |
|
— |
Article 2, introductory words and point 1 |
Article 2, introductory words and point 1 |
|
|
— |
Article 2, point 2 |
|
|
Article 2, point 2 |
Article 2, point 3 |
|
|
Article 2, point 3 |
Article 2, point 4 |
|
|
Article 2, point 4 |
— |
|
|
Article 2, point 5 |
Article 2, point 5 |
|
— |
— |
Article 3 |
|
— |
— |
Article 4 |
|
— |
— |
Article 5 |
|
— |
— |
Article 6 |
|
Article 1 |
— |
Article 7 |
|
Article 2(1) |
— |
Article 8(1) |
|
Article 2(2) |
— |
Article 8(2) |
|
Article 2(3) |
— |
Article 8(3) |
|
Article 2(4) |
— |
Article 8(3) and 9(2) |
|
— |
— |
Article 8(4) |
|
Article 2(5) |
|
Article 9(1) |
|
Article 2(6) |
|
Article 9(3) and 9(4) |
|
— |
— |
Article 10 |
|
— |
— |
Article 11 |
|
— |
— |
Article 12 |
|
— |
Articles 3 and 4 |
Article 13 |
|
— |
Article 5 |
Article 14 |
|
— |
Article 6 |
Article 15 |
|
— |
— |
Article 16 |
|
— |
— |
Article 17 |
|
— |
Article 7(1) |
— |
|
— |
Article 7(2) |
— |
|
— |
— |
Article 18 |
|
— |
— |
Article 19 |
|
Article 3 |
Article 8 |
Article 20 |
|
Article 4 |
Article 9 |
Article 21 |
|
Annex II |
— |
— |
|
— |
— |
Annex I |
|
— |
— |
Annex II |
|
Annex I |
— |
Annex III |
|
— |
— |
Annex IV |
|
— |
Annex I |
Annex V |
|
— |
Annex II |
Annex VI |
|
Annex III |
Annex IV |
Annex VII |
( 1 ) OJ L 189, 20.7.2007, p. 1.
( 2 ) OJ L 108, 18.4.2008, p. 8.
( 3 ) OJ L 114, 30.4.2002, p. 132.
( 4 ) OJ L 114, 30.4.2002, p. 1.
( 5 ) OJ L 166, 27.6.2008, p. 3.
( 6 ) OJ L 250, 18.9.2008, p. 1.
( 7 ) OJ L 13, 19.1.2000, p. 12.
( 8 ) OJ L 251, 27.7.2004, p. 9.
( 9 ) OJ L 302, 19.10.1992, p. 1.
( 10 ) Ingredients have to be certified by a recognised control body or control authority according to Article 33(3) or produced and certified within the scope of a recognised third country according to Article 33(2) of Regulation (EC) No 834/2007 or produced and certified in the Union in accordance with Regulation (EC) No 834/2007.
