

This document is an excerpt from the EUR-Lex website
Document 02013R0294-20130315
Commission Regulation (EU) No 294/2013 of 14 March 2013 amending and correcting Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive (Text with EEA relevance)
Consolidated text: Commission Regulation (EU) No 294/2013 of 14 March 2013 amending and correcting Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive (Text with EEA relevance)
Commission Regulation (EU) No 294/2013 of 14 March 2013 amending and correcting Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive (Text with EEA relevance)
- Date of document:
- 15/03/2013
- Date of effect:
- 15/03/2013
- Author:
- Not available
- Form:
- Consolidated text
- Additional information:
- LASTMODIN
- Link
- Link
- Link
- Select all documents mentioning this document
- Consolidation: basic act:
- 32013R0294
2013R0294 — EN — 15.03.2013 — 000.002
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
COMMISSION REGULATION (EU) No 294/2013 of 14 March 2013 amending and correcting Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive (OJ L 098, 6.4.2013, p.1) |
Corrected by:
COMMISSION REGULATION (EU) No 294/2013
of 14 March 2013
amending and correcting Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 ( 1 ), and in particular Article 5(2), points (b) and (c) of the first subparagraph of Article 15(1) and the second subparagraph of Article 15(1), Article 18(3), points (a), (b) and (c) of the first subparagraph of Article 19(4) and the second subparagraph of Article 19(4), Articles 21(6)(c) and 32(3)(a), point (d) of the first subparagraph of Article 40, the first and third subparagraphs of Article 41(3) and Articles 42(2) and 45(4) thereof,
Whereas:|
(1) |
Regulation (EC) No 1069/2009 lays down public and animal health rules for animal by-products and derived products, in order to prevent and minimise risks to public and animal health arising from those products. It also provides for the determination of an end point in the manufacturing chain for certain derived products, beyond which they are no longer subject to the requirements of that Regulation. |
|
(2) |
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive ( 2 ), lays down implementing rules for Regulation (EC) No 1069/2009, including rules on the determination of end points for certain derived products. |
|
(3) |
In its opinion of 7 February 2011 on the capacity of oleochemical processes to minimise possible risks linked to TSE in Category 1 animal by-products ( 3 ), the European Food Safety Authority (EFSA) concluded that the risks concerning the spread of Transmissible Spongiform Encephalopathy (TSE) are significantly reduced after processing Category 1 material with hydrolytic fat splitting and hydrogenation. However, some uncertainties exist with regard to the reduction of TSE infectivity in oleochemical products derived from Category 1 material. For that reason, it cannot be safely assumed that those products are free from infectivity and therefore they could pose a risk if they entered the food and feed chain. Consequently, Article 3 of Regulation (EU) No 142/2011 and Annexes XIV and XV thereto should be amended accordingly. |
|
(4) |
Article 18(1) of Regulation (EC) No 1069/2009 provides for derogations for the use of Categories 2 and 3 materials for feeding certain animals which do not enter the food chain, including circus animals. Because certain circus animals belong to species normally used for food production, it is necessary to subject the feeding of those materials to circus animals to the conditions laid down in Article 13 of Regulation (EU) No 142/2011. |
|
(5) |
Article 19(1)(f) of Regulation (EC) No 1069/2009 provides for a derogation for the disposal of bees and apiculture by-products by burning or burial on site, under conditions which prevent the transmission of risk to public or animal health. Article 15(c) of Regulation (EU) No 142/2011 refers to special rules for collection and disposal of bees and apiculture by-products. The introductory phrase of that Article should therefore be corrected accordingly with a reference to special rules for collection and disposal of bees and apiculture by-products. |
|
(6) |
Article 36(3) of Regulation (EU) No 142/2011 provides for a transitional period until 31 December 2012 for the disposal of small quantities of Category 3 material referred to in Article 10(f) of Regulation (EC) No 1069/2009. That transitional period should be extended for two additional years during which further data should be gathered on the collection, transport and disposal of the Category 3 material concerned. |
|
(7) |
Processed animal protein derived from animal by-products, other than Category 3 materials referred to in Article 10(n), (o) and (p) of Regulation (EC) No 1069/2009, may be used as an ingredient for the production of processed petfood. Processed animal protein should not be declared as petfood unless it is mixed in appropriate proportions with other feeding substances which are normally consumed by the relevant species of pet animals. However, the producer of processed animal protein may dispatch the product to keepers of recognised kennels or packs of hounds and for feeding of dogs and cats in shelters for the production of mixed feed for dogs and cats. In such case, the product must be declared and labelled as processed animal protein. In the case of export of processed animal protein in addition to animal by-products legislation, the provisions of Regulation (EC) No 999/2001 of the European Parliament and of the Council ( 4 ) also apply. In accordance with point E2 of Part III of Annex IV to the aforementioned Regulation export of processed animal protein must be subject to a written agreement between Member States of origin of the processed animal protein and the third country of destination. Such an obligation does not exist in case of export of petfood. Given the observed risk of inappropriate use of rules on export of processed animal protein a more precise definition of petfood is required. |
|
(8) |
Transformation of animal by-products and derived products into biogas is authorised pursuant to Regulation (EC) No 1069/2009. The production of biogas leads to the generation of solid or liquid fractions. It is necessary to clarify that the requirements on the disposal of those residues apply to both fractions. |
|
(9) |
In its opinion of 30 November 2010 on the abiotic risks for public and animal health of glycerine as a co-product from the biodiesel production from Category 1 animal by-products (ABP) and vegetable oils ( 5 ), EFSA acknowledged that glycerine which had been processed with method 1 referred to in Chapter III of Annex IV to Regulation (EC) No 142/2011 for the production of biodiesel is a safe material regarding the TSE risk. Glycerine as a co-product from biodiesel production may be transformed into biogas and digestion residues after biogas production and applied to land without risk to public and animal health within the national territory of the producing Member State, subject to the decision of the competent authority. |
|
(10) |
Animal by-products referred to in Article 13(f) of Regulation (EC) No 1069/2009 may be applied to land without processing if the competent authority does not consider they present a risk for the spread of any serious transmissible disease. The same products may be composted or transformed into biogas without prior processing. |
|
(11) |
The standard wording for the description of animal by-products and derived products in trade between Member States set out in Annex VIII to Regulation (EU) No 142/2011 must be visibly and legibly displayed on the packaging, container or vehicle during transport and storage. The list of standard wordings should be extended in order to take account of trade in processed manure. |
|
(12) |
Article 48 of Regulation (EC) No 1069/2009 requires operators to inform the competent authority of the Member State of destination of their intention to dispatch consignments of Category 1 or 2 materials. Member States may conclude bilateral agreements to provide the services of their facilities for the purpose of cremating pet animals from other Member States sharing a common border. In such cases, the requirement laid down in Article 48(1) to (3) of Regulation (EC) No 1069/2009 presents unnecessary additional administrative burdens. |
|
(13) |
Chapter II of Annex X to Regulation (EU) No 142/2011 sets out specific requirements for derived products which are intended for the production of feed materials. The wording of the derogation for the placing on the market of milk processed in accordance with national standards should be amended in order to also refer to milk-based and milk-derived products and thus to align Part II of Section 4 of that Chapter with the provisions in Article 10 of Regulation (EC) No 1069/2009, in particular to its point (f) with authorisation of processing certain former foodstuffs into material for feeding of farmed animals other than fur animals. |
|
(14) |
When former foodstuffs containing ingredients of animal origin are used as source material for the production of feed for farmed animals, specific requirements apply to prevent the risk of disease transmission to animals. However, if the former foodstuffs do not contain meat, fish or their products, their use for the production of feed destined to farmed animals should be permitted, provided that they do not pose any risk of transmission of diseases communicable to humans or animals. |
|
(15) |
Article 32 of Regulation (EC) No 1069/2009 lays down conditions for placing on the market and use of organic fertilisers and soil improvers. Those products may be produced from Categories 2 and 3 materials in accordance with the requirements set out in Annex XI to Regulation (EU) No 142/2011. In case of processed animal protein of Category 3 material, specific production requirements laid down in Chapter II of Annex X to Regulation (EU) No 142/2011 must be respected including for processed animal protein where it is exclusively destined for use in petfood. For the sake of clarity it is necessary to amend Annex XI to Regulation (EU) No 142/2011 and to introduce references to any processing standards for processed animal proteins. |
|
(16) |
For the promotion of science and biodiversity research, a derogation should be granted to repositories, scientific organisations and museums as regards the collection, transport and use of animals or parts of animals preserved in media, embedded completely on micro-slides or as processed genetic samples. The requirements on game trophies and other preparations as set out in Chapter VI of Annex XIII to Regulation (EU) No 142/2011 should be amended accordingly. |
|
(17) |
Table 2 of Section 1 of Chapter II of Annex XIV to Regulation (EU) No 142/2011 sets out requirements for imports of animal by-products into the Union. The wording of certain parts of Table 2 should be improved in order to provide clearer information. In case of certain commodities which may consist of animal by-products of different animals, the list of third countries authorised for the import of animal by-products of the relevant species in Table 2 should be amended accordingly. The changes should be reflected in the corresponding certificates set out in Annex XV to that Regulation. |
|
(18) |
Petfood may be produced from any Category 3 material other than the Category 3 materials referred to in Article 10(n), (o) and (p) of Regulation (EC) No 1069/2009. The same rules which apply for placing on the market of petfood within the EU are to be applicable also for the import from third countries. Certificate Chapter 3(B) of Annex XV to Regulation (EU) No 142/2011 should be extended with reference to Article 10(c) of Regulation (EC) No 1069/2009. |
|
(19) |
Certain requirements on the import of blood and blood products should be clarified, in particular those concerning the origin of the blood. Blood must come from safe sources which may be a slaughterhouse approved in accordance with the EU legislation, a slaughterhouse approved with national legislation of the third country or from live animals bred for such purposes. Blood from such safe sources may also be mixed. It is necessary to change the text of the relevant certificates accordingly. Annex XIV and the health certificates set out in Chapters 4(A), 4(C) and 4(D) of Annex XV to Regulation (EU) No 142/2011 should therefore be amended. |
|
(20) |
Annex XVI to Regulation (EU) No 142/2011 sets out rules on official controls regarding the feeding of necrophagous birds with Category 1 material. In accordance with Article 18 of Regulation (EC) No 1069/2009, the competent authority may authorise the feeding of Category 1 material to endangered or protected species of necrophagous birds and other species living in their natural habitat. The existing rules on official controls regarding the feeding of necrophagous birds should therefore be extended to all animals to which feeding of Category 1 material may be authorised according to Annex VI to Regulation (EU) No 142/2011. |
|
(21) |
Regulation (EU) No 142/2011 should therefore be amended accordingly. |
|
(22) |
In order to avoid disruptions of trade, a transitional period should be laid down during which imports of the commodities to which the provisions of Regulation (EU) No 142/2011, as amended by this Regulation, apply should be accepted by Member States in accordance with the rules in force prior to the entry into force/date of application of this Regulation. |
|
(23) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, and neither the European Parliament nor the Council has opposed them, |
HAS ADOPTED THIS REGULATION:
Article 1
Regulation (EU) No 142/2011 is amended as follows:
(1) in Article 3, point (i) is replaced by the following:
‘(i) gasoline and fuels which fulfil the specific requirements for products from the multi-step catalytic process for the production of renewable fuels set out in point 2(c) of Section 3 of Chapter IV of Annex IV;
(j) oleochemical products derived from rendered fats and which fulfil the requirements set out in Chapter XI of Annex XIII.’;
(2) Article 13 is amended as follows:
(a) in paragraph 1, point (e) is replaced by the following:
‘(e) maggots and worms for fishing bait;
(f) circus animals.’;
(b) in paragraph 2, point (e) is replaced by the following:
‘(e) maggots and worms for fishing bait;
(f) circus animals.’;
(3) in Article 15, the introductory phrase is replaced by the following:
‘If the competent authority authorises the disposal of animal by-products by way of the derogation provided for in Article 19(1)(a), (b), (c), (e) and (f) of Regulation (EC) No 1069/2009, the disposal shall comply with the following special rules set out in Chapter III of Annex VI:’;
(4) in Article 36(3), the date ‘31 December 2012’ is replaced by ‘31 December 2014’;
(5) Annexes I, IV, V, VI, VIII, X, XI and Annexes XIII to XVI are amended in accordance with the text in the Annex to this Regulation.
Article 2
For a transitional period until 26 December 2013, consignments of animal by-products and of derived products accompanied by a health certificate, which has been completed and signed in accordance with the model set out in Chapters 3(B), 3(D), 4(A), 4(C), 4(D), 6(A), 8, 10(B), 11, 14(A) and 15 of Annex XV to Regulation (EU) No 142/2011 in its version before the date of entry into force of this Regulation, shall continue to be accepted for importation into the Union, provided that such certificates were completed and signed before 26 October 2013.
Article 3
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
It shall apply from 15 March 2013.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
ANNEX
The Annexes to Regulation (EU) No 142/2011 are amended as follows:
(1) Annex I is amended as follows:
(a) point 19 is replaced by the following:
‘19. “petfood” means feed, other than material referred to in Article 24(2), for use as feed for pet animals, and dogchews consisting of animal by-products or derived products which:
(a) contain Category 3 material, other than material referred to in Article 10(n), (o) and (p) of Regulation (EC) No 1069/2009; and
(b) may contain imported Category 1 material comprising of animal by-products derived from animals which have been submitted to illegal treatment as defined in Article 1(2)(d) of Directive 96/22/EC or Article 2(b) of Directive 96/23/EC;’;
(b) point 23 is replaced by the following:
‘23. “digestion residues” means residues, including the liquid fraction, resulting from the transformation of animal by-products in a biogas plant;’;
(2) in Annex IV, Chapter IV, Section 3 is amended as follows:
(a) point 1 is amended as follows:
(i) point (a)(iii) is replaced by the following:
‘(iii) transformed into biogas. In such case the digestion residues must be disposed of in accordance with point (i) or (ii), except where the material results from processing in accordance with point 2(a) or (b) where the residues can be used in accordance with the conditions set out in point 2(a) or point 2(b)(iii) as appropriate; or’;
(ii) point (b)(i) is replaced by the following:
‘(i) disposed of as provided for in point 1(a)(i) or (ii), with or without prior processing as provided for in Article 13(a) and (b) and Article 14(a) and (b) of Regulation (EC) No 1069/2009;’;
(b) points 2(b)(ii) and (iii) are replaced by the following:
‘(ii) in the case of potassium sulphate, used for direct application to land or for the production of derived products for application to land;
(iii) in the case of glycerine derived from Categories 1 and 2 material which has been processed in accordance with processing method 1 as set out in Chapter III:
— used for technical purposes,
— transformed into biogas, in which case the digestion residues may be applied to land within the national territory of the producing Member State, subject to the decision of the competent authority, or
— used for denitrification in a waste water treatment plant, in which case the residues of the denitrification may be applied to land in accordance with Council Directive 91/271/EEC ( 6 );
(iv) in the case of glycerine derived from Category 3 material:
— used for technical purposes,
— transformed into biogas, in which case the digestion residues may be applied to land, or
— used for feeding, provided that the glycerine is not derived from Category 3 material referred to in Article 10(n), (o) and (p) of Regulation (EC) No 1069/2009;
(c) point 3 is replaced by the following:
‘3. Any waste other than animal by-products and derived products provided for in point 2, resulting from the processing of animal by-products in accordance with this Section, such as sludge, filter contents, ash and digestion residues, shall be disposed of in accordance with Regulation (EC) No 1069/2009 and with this Regulation.’;
(3) in Annex V, Chapter I, Section 1, point 2(d) is replaced by the following:
‘(d) animal by-products which may be applied to land without processing in accordance with Article 13(f) of Regulation (EC) No 1069/2009 and with this Regulation, if the competent authority does not consider them to present a risk of spreading any serious transmissible disease to humans or animals;’;
(4) in Annex VI, Chapter II, Section 1, the introductory phrase is replaced by following:
‘Categories 2 and 3 materials as referred to in Article 18(1) of Regulation (EC) No 1069/2009 may be fed to the animals referred to in paragraph (1)(a), (b), (d), (f), (g) and (h) of that Article subject to compliance with at least the following conditions, in addition to any conditions laid down by the competent authority in accordance with Article 18(1) of that Regulation:’;
(5) Annex VIII is amended as follows:
(a) in Chapter II, point 2(b), point (xix) is replaced by the following:
‘(xix) in the case of manure which has been subject to the lime treatment set out in point I of Section 2 of Chapter IV of Annex IV, the words “manure-lime-mixture”;
(xx) in the case of processed manure which has been subject to the treatment set out in point (b) and (c) of Section 2 of Chapter I of Annex XI, the words “processed manure”.’;
(b) the following Chapter VI is added:
‘CHAPTER VI
TRANSPORT OF DEAD PET ANIMALS
The conditions in points 1 to 3 of Article 48 of Regulation (EC) No 1069/2009 regarding the advance authorisation by the competent authority in the Member States of destination and the use of TRACES shall not be required in the case of the transport of a dead pet animal for incineration in an establishment or plant located in the border region of another Member State sharing a common border when the Member States conclude a bilateral agreement on the condition of the transport.’;
(6) in Annex X, Chapter II is amended as follows:
(a) in Section 4, Part II, point 1 is replaced by the following:
‘1. The requirements laid down in points 2 and 3 of this Part shall apply to the processing, use and storage of milk, milk-based products and milk-derived products which are Category 3 material, as referred to in Article 10(e) of Regulation (EC) No 1069/2009, other than centrifuge or separator sludge, and milk, milk-based products and milk-derived products referred to in Article 10(f) and (h) of that Regulation, that have not been processed in accordance with Part I of this Section.’;
(b) Section 10 is replaced by the following:
‘Section 10
Specific requirements for feeding to farmed animals, other than fur animals, of certain Category 3 material referred to Article 10(f) of Regulation (EC) No 1069/2009
Category 3 material comprising of foodstuffs containing products of animal origin originating from Member States which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise, referred to in Article 10(f) of Regulation (EC) No 1069/2009, may be placed on the market for feeding to farmed animals, other than fur animals, without further treatment, provided that the material:
(i) has undergone processing as defined in Article 2(1)(m) of Regulation (EC) No 852/2004 or in accordance with this Regulation;
(ii) is composed of or contain one or more of the following Category 3 materials referred to in Article 10(f) of Regulation (EC) No 1069/2009:
— milk,
— milk-based products,
— milk-derived products,
— eggs,
— egg products,
— honey,
— rendered fats,
— collagen,
— gelatine;
(iii) has not been in contact with any other Category 3 materials; and
(iv) all necessary precautions have been taken to prevent the contamination of the material.’;
(7) in Annex XI, Chapter II, Section 1, point 1(b) is replaced by the following:
‘(b) using processed animal protein, including processed animal protein produced in accordance with point B.1(b)(ii) of Section 1 of Chapter II of Annex X, which has been produced from Category 3 material in accordance with Section 1 of Chapter II of Annex X, or materials which have been subject to another treatment, where such materials may be used for organic fertilisers and soil improvers in accordance with this Regulation; or’;
(8) Annex XIII is amended as follows:
(a) in Chapter VI, points C(1)(c) and (d) are replaced by the following:
‘(c) have been subject to an anatomical preparation such as by plastination;
(d) are animals of the biological class Insecta or Arachnida which have been subject to a treatment, such as drying, to prevent any transmission of diseases communicable to humans or animals; or
(e) are objects in natural history collections or for the promotion of science and they have been:
(i) preserved in media, such as alcohol or formaldehyde, which allow display of the items; or
(ii) embedded completely on micro-slides;
(f) are processed DNA samples intended for repositories for the promotion of biodiversity research, ecology, medical and veterinary science or biology.’;
(b) in Chapter XI, the following point is added:
‘3. End point for products derived from rendered fats:
Fat derivatives which have been processed as referred to in point 1 may be placed on the market for uses indicated in point 2 without restrictions in accordance with this Regulation.’;
(9) Annex XIV is amended as follows:
(a) in Chapter I, Section 1 is amended as follows:
(i) points (c), (d) and (e) are replaced by the following:
‘(c) they must come from a third country or part of a third country listed in the column “third countries’ list” of Table 1;
(d) they must come from an establishment or plant which is registered or approved by the competent authority of the third country, as applicable, and which is on the list of such establishments and plants referred to in Article 30; and
(e) they must be:
(i) accompanied during transportation to the point of entry into the Union where the veterinary checks take place by the health certificate referred to in the column “certificates/model documents” of Table 1; or
(ii) presented at the point of entry into the Union where the veterinary checks take place accompanied by a document corresponding to the model referred to in the column “certificates/model documents” of Table 1.’;
(ii) point (f) is deleted;
(b) in Chapter II, Section 1 is amended as follows:
(i) points (c), (d) and (e) are replaced by the following:
‘(c) they must come from a third country or part of a third country listed in the column “third countries’ list” of Table 2;
(d) they must come from an establishment or plant which is registered or approved by the competent authority of the third country, as applicable, and which is on the list of such establishments and plants referred to in Article 30; and
(e) they must be:
(i) accompanied during transportation to the point of entry into the Union where the veterinary checks take place by the health certificate referred to in the column “certificates/model documents” of Table 2; or
(ii) presented at the point of entry into the Union where the veterinary checks take place accompanied by a document corresponding to the model referred to in the column “certificates/model documents” of Table 2.’;
(ii) point (f) is deleted;
(iii) Table 2 is amended as follows:
— row No 13 is replaced by the following:
—
|
‘13 |
Flavouring innards for the manufacture of petfood |
Materials referred to in Article 35(a) |
The flavouring innards must have been produced in accordance with Chapter III of Annex XIII. |
Third countries listed in Part 1 of Annex II to Regulation (EU) No 206/2010, from which Member States authorise imports of fresh meat from the same species and where only bone in meat is authorised. In the case of flavouring innards from fish materials, third countries listed in Annex II to Decision 2006/766/EC. In the case of flavouring innards from poultry meat third countries listed in Part 1 of Annex I to Regulation (EC) No 798/2008, from which Member States authorise imports of fresh poultry meat. |
Annex XV, Chapter 3(E).’ |
— in row No 14, point (a) in the third column is replaced by the following:
—‘(a) Category 3 materials referred to in Article 10(a) to (m).’;
— rows Nos 15 and 16 are replaced by the following:
—
|
‘15 |
Animal by-products for use as raw petfood |
Category 3 materials referred to in Article 10(a) and Article 10(b)(i) and (ii). |
The products shall comply with the requirements set out in Section 8. |
Third countries listed in Part 1 of Annex II to Regulation (EU) No 206/2010 or in Annex I to Regulation (EC) No 798/2008, from which Member States authorise imports of fresh meat from the same species and where only bone in meat is authorised. In the case of fish materials, third countries listed in Annex II to Decision 2006/766/EC. |
Annex XV, Chapter 3(D). |
|
16 |
Animal by-products for use in feed for fur animals |
Category 3 materials referred to in Article 10(a) to (m) |
The products shall comply with the requirements set out in Section 8. |
Third countries listed in part 1 of Annex II to Commission Regulation (EU) No 206/2010, or in Annex I to Regulation (EC) No 798/2008, from which Member States authorise imports of fresh meat from the same species and where only bone in meat is authorised. In the case of fish materials, third countries listed in Annex II to Decision 2006/766/EC. |
Annex XV, Chapter 3(D).’ |
— in row No 17, third column, point (a) is replaced by the following:
—‘(a) In the case of materials destined for the production of biodiesel or oleochemical products: Categories 1, 2 and 3 materials referred to in Articles 8, 9 and 10.’;
— row No 18 is replaced by the following:
—
|
‘18 |
Fat derivatives |
(a) In the case of fat derivatives for uses outside the feed chain for farmed animals: Category 1 materials referred to in Article 8(b), (c) and (d), Category 2 materials referred to in Article 9(c) and (d) and Article 9(f)(i) and Category 3 materials referred to in Article 10. (b) In the case of fat derivatives for use as feed: Category 3 materials other than materials referred to in Article 10(n), (o) and (p); |
The fat derivatives shall comply with the requirements set out in Section 10. |
Any third country. |
(a) In the case of fat derivatives for uses outside the feed chain for farmed animals: Annex XV, Chapter 14(A). (b) In the case of fat derivatives for use as feed: Annex XV, Chapter 14(B).’ |
(c) in Chapter II, Section 2, point 2 is replaced by the following:
‘2. The blood from which blood products for the manufacture of derived products for uses outside the feed chain for farmed animals are produced must have been collected under veterinary supervision:
(a) in slaughterhouses:
(i) approved in accordance with Regulation (EC) No 853/2004; or
(ii) approved and supervised by the competent authority of the country of collection; or
(b) from live animals in facilities approved and supervised by the competent authority of the country of collection.’;
(d) in Chapter II, Section 3, point 1 is replaced by the following:
‘1. The blood must comply with the conditions set out in point 1(a) of Chapter IV of Annex XIII and must be collected under veterinary supervision:
(a) in slaughterhouses:
(i) approved in accordance with Regulation (EC) No 853/2004; or
(ii) approved and supervised by the competent authority of the country of collection; or
(b) from live equidae in facilities approved and furnished with a veterinary approval number and supervised by the competent authority of the country of collection for the purpose of collecting blood from equidae for the production of blood products for purposes other than feeding.’;
(e) in Chapter II, Section 3, point 2(d) is replaced by the following:
‘(d) in the case of blood products other than serum and plasma, vesicular stomatitis for a period of at least six months.’;
(f) in Chapter II, Section 9, point (a)(i) is replaced by the following:
‘(i) in the case of materials destined for the production of biodiesel or oleochemical products, animal by-products referred to in Articles 8, 9 and 10 of Regulation (EC) No 1069/2009;’;
(10) Annex XV is amended as follows:
(a) Chapter 3(B) is replaced by the following:
‘CHAPTER 3(B)
Health certificate
For processed petfood other than canned petfood, intended for dispatch to or for transit through (2) the European Union
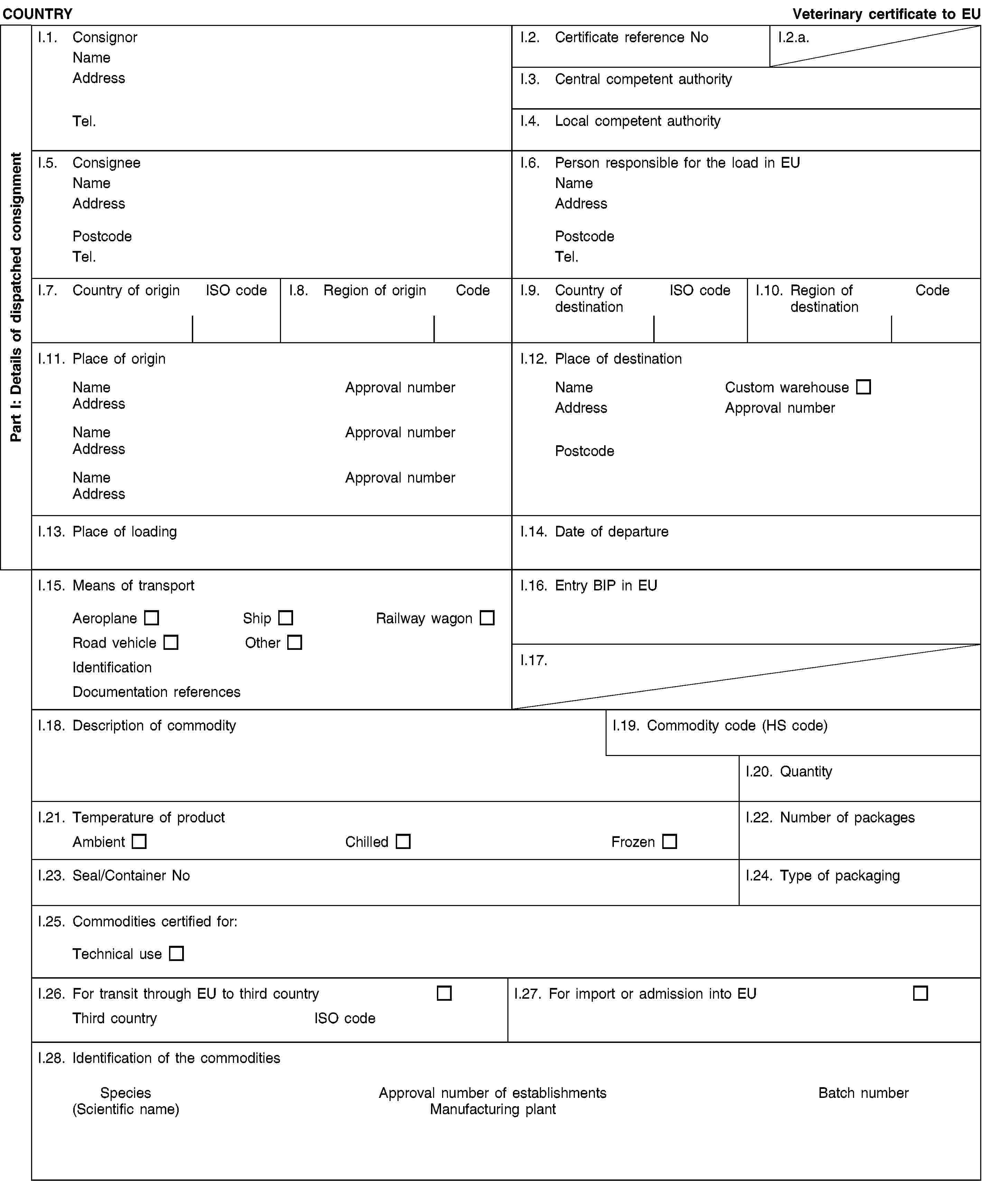
 and/or [- carcases and the following parts originating either from animals that have been slaughtered in a slaughterhouse and were considered fit for slaughter for human consumption following an ante-mortem inspection or bodies and the following parts of animals from game killed for human consumption in accordance with Union legislation:(i) carcases or bodies and parts of animals which are rejected as unfit for human consumption in accordance with Union legislation, but which did not show any signs of disease communicable to humans or animals;(ii) heads of poultry;(iii) hides and skins, including trimmings and splitting thereof, horns and feet, including the phalanges and the carpus and metacarpus bones, tarsus and metatarsus bones;(iv) pig bristles;(v) feathers;](2) and/or [- animal by-products from poultry and lagomorphs slaughtered on the farm as referred to in Article 1(3)(d) of Regulation (EC) No 853/2004, which did not show any signs of disease communicable to humans or animals;](2) and/or [- blood of animals which did not show any signs of disease communicable through blood to humans or animals, obtained from animals other than ruminants that have been slaughtered in a slaughterhouse after having been considered fit for slaughter for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- animal by-products arising from the production of products intended for human consumption, including degreased bone, greaves and centrifuge or separator sludge from milk processing;](2) and/or [- products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise;](2) and/or [- petfood and feedingstuffs of animal origin, or feedingstuffs containing animal by-products or derived products, which are no longer intended for feeding for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- blood, placenta, wool, feathers, hair, horns, hoof cuts and raw milk originating from live animals that did not show signs of any disease communicable through that product to humans or animals;](2) and/or [- aquatic animals, and parts of such animals, except sea mammals, which did not show any signs of diseases communicable to humans or animals;](2) and/or [- animal by-products from aquatic animals originating from plants or establishments manufacturing products for human consumption;](2) and/or [- the following material originating from animals which did not show any signs of disease communicable through that material to humans or animals:(i) shells from shellfish with soft tissue or flesh;(ii) the following originating from terrestrial animals:hatchery by-products,eggs,egg by-products, including egg shells;(iii) day-old chicks killed for commercial reasons;]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01001101.tif.jpg)
 and/or [- animals and parts thereof of the zoological orders of Rodentia and Lagomorpha, except Category 1 material as referred to in Article 8(a)(iii), (iv) and (v) of Regulation (EC) No 1069/2009 and Category 2 material as referred to in Article 9(a) to (g) of that Regulation;](2) and/or [- material from animals which have been treated with certain substances which are prohibited pursuant to Directive 96/22/EC, the import of the material being permitted in accordance with Article 35(a)(ii) of Regulation (EC) No 1069/2009;]II.3.(2) either [was subjected to a heat treatment of at least 90 °C throughout its substance;](2) or [was produced as regards ingredients of animal origin using exclusively products which had been:(a) in the case of animal by-products or derived products from meat or meat products subjected to a heat treatment of at least 90 °C throughout its substance;(b) in the case of milk and milk based products,(i) if they are from third countries or parts of third countries listed in column B of Annex I to Commission Regulation (EU) No 605/2010 (3) submitted to a pasteurisation treatment sufficient to produce a negative phosphatase test;(ii) with a pH reduced to less than 6 from third countries or parts of third countries listed in column C of Annex I to Commission Regulation (EU) No 605/2010, first submitted to a pasteurisation treatment sufficient to produce a negative phosphatase test;(iii) if they are from third countries or parts of third countries listed in column C of Annex I to Regulation (EU) No 605/2010, submitted to a sterilisation process or a double heat treatment where each treatment was sufficient to produce a negative phosphatase test on its own;(iv) if they are from third countries or parts of third countries listed in column C of Annex I to Regulation (EU) No 605/2010, where there has been an outbreak of foot-and-mouth disease in the last 12 months or where vaccination against foot-and-mouth disease has been carried out in the last 12 months, submitted toeithera sterilisation process whereby an Fc value equal or greater than 3 is achievedoran initial heat treatment with a heating effect at least equal to that achieved by a pasteurisation process of at least 72 °C for at least 15 seconds and sufficient to produce a negative reaction to a phosphatase test, followed byeithera second heat treatment with a heating effect at least equal to that achieved by the initial heat treatment, and which would be sufficient to produce a negative reaction to a phosphatase test, followed, in the case of dried milk, or dried milk-based products by a drying processoran acidification process such that the pH has been maintained at less than 6 for at least one hour;(c) in the case of gelatine, produced using a process that ensures that unprocessed Category 3 material is subjected to a treatment with acid or alkali, followed by one or more rinses with subsequent adjustment of the pH and subsequent, if necessary repeated, extraction by heat, followed by purification by means of filtration and sterilisation;(d) in the case of hydrolysed protein produced using a production process involving appropriate measures to minimise contamination of raw Category 3 material, and, in the case of hydrolysed protein entirely or partly derived from ruminant hides and skins produced in a processing plant dedicated only to hydrolysed protein production, using only material with a molecular weight below 10000 Dalton and a process involving the preparation of raw Category 3 material by brining, liming and intensive washing followed by:(i) exposure of the material to a pH of more than 11 for more than three hours at a temperature of more than 80 °C and subsequently by heat treatment at more than 140 °C for 30 minutes at more than 3,6 bar; or](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01001201.tif.jpg)
 or [was subject to a treatment such as drying or fermentation, which has been authorised by the competent authority;](2) or [in the case of aquatic and terrestrial invertebrates other than species pathogenic to humans or animals, be subject to a treatment which has been authorised by the competent authority and which ensures that the petfood poses no unacceptable risks to public and animal health;]II.4. was analysed by a random sampling of at least five samples from each processed batch taken during or after storage at the processing plant and complies with the following standards (5):Salmonella: absence in 25g: n = 5, c = 0, m = 0, M = 0,Enterobacteriaceae: n = 5, c = 2, m = 10, M = 300 in 1 gram;](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01001301.tif.jpg)
 or [the product does not contain and is not derived from bovine, ovine or caprine materials other than those derived from animals born, continuously reared and slaughtered in a country or region classified as posing a negligible BSE risk by a decision in accordance with Article 5(2) of Regulation (EC) No 999/2001.]II.8. in addition as regards TSE:(2) either [in case of animal by-products intended for feeding ruminants and containing milk or milk products of ovine or caprine origin, the ovine and caprine animals from which these products are derived have been kept continuously since birth or for the last three years on a holding where no official movement restriction is imposed due to a suspicion of TSE and which has satisfied the following requirements for the last three years:(i) it has been subject to regular official veterinary checks;(ii) no classical scrapie case, as defined in point 2(g) of Annex I to Regulation (EC) No 999/2001, has been diagnosed or, following the confirmation of a classical scrapie case:all animals in which classical scrapie was confirmed have been killed and destroyed, andall goats and sheep on the holding have been killed and destroyed, except for breeding rams of the ARR/ARR genotype and breeding ewes carrying at least one ARR allele and no VRQ allele;(iii) ovine and caprine animals, with the exception of sheep of the ARR/ARR prion genotype, are introduced into the holding only if they come from a holding which complies with the requirements set out in points (i) and (ii).](2) or [in case of animal by-products intended for feeding ruminants and containing milk or milk products of ovine or caprine origin, and destined to a Member State listed in the Annex to Commission Regulation (EC) No 546/2006 (7), the ovine and caprine animals from which these products are derived have been kept continuously since birth or for the last seven years on a holding where no official movement restriction is imposed due to a suspicion of TSE and which has satisfied the following requirements for the last seven years:(i) it has been subject to regular official veterinary checks;(ii) no classical scrapie case, as defined in point 2(g) of Annex I to Regulation (EC) No 999/2001, has been diagnosed or, following the confirmation of a classical scrapie case:all animals in which classical scrapie was confirmed have been killed and destroyed, andall goats and sheep on the holding have been killed and destroyed, except for breeding rams of the ARR/ARR genotype and breeding ewes carrying at least one ARR allele and no VRQ allele;(iii) ovine and caprine animals, with the exception of sheep of the ARR/ARR prion genotype, are introduced into the holding only if they come from a holding which complies with the requirements set out in points (i) and (ii).]NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01001401.tif.jpg)

(b) Chapter 3(D) is replaced by the following:
‘CHAPTER 3(D)
Health certificate
For raw petfood for direct sale or animal by-products to be fed to fur animals, intended for dispatch to or for transit through (2) the European Union

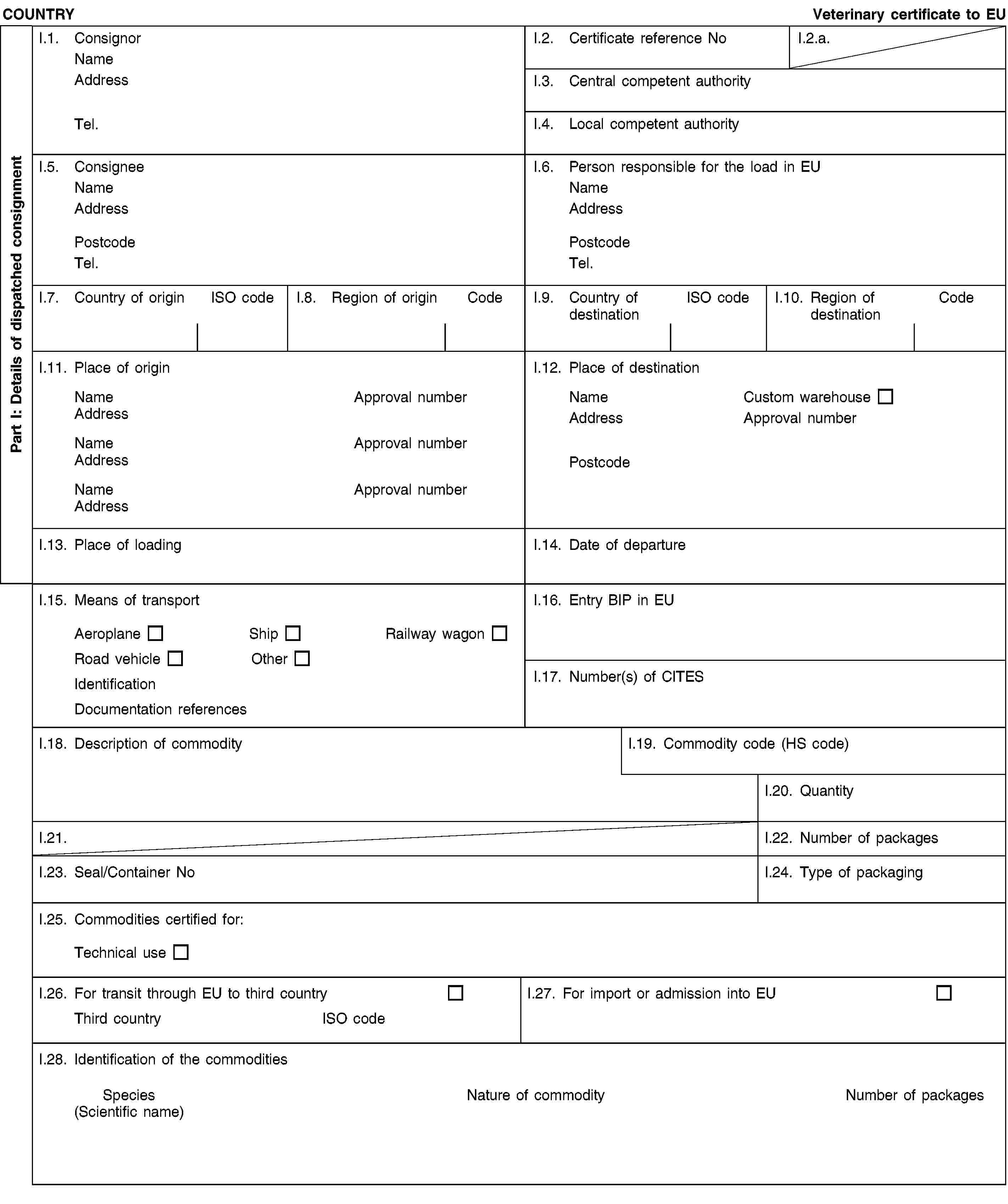
 and/or [- blood of animals which did not show any signs of disease communicable through blood to humans or animals, obtained from animals other than ruminants that have been slaughtered in a slaughterhouse after having been considered fit for slaughter for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- animal by-products arising from the production of products intended for human consumption, including degreased bone, greaves and centrifuge or separator sludge from milk processing;](2) and/or [- products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- petfood and feedingstuffs of animal origin, or feedingstuffs containing animal by-products or derived products, which are no longer intended for feeding for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- blood, placenta, wool, feathers, hair, horns, hoof cuts and raw milk originating from live animals that did not show signs of any disease communicable through that product to humans or animals;](2) and/or [- aquatic animals, and parts of such animals, except sea mammals, which did not show any signs of diseases communicable to humans or animals;]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01001701.tif.jpg)
 and/or [- the following material originating from animals which did not show any signs of disease communicable through that material to humans or animals:(i) shells from shellfish with soft tissue or flesh;(ii) the following originating from terrestrial animals:hatchery by-products,eggs,egg by-products, including egg shells;(iii) day-old chicks killed for commercial reasons;](2) and/or [- animal by-products from aquatic or terrestrial invertebrates other than species pathogenic to humans or animals;](2) and/or [- animals and parts thereof of the zoological orders of Rodentia and Lagomorpha, except Category 1 material as referred to in Article 8(a)(iii), (iv) and (v) of Regulation (EC) No 1069/2009 and Category 2 material as referred to in Article 9(a) to (g) of that Regulation;]II.4. have been obtained and prepared without contact with other material not complying with the conditions laid down in the Regulation (EC) No 1069/2009, and it has been handled so as to avoid contamination with pathogenic agents;II.5. have been packed in final packaging which bear labels indicating “RAW PET FOOD — NOT FOR HUMAN CONSUMPTION” or ”ANIMAL BY-PRODUCTS FOR FEED FOR FUR ANIMALS — NOT FOR HUMAN CONSUMPTION” and then in leak-proof and officially sealed boxes/containers or in new packaging preventing any leakage and officially sealed boxes/containers which bear labels indicating “RAW PET FOOD — NOT FOR HUMAN CONSUMPTION” or “ANIMAL BY-PRODUCTS FOR FEED FOR FUR ANIMALS — NOT FOR HUMAN CONSUMPTION”, and the name and the address of the establishment of destination;II.6. in the case of raw petfood:(a) have been prepared and stored in a plant approved and supervised by the competent authority in accordance with Article 24 of Regulation (EC) No 1069/2009 and(b) were examined by random sampling of at least five samples from each batch taken during storage (before dispatch) and complies with the following standards (8):Salmonella: absence in 25 g: n=5, c=0, m=0, M=0Enterobacteriaceae: n=5, c=2, m=10, M=5000 in 1 gram;II.7.(2) either [the product does not contain and is not derived from specified risk material as defined in Annex V to Regulation (EC) No 999/2001 of the European Parliament and of the Council (9) or mechanically separated meat obtained from bones of bovine, ovine or caprine animals; and the animals from which this product is derived have not been slaughtered after stunning by means of gas injected into the cranial cavity or killed by the same method or slaughtered by laceration of central nervous tissue by means of an elongated rod-shaped instrument introduced into the cranial cavity;](2) or [the product does not contain and is not derived from bovine, ovine or caprine materials other than those derived from animals born, continuously reared and slaughtered in a country or region classified as posing a negligible BSE risk by a decision in accordance with Article 5(2) of Regulation (EC) No 999/2001;]II.8. in addition as regards TSE:(2) either [in case of animal by-products intended for feeding ruminants and containing milk or milk products of ovine or caprine origin, the ovine and caprine animals from which these products are derived have been kept continuously since birth or for the last three years on a holding where no official movement restriction is imposed due to a suspicion of TSE and which has satisfied the following requirements for the last three years:(i) it has been subject to regular official veterinary checks;(ii) no classical scrapie case, as defined in point 2(g) of Annex I to Regulation (EC) No 999/2001, has been diagnosed or, following the confirmation of a classical scrapie case:all animals in which classical scrapie was confirmed have been killed and destroyed, andall goats and sheep on the holding have been killed and destroyed, except for breeding rams of the ARR/ARR genotype and breeding ewes carrying at least one ARR allele and no VRQ allele;(iii) ovine and caprine animals, with the exception of sheep of the ARR/ARR prion genotype, are introduced into the holding only if they come from a holding which complies with the requirements set out in points (i) and (ii).]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01001801.tif.jpg)
![COUNTRYRaw petfood for direct sale or animal by-products to be fed to fur animalsII. Health informationII.a. Certificate reference NoII.b.(2) or [in case of animal by-products intended for feeding ruminants and containing milk or milk products of ovine or caprine origin, and destined to a Member State listed in the Annex to Commission Regulation (EC) No 546/2006 (10), the ovine and caprine animals from which these products are derived have been kept continuously since birth or for the last seven years on a holding where no official movement restriction is imposed due to a suspicion of TSE and which has satisfied the following requirements for the last seven years:(i) it has been subject to regular official veterinary checks;(ii) no classical scrapie case, as defined in point 2(g) of Annex I to Regulation (EC) No 999/2001, has been diagnosed or, following the confirmation of a classical scrapie case:all animals in which classical scrapie was confirmed have been killed and destroyed, andall goats and sheep on the holding have been killed and destroyed, except for breeding rams of the ARR/ARR genotype and breeding ewes carrying at least one ARR allele and no VRQ allele;(iii) ovine and caprine animals, with the exception of sheep of the ARR/ARR prion genotype, are introduced into the holding only if they come from a holding which complies with the requirements set out in points (i) and (ii).]NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the BIP of entry into the EU.Box I.19: use the appropriate Harmonized System (HS) code under the following heading: 05.11.Box reference I.23: for bulk containers, the container number and the seal number (if applicable) should be given.Box reference I.25: technical use: any use other than for animal consumption.Box reference I.26 and I.27: fill in according to whether it is a transit or an import certificate.Box reference I.28:Nature of commodity: select raw petfood or animal by-product.In case of raw material for manufacture of raw pet food indicate scientific name of the species.In case of raw material for manufacture of feed for fur animals select from the following: Aves, Ruminantia, Mammalia - Ruminantia, Pesca, Mollusca, Crustacea, Invertebrata.Part II:(1a) OJ L 300, 14.11.2009, p. 1.(1b) OJ L 54, 26.2.2011, p. 1(2) Delete as appropriate.(3) OJ L 73, 20.3.2010, p. 1.(4) OJ L 226, 23.8.2008, p. 1.(5) OJ L 39, 10.2.2009, p. 12.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01001901.tif.jpg)

(c) Chapter 4(A) is replaced by the following:
‘CHAPTER 4(A)
Health certificate
For the import of blood and blood products from equidae to be used outside the feed chain, for dispatch to or for transit through (2) the European Union
 or [all the animals of species susceptible to the disease located on the holding have been slaughtered and the premises were disinfected, in which case the period of prohibition must be 30 days, beginning on the date on which the animals were slaughtered and the premises disinfected, except in the case of anthrax, where the period of prohibition shall be 15 days;]II.6. blood products come from an establishment or plant approved or registered by the competent authority of the third country meeting the specific conditions set out in Article 23 or 24 of Regulation (EC) No 1069/2009;II.7. blood products have been produced from blood which fulfils the conditions referred in II.4 and II.5 and(2) either [has been collected from equidae which have been kept for a period of at least three months, or since birth if less than three months old, prior to the date of collection on holdings under veterinary supervision in the country of collection which during that period and the period of blood collection has been free of:(a) African horse sickness for two years;](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01002201.tif.jpg)
 or [for a period of six months where the animals have passed the post-mortem inspection for glanders in the slaughterhouse referred to in II.4, including a careful examination of mucous membranes from the trachea, larynx, nasal cavities and sinuses and their ramifications, after splitting the head in the median plane and excising the nasal septum;](d) in the case of blood products other than serum and plasma, vesicular stomatitis for six months;]](2) or [has been subjected to at least one of the following treatments, followed by an effectiveness check, for the inactivation of possible causative pathogens for African horse sickness, equine encephalomyelitis of all types including Venezuelan equine encephalomyelitis, equine infectious anaemia, vesicular stomatitis and glanders (Burkholderia mallei):(2) either [heat treatment at a temperature of 65°C for at least three hours;](2) and/or [irradiation at 25 kGy by gamma rays;](2) and/or [change in pH to pH 5 for two hours;](2) and/or [heat treatment of at least 80°C throughout their substance;]]II.8. all precautions have been taken to avoid contamination of the blood and blood products with pathogenic agents during production, handling and packaging;II.9. blood and blood products were packed in sealed impermeable containers clearly labelled “NOT FOR HUMAN OR ANIMAL CONSUMPTION” and bearing :(a) in the case of blood, the approval number of the establishment of collection;(b) in the case of blood products, the approval number of the establishment of production;II.10. the products were stored in enclosed storage.NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.11 and I.12: Approval number: the registration number of the establishment or plant, which has been issued by the competent authority.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the BIP of entry into the EU.Box I.19: use the appropriate Harmonized System (HS) code under the following heading: 30.02.Box reference I.23: for bulk containers, the container number and the seal number (if applicable) must be included.Box reference I.25: technical use: any use other than for animal consumption.Box reference I.26 and I.27: fill in according to whether it is a transit or an import certificate.Box reference I.28:(a) Manufacturing plant:(i) in the case of blood, provide the approval number of the registered establishment of collection;(ii) in the case of blood products, provide the approval number of the establishment of production;(b) Species: select amongst the following: Equus cabalus, Equus asinus, Equus cabalus*asinus.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01002301.tif.jpg)
(d) Chapter 4(C) is replaced by the following:
‘CHAPTER 4(C)
Health certificate
For untreated blood products, excluding of equidae, for the manufacture of derived products for purposes outside the feed chain for farmed animals, intended for dispatch to or for transit through (2) the European Union
 and/or [- blood of slaughtered animals, which is rejected as unfit for human consumption in accordance with Union legislation, but which did not show any signs of diseases communicable to humans or animals, derived from carcases that have been slaughtered in a slaughterhouse and were considered fit for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- blood of slaughtered animals, which did not show any signs of diseases communicable to humans or animals, obtained from animals that have been slaughtered in a slaughterhouse after having been considered fit for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- blood and blood products derived from the production of products intended for human consumption;](2) and/or [- blood and blood products originating from live animals that did not show signs of any disease communicable through that product to humans or animals;](2) and/or [- animal by-products derived from animals which have been submitted to illegal treatment as defined in Article 1(2)(d) of Directive 96/22/EC or Article 2(b) of Directive 96/23/EC;](2) and/or [- animal by-products containing residues of other substances and environmental contaminants listed in Group B(3) of Annex I to Directive 96/23/EC, if such residues exceed the permitted level laid down in Union legislation or, in the absence thereof, in national legislation;]II.4. the blood from which such products are manufactured has been collected in slaughterhouses approved in accordance with Union legislation, in slaughterhouses approved and supervised by the competent authority of the country of collection or from live animals in facilities approved and supervised by the competent authority of the country of collection.(2) [II.5. in the case of blood products derived from animals belonging to the taxa Artiodactyla, Perissodactyla and Proboscidea, including their crossbreds, the products come:II.5.1. from a country where no case of rinderpest, peste des petits ruminants and Rift Valley fever has been recorded for 12 months and in which vaccination has not been carried out against those diseases for at least 12 months;(2) [II.5.2. either [from the third countries, territories or parts thereof … (ISO code in case of country or codes for territories or parts thereof) (3) where no case of foot-and-mouth disease has been recorded for 12 months and in which vaccination has not been carried out against this disease for at least 12 months;]or [from the countries, territories or parts thereof … (ISO code in case of country or codes for territories or parts thereof) (3) where no case of foot-and-mouth disease has been recorded for 12 months and in which vaccination programmes against foot-and-mouth disease are being officially carried out and controlled in domestic ruminant animals for at least 12 months (4);]](2) [II.5.3. In addition, in case of animals other than Suidae and Tayassuidae:(2) either [in the country or region of origin no case of vesicular stomatitis and bluetongue (2) (including the presence of seropositive animals) has been recorded for 12 months and in which vaccination has not been carried out against those diseases for at least 12 months;](2) or [in the country or region of origin vesicular stomatitis and bluetongue (2) seropositive animals are present (4);]](2) [II.5.4. In addition, in case of Suidae and Tayassuidae:II.5.4.1. in the country or region of origin no case of swine vesicular disease, classical swine fever and African swine fever has been recorded for at least 12 months and vaccination has not been carried out against those diseases for at least 12 months in the susceptible species and](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01002601.tif.jpg)
 [II.5.4.2. or [in the country or region of origin vesicular stomatitis seropositive animals are present (4);](2) [II.6. in the case of blood products derived from poultry or other avian species the animals and the products come from the territory of the country or region with code … (5)which has been free from Newcastle disease and highly pathogenic avian influenza as defined in the Terrestrial Animal Health Code of the OIE,which for at least 12 months has not carried out vaccination against avian influenza,where the animals from which the products derive have not been vaccinated against Newcastle disease with vaccines prepared from a Newcastle disease master strain showing a higher pathogenicity than lentogenic virus strains;]II.7. the products were:(2) either [packed in new or sterilised bags or bottles,](2) or [transported in bulk in containers or other means of transport that were thoroughly cleaned and disinfected with a disinfectant approved by the competent authority before use,]the outer packaging or containers bear labels indicating “NOT FOR HUMAN OR ANIMAL CONSUMPTION”;II.8. the products were stored in enclosed storage;II.9. all precautions were taken to avoid contamination of the products with pathogenic agents during transport;II.10.(2) either [the product does not contain and is not derived from specified risk material as defined in Annex V to Regulation (EC) No 999/2001 of the European Parliament and of the Council (6) or mechanically separated meat obtained from bones of bovine, ovine or caprine animals; and the animals from which the product is derived have not been slaughtered after stunning by means of gas injected into the cranial cavity or killed by the same method or slaughtered by laceration of central nervous tissue by means of an elongated rod-shaped instrument introduced into the cranial cavity.](2) or [the product does not contain and is not derived from bovine, ovine or caprine materials other than those derived from animals born, continuously reared and slaughtered in a country or region classified as posing a negligible BSE risk by a decision in accordance with Article 5(2) of Regulation (EC) No 999/2001.]NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.11 and I.12: Approval number: the registration number of the establishment or plant, which has been issued by the competent authority.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the border inspection post of entry into the EU.Box I.19: use the appropriate Harmonized System (HS) code under the following headings: 30.02 or 35.02.Box reference I.23: for bulk containers, the container number and the seal number (if applicable) should be included.Box reference I.25: technical use: any use other than for animal consumption.Box reference I.26 and I.27: fill in according to whether it is a transit or an import certificate.Box reference I.28 Species: select from the following: Aves, Bovidae, Suidae, Otra Mammalia, Pesca, Reptilia.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01002701.tif.jpg)

(e) Chapter 4(D) is replaced by the following:
‘CHAPTER 4(D)
Health certificate
For treated blood products, excluding of equidae, for the manufacture of derived products for purposes outside the feed chain for farmed animals, intended for dispatch to or for transit through (2) the European Union
 and/or [- blood of slaughtered animals, which is rejected as unfit for human consumption in accordance with Union legislation, but which did not show any signs of diseases communicable to humans or animals, derived from carcases that have been slaughtered in a slaughterhouse and were considered fit for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- blood of slaughtered animals, which did not show any signs of diseases communicable to humans or animals, obtained from animals that have been slaughtered in a slaughterhouse after having been considered fit for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- blood and blood products originating from live animals that did not show clinical signs of any disease communicable through these products to humans or animals;](2) and/or [- animal by-products which have been derived from animals which have been submitted to illegal treatment as defined in Article 1(2)(d) of Directive 96/22/EC or Article 2(b) of Directive 96/23/EC;](2) and/or [- animal by-products containing residues of other substances and environmental contaminants listed in Group B(3) of Annex I to Directive 96/23/EC, if such residues exceed the permitted levels laid down by Union legislation or, in the absence thereof, in national legislation;]II.4. the blood from which such products are manufactured has been collected in slaughterhouses approved in accordance with Union legislation, in slaughterhouses approved and supervised by the competent authority of the country of collection or from live animals in facilities approved and supervised by the competent authority of the country of collection.(2) [II.5. In case of blood products derived from Artiodactyla, Perissodactyla and Proboscidea including their crossbreeds, other than Suidae and Tayassuidae, the products have undergone one of the following treatments, guaranteeing the absence of pathogens of foot-and-mouth disease, vesicular stomatitis, rinderpest, peste des petits ruminants, Rift Valley fever and bluetongue:(2) either [heat treatment at a temperature of 65 °C for at least three hours, followed by an effectiveness check;](2) and/or [irradiation at 25 kGy by gamma rays, followed by an effectiveness check;](2) and/or [change in pH to pH 5 for two hours, followed by an effectiveness check;](2) and/or [heat treatment of at least 80 °C throughout their substance, followed by an effectiveness check.]](2) [II.6. In the case of blood products derived from Suidae, Tayassuidae, poultry and other avian species, the products have undergone one of the following treatments guaranteeing the absence of pathogens of the following diseases: foot-and-mouth disease, vesicular stomatitis, swine vesicular disease, classical swine fever, African swine fever, Newcastle disease and highly pathogenic avian influenza, as appropriate to the species:(2) either [heat treatment at a temperature of 65 °C for at least three hours, followed by an effectiveness check;](2) and/or [irradiation at 25 kGy by gamma rays, followed by an effectiveness check;](2) and/or [heat treatment of at least 80 °C for Suidae/Tayassuidae (2) and at least 70 °C for poultry and other avian species (2) throughout their substance, followed by an effectiveness check]].](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01003001.tif.jpg)
![COUNTRYTreated blood products, excluding of equidae, for the manufacture of derived products for purposes outside the feed chain for farmed animalsII. Health informationII.a. Certificate reference NoII.b.(2) [II.7 In the case of blood products derived from species other than listed in points II.5 or II.6 the products have undergone of the following treatment (please specify):…]II.8. The products were:(2) either [packed in new or sterilised bags or bottles;](2) or [transported in bulk in containers or other means of transport that were thoroughly cleaned and disinfected with a disinfectant approved by the competent authority before use;] andthe outer packaging or containers bear labels indicating “NOT FOR HUMAN OR ANIMAL CONSUMPTION”;II.9. the products were stored in enclosed storage;II.10. all precautions were taken to avoid contamination of the products with pathogenic agents after treatment;II.11.(2) either [the product does not contain and is not derived from specified risk material as defined in Annex V to Regulation (EC) No 999/2001 of the European Parliament and of the Council (3) or mechanically separated meat obtained from bones of bovine, ovine or caprine animals; and the animals from which the product is derived have not been slaughtered after stunning by means of gas injected into the cranial cavity or killed by the same method or slaughtered by laceration of central nervous tissue by means of an elongated rod-shaped instrument introduced into the cranial cavity.](2) or [the product does not contain and is not derived from bovine, ovine or caprine materials other than those derived from animals born, continuously reared and slaughtered in a country or region classified as posing a negligible BSE risk by a decision in accordance with Article 5(2) of Regulation (EC) No 999/2001.]NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.11 and I.12: Approval number: the registration number of the establishment or plant, which has been issued by the competent authority.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the BIP of entry into the EU.Box I.19: use the appropriate Harmonized System (HS) code under the following headings: 05.11, 30.02 or 35.02.Box reference I.23: for bulk containers, the container number and the seal number (if applicable) should be included.Box reference I.25: technical use: any use other than for animal consumption.Box reference I.26 and I.27: fill in according to whether it is a transit or an import certificate.Box reference I.28 in case of Species: select from the following: Aves, Bovidae, Suidae, Otra Mammalia, Pesca, Reptilia.Part II:(1a) OJ L 300, 14.11.2009, p. 1.(1b) OJ L 54, 26.2.2011, p. 1.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01003101.tif.jpg)

(f) Chapter 6(A) is replaced by the following:
‘CHAPTER 6(A)
Health certificate
For treated game trophies and other preparations of birds and ungulates, consisting only of bones, horns, hooves, claws, antlers, teeth, hides or skins, for dispatch to or for transit through (2) the European Union
 and/or [have been dry-salted or wet-salted for a minimum of 14 days before dispatch;](2) and/or [were dry-salted or wet-salted on … (date) and, according to the declaration of the transporter, will be transported by ship and the duration of the transport will be such that they will have undergone a minimum of 14 days salting before they reach the EU border inspection post;]](2) and/or [II.2.2 in the case of game trophies or other preparations consisting only of bone, horns, hooves, claws, antlers or teeth:(a) have been immersed in boiling water for an appropriate time so as to ensure that any matter other than bone, horns, hooves, claws, antlers or teeth is removed, and(b) have been disinfected with a product authorised by the competent authority, in particular with hydrogen peroxide where parts consisting of bone are concerned.]NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.11 and I.12: Approval number: the registration number of the establishment or plant, which has been issued by the competent authority.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the BIP of entry into the EU.Box I.19: use the appropriate Harmonized System (HS) code under the following headings: 05.05, 05.06, 05.07 or 97.05.Box reference I.23: for bulk containers, the container number and the seal number (if applicable) should be included.Box reference I.25: technical use: any use other than for animal consumption.Box reference I.26 and I.27: fill in according to whether it is a transit or an import certificate.Box reference I.28:(a) for nature of commodity, select one or more of the following: [bones], [horns], [hooves], [claws], [antlers], [teeth], [hides] and/or [skins];(b) in case of Species: select from the following: Aves, Equidae, Tapiridae, Rhinoceritidae, Antilocaparidae, Bovidae, Camelidae, Cervidae, Giraffidae, Hippopotamindae, Moschidae Suidae, Tayassuidae, Tragulidae and Elephantidae.Part II:(1a) OJ L 300, 14.11.2009, p. 1.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01003401.tif.jpg)

(g) Chapter 8 is replaced by the following:
‘CHAPTER 8
Health certificate
For animal by-products to be used for purposes outside the feed chain or for trade samples (2), intended for dispatch to or for transit through (2) the European Union

 and/or [(b) obtained in the exporting country, territory or part thereof: … (3) from animalseither(i) That have remained in this territory or in a region eligible to export fresh meat of the species to the EU since birth or for at least the last three months before slaughter; and/or(ii) Killed in the wild in this territory (4);](2) and/or [(c) are derived from eggs, milk, rodents, lagomorphs, or aquatic animals or terrestrial or aquatic invertebrates;]II.2.2. (2) in the case of materials other than derived from eggs, milk, rodents, lagomorphs, or aquatic animals or terrestrial or aquatic invertebrates, have been obtained from animals:(2) either [(a) coming from holdings:(i) where, for the following diseases for which the animals are susceptible, there has been neither case/outbreak of rinderpest, swine vesicular disease, Newcastle disease or highly pathogenic avian influenza during the prior 30 days, nor of classical or African swine fever during the prior 40 days; nor in the holdings situated in their vicinity within 10 km, during the prior 30 days; and(ii) where there has been neither case/outbreak of foot-and-mouth disease during the prior 60 days, nor in the holdings situated in their vicinity within 25 km, during the prior 30 days; and(b) which:(i) were not killed to eradicate any epizootic disease;(ii) have remained in their holdings of origin for at least 40 days before departure and which have been transported directly to the slaughterhouse without contact with other animals which did not comply with the same health conditions;(iii) at the slaughterhouse, have passed the ante-mortem health inspection during the 24 hours before the slaughter and have shown no evidence of the diseases referred to above for which the animals are susceptible; and(iv) have been treated in the slaughterhouse before and at the time of slaughter or killing in accordance with the relevant provisions of Council Directive 93/119/EC) (5) on the protection of animals at the time of slaughter or killing;](2) or [(a) captured and killed in the wild in an area:(i) in which within 25 km there has been no case/outbreak of any of the following diseases for which the animals are susceptible: foot-and-mouth disease, rinderpest, Newcastle disease or highly pathogenic avian influenza during the prior 30 days nor of classical or African swine fever during the prior 40 days; and(ii) that is situated at a distance that exceeds 20 km from the borders separating another territory of a country or part thereof, which is not authorised at these dates for exporting this material to the European Union; and(b) which after killing were transported within 12 hours for chilling either to a collection centre and immediately afterwards to a game establishment, or directly to a game establishment;]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01003701.tif.jpg)
 and/or [- carcases and the following parts originating either from animals that have been slaughtered in a slaughterhouse and were considered fit for slaughter for human consumption following an ante-mortem inspection or bodies and the following parts of animals from game killed for human consumption in accordance with Union legislation:(i) carcases or bodies and parts of animals which are rejected as unfit for human consumption in accordance with Union legislation, but which did not show any signs of disease communicable to humans or animals;(ii) heads of poultry;(iii) hides and skins, including trimmings and splitting thereof, horns and feet, including the phalanges and the carpus and metacarpus bones, tarsus and metatarsus bones;(iv) pig bristles;(v) feathers;](2) and/or [- animal by-products from poultry and lagomorphs slaughtered on the farm as referred to in Article 1(3)(d) of Regulation (EC) No 853/2004, which did not show any signs of disease communicable to humans or animals;](2) and/or [- blood of animals which did not show any signs of disease communicable through blood to humans or animals, obtained from animals other than ruminants that have been slaughtered in a slaughterhouse after having been considered fit for slaughter for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- animal by-products arising from the production of products intended for human consumption, including degreased bone, greaves and centrifuge or separator sludge from milk processing;](2) and/or [- products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- petfood and feedingstuffs of animal origin, or feedingstuffs containing animal by-products or derived products, which are no longer intended for feeding for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- blood, placenta, wool, feathers, hair, horns, hoof cuts and raw milk originating from live animals that did not show signs of any disease communicable through that product to humans or animals;](2) and/or [- aquatic animals, and parts of such animals, except sea mammals, which did not show any signs of diseases communicable to humans or animals;](2) and/or [- animal by-products from aquatic animals originating from establishments or plants manufacturing products for human consumption;]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01003801.tif.jpg)
 and/or [- animal by-products from aquatic or terrestrial invertebrates, other than species pathogenic to humans or animals;](2) and/or [- animals and parts thereof of the zoological orders of Rodentia and Lagomorpha, except Category 1 material as referred to in Article 8(a)(iii), (iv) and (v) of Regulation (EC) No 1069/2009 and Category 2 material as referred to in Article 9(a) to (g) of that Regulation;](2) and/or [- fur originating from dead animals that did not show clinical signs of any disease communicable through that product to humans or animals;]II.2.7. have been deep-frozen at the plant of origin or have been preserved in accordance with EU legislation in such a way that they will not spoil between dispatch and delivery to the plant of destination.(2) (6) [II.2.8. Specific requirements(2) (7) II.2.8.1. The by-products in this consignment come from animals that have been obtained in the territory mentioned under (II.2.1), where vaccination programmes against foot-and-mouth disease are being regularly carried out and officially controlled in domestic bovine animals.(2) (8) II.2.8.2. The by-products in this consignment consist of animal by-products derived from offal or deboned meat.]II.2.9.(2) either [the product does not contain and is not derived from specified risk material as defined in Annex V to Regulation (EC) No 999/2001 of the European Parliament and of the Council (9) or mechanically separated meat obtained from bones of bovine, ovine or caprine animals; and the animals from which this product is derived have not been slaughtered after stunning by means of gas injected into the cranial cavity or killed by the same method or slaughtered by laceration of central nervous tissue by means of an elongated rod-shaped instrument introduced into the cranial cavity;](2) or [the product does not contain and is not derived from bovine, ovine or caprine materials other than those derived from animals born, continuously reared and slaughtered in a country or region classified as posing a negligible BSE risk by a decision in accordance with Article 5(2) of Regulation (EC) No 999/2001;]II.2.10. in addition as regards TSE:(2) either [in case of animal by-products intended for feeding ruminants and containing milk or milk products of ovine or caprine origin, the ovine and caprine animals from which these products are derived have been kept continuously since birth or for the last three years on a holding where no official movement restriction is imposed due to a suspicion of TSE and which has satisfied the following requirements for the last three years:(i) it has been subject to regular official veterinary checks;(ii) no classical scrapie case, as defined in point 2(g) of Annex I to Regulation (EC) No 999/2001, has been diagnosed or, following the confirmation of a classical scrapie case:all animals in which classical scrapie was confirmed have been killed and destroyed, andall goats and sheep on the holding have been killed and destroyed, except for breeding rams of the ARR/ARR genotype and breeding ewes carrying at least one ARR allele and no VRQ allele;(iii) ovine and caprine animals, with the exception of sheep of the ARR/ARR prion genotype, are introduced into the holding only if they come from a holding which complies with the requirements set out in points (i) and (ii).]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01003901.tif.jpg)
![COUNTRYAnimal by-products to be used for purposes outside the feed chain or for trade samples (2)II. Health informationII.a. Certificate reference NoII.b.(2) or [in case of animal by-products intended for feeding ruminants and containing milk or milk products of ovine or caprine origin, and destined to a Member State listed in the Annex to Commission Regulation (EC) No 546/2006 (10), the ovine and caprine animals from which these products are derived have been kept continuously since birth or for the last seven years on a holding where no official movement restriction is imposed due to a suspicion of TSE and which has satisfied the following requirements for the last seven years:(i) it has been subject to regular official veterinary checks;(ii) no classical scrapie case, as defined in point 2(g) of Annex I to Regulation (EC) No 999/2001, has been diagnosed or, following the confirmation of a classical scrapie case:all animals in which classical scrapie was confirmed have been killed and destroyed, andall goats and sheep on the holding have been killed and destroyed, except for breeding rams of the ARR/ARR genotype and breeding ewes carrying at least one ARR allele and no VRQ allele;(iii) ovine and caprine animals, with the exception of sheep of the ARR/ARR prion genotype, are introduced into the holding only if they come from a holding which complies with the requirements set out in points (i) and (ii).]NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.11: In case of consignments for the particular technological studies or analyses: indicate name and address of establishment only.Box reference I.11 and I.12: Approval number: the registration number of the establishment or plant, which has been issued by the competent authority.Box reference I.12: Place of destination: this box is to be filled in:products for the manufacture of derived products for uses outside the feed chain: only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.products for the particular technological studies or analyses: the EU plant indicated in authorisation of competent authority when appropriate.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the BIP of entry into the EU.Box reference I.19: use the appropriate Harmonized System (HS) code under the following headings: 05.11.91; 05.11.99 or 30.01.Box reference I.23: for bulk containers, the container number and the seal number (if applicable) should be included.Box reference I.25: technical use: any use other than for animal consumption.Box reference I.25: for the purposes of the certificate, “technical use” includes use as a trade sample.Box reference I.26 and I.27: except for trade samples, which are not sent in transit, fill in according to whether it is a transit or an import certificate.Box reference I.28:products for the manufacture of derived products for uses outside the feed chain: Manufacturing plant: provide the veterinary control number of the approved establishment.products for the particular technological studies or analyses: the EU plant indicated in authorisation of competent authority when appropriate.Species: select from the following: Aves, Ruminantia, Mammalia - Ruminantia, Pesca, Mollusca, Crustacea, Invertebrata.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01004001.tif.jpg)

(h) Chapter 10(B) is replaced by the following:
‘CHAPTER 10(B)
Health certificate
For rendered fats not intended for human consumption to be used for certain purposes outside the feed chain, intended for dispatch to or for transit through (2) the European Union
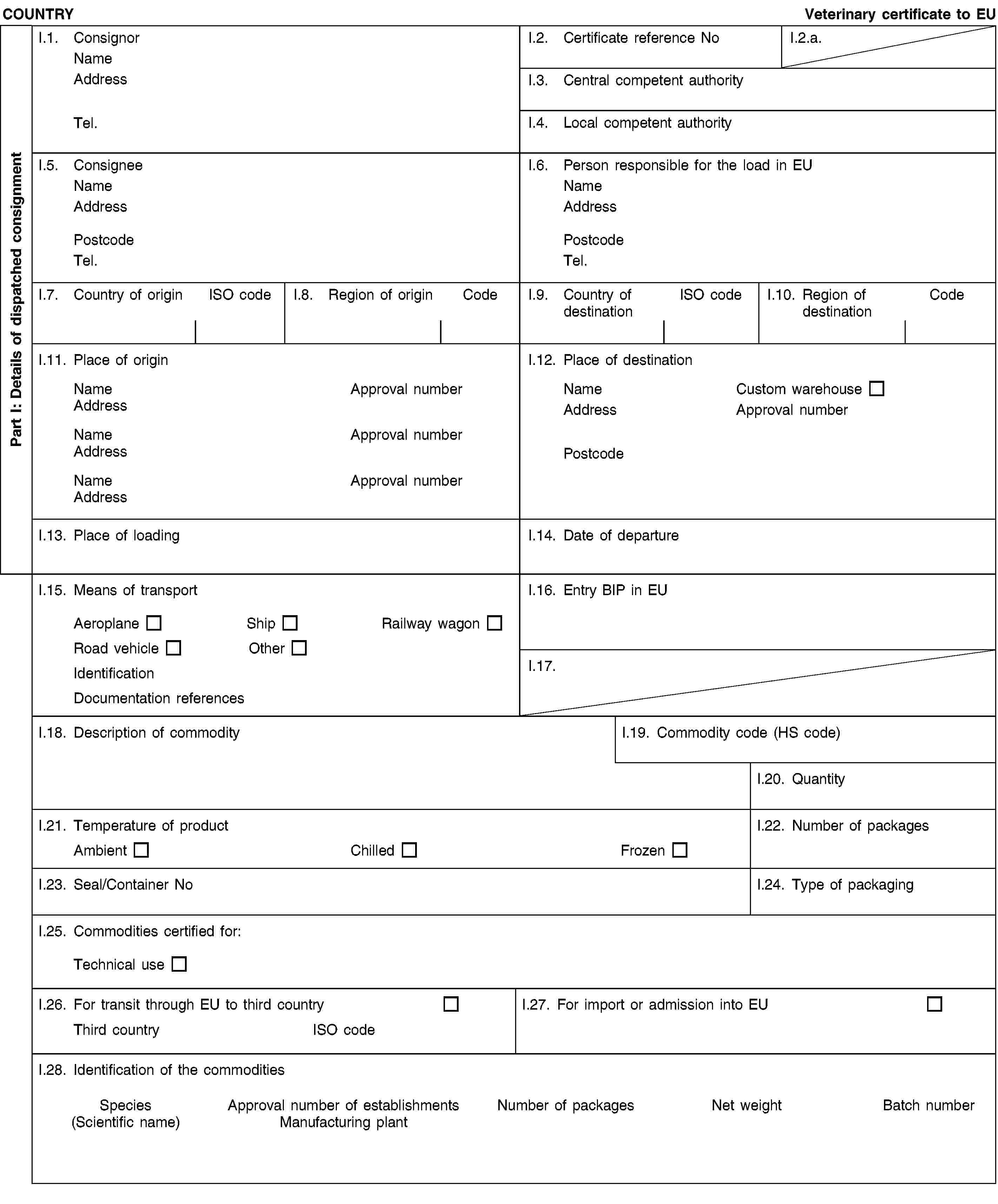
 and/or [- products of animal origin which have been declared unfit for human consumption due to the presence of foreign bodies in those products;](2) and/or [- animals and parts of animals, other than those referred to in Articles 8 and 10 of Regulation (EC) No 1069/2009, that died other than being slaughtered or killed for human consumption, including animals killed for disease control purposes;](2) and/or [- carcases and parts of animals slaughtered or, in the case of game, bodies or parts of animals killed, and which are fit for human consumption in accordance with Union legislation, but are not intended for human consumption for commercial reasons;](2) and/or [- carcases and the following parts originating either from animals that have been slaughtered in a slaughterhouse and were considered fit for slaughter for human consumption following an ante-mortem inspection or bodies and the following parts of animals from game killed for human consumption in accordance with Union legislation:(i) carcases or bodies and parts of animals which are rejected as unfit for human consumption in accordance with Union legislation, but which did not show any signs of disease communicable to humans or animals;(ii) heads of poultry;(iii) hides and skins, including trimmings and splitting thereof, horns and feet, including the phalanges and the carpus and metacarpus bones, tarsus and metatarsus bones;(iv) pig bristles;(v) feathers;](2) and/or [- blood of animals which did not show any signs of disease communicable through blood to humans or animals obtained from animals other than ruminants that have been slaughtered in a slaughterhouse after having been considered fit for slaughter for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- animal by-products arising from the production of products intended for human consumption, including degreased bone, greaves and centrifuge or separator sludge from milk processing;](2) and/or [- products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- petfood and feeding stuffs of animal origin, or feeding stuffs containing animal by-products or derived products, which are no longer intended for feeding for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- blood, placenta, wool, feathers, hair, horns, hoof cuts and raw milk originating from live animals that did not show signs of any disease communicable through that product to humans or animals;](2) and/or [- aquatic animals, and parts of such animals, except sea mammals, which did not show any signs of diseases communicable to humans or animals;](2) and/or [- animal by-products from aquatic animals originating from plants or establishments manufacturing products for human consumption;]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01004301.tif.jpg)
 and/or [- aquatic and terrestrial invertebrates other than species pathogenic to humans or animals;](2) and/or [- animals and parts thereof of the zoological orders of Rodentia and Lagomorpha, except Category 1 material as referred to in Article 8(a)(iii), (iv) and (v) of Regulation (EC) No 1069/2009and Category 2 material as referred to in Article 9(a) to (g) of that Regulation;](2) and/or [- hides and skins, hooves, feathers, wool, horns, hair and fur originating from dead animals that did not show any signs of disease communicable through that product to humans or animals;](2) and/or [- adipose tissue from animals which did not show any signs of disease communicable through that material to humans or animals, which were slaughtered in a slaughterhouse and which were considered fit for slaughter for human consumption following an ante-mortem inspection in accordance with Union legislation;]II.2.4. in the case of materials destined for purposes other than the production of organic fertilisers or soil improvers, cosmetics, pharmaceutical or medical devices or renewable fuels referred to in point J of Section 2 of Chapter IV of Annex IV to Regulation (EU) No 142/2011:(2) either [- specified risk material as defined in Article 3(1)(g) of Regulation (EC) No 999/2001 of the European Parliament and of the Council (3);](2) and/or [- entire bodies or parts of dead animals containing specified risk material as defined in Article 3(1)(g) of Regulation (EC) No 999/2001 at the time of disposal;](2) and/or [- animal by-products which have been derived from animals which have been submitted to illegal treatment as defined in Article 1(2)(d) of Directive 96/22/EC or Article 2(b) of Directive 96/23/EC;](2) and/or [- animal by-products containing residues of other substances and environmental contaminants listed in Group B(3) of Annex I to Directive 96/23/EC, if such residues exceed the permitted levels laid down by Union legislation or, in the absence thereof, by legislation of the Member State of importation;]II.3. the rendered fats:(a) have been subjected to processing in accordance with method … as laid down in Chapter III of Annex IV to Regulation (EU) No 142/2011, in order to kill pathogenic agents,(b) have been marked before shipment to the European Union with glyceroltriheptanoate (GTH), so that a homogenous minimum concentration of at least 250 mg GTH per kilogram fat is achieved,(c) in the case of rendered fats of ruminant origin, insoluble impurities in excess of 0.15% in weight have been removed,(d) have been transported under conditions which prevent their contamination, and(e) bear labels on the packaging or container indicating “NOT FOR HUMAN OR ANIMAL CONSUMPTION”;](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01004401.tif.jpg)
 or [the product does not contain and is not derived from bovine, ovine or caprine materials other than those derived from animals born, continuously reared and slaughtered in a country or region classified as posing a negligible BSE risk by a decision in accordance with Article 5(2) of Regulation (EC) No 999/2001.]NotesPart I:Box reference I.6: Person responsible for the consignment in EU: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.11 and I.12: Approval number: the registration number of the establishment or plant, which has been issued by the competent authority.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for a transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the BIP of entry into the EU.Box I.19: use the appropriate Harmonized System (HS) code under the following headings: 15.01, 15.02; 15.03; 15.04; 15.05; 15.06; 15.16; 15.17 or 15.18.Box reference I.23: for bulk containers, the container number and the seal number (if applicable) should be included.Box reference I.25: technical use: any use other than for animal consumption.Box reference I.26 and I.27: fill in according to whether it is a transit or an import certificate.Box reference I.28:Species: select from the following: Ruminantia, OtherManufacturing plant: provide the registration number of the treatment/processing establishment.Part II:(1a) OJ L 300, 14.11.2009, p. 1.(1b) OJ L 54, 26.2.2011, p. 1.(2) Delete as appropriate.(3) OJ L 147, 31.5.2001, p. 1.The signature and the stamp must be in a different colour to that of the printing.Note for the person responsible for the consignment in EU: this certificate is only for veterinary purposes and has to accompany the consignment until it reaches the border inspection post.Official veterinarian/Official inspectorName (in capital letters):Qualification and title:Date:Signature:Stamp:](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.CL2013R0294EN0000020.0001.tif.jpg)
(i) Chapter 11 is replaced by the following:
‘CHAPTER 11
Health certificate
For gelatine and collagen not intended for human consumption to be used as feed material or for purposes outside the feed chain, intended for dispatch to or for transit through (2) the European Union

 and/or [- carcases and the following parts originating either from animals that have been slaughtered in a slaughterhouse and were considered fit for slaughter for human consumption following an ante-mortem inspection or bodies and the following parts of animals from game killed for human consumption in accordance with Union legislation:(i) carcases or bodies and parts of animals which are rejected as unfit for human consumption in accordance with Union legislation, but which did not show any signs of disease communicable to humans or animals;(ii) heads of poultry;(iii) hides and skins, including trimmings and splitting thereof, horns and feet, including the phalanges and the carpus and metacarpus bones, tarsus and metatarsus bones;(iv) pig bristles;(v) feathers;](2) and/or [- animal by-products arising from the production of products intended for human consumption, including degreased bone, greaves and centrifuge or separator sludge from milk processing;](2) and/or [- products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- petfood and feedingstuffs of animal origin, or feedingstuffs containing animal by-products or derived products, which are no longer intended for feeding for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- aquatic animals, and parts of such animals, except sea mammals, which did not show any signs of diseases communicable to humans or animals;](2) and/or [- animal by-products from aquatic animals originating from plants or establishments manufacturing products for human consumption;]II.5. the gelatine/collagen (2):(a) was wrapped, packaged, stored and transported under satisfactory hygiene conditions, and in particular wrapping and packaging took place in a dedicated room, and only preservatives permitted under Union legislation were used.Wrappings and packages containing gelatine/collagen (2) carry the words “GELATINE/COLLAGEN (2) SUITABLE FOR ANIMAL CONSUMPTION”; and(2) either [(b) in the case of gelatine, has been produced by a process that is ensuring that unprocessed Category 3 material is subjected to a treatment with acid or alkali, followed by one or more rinses, involving pH adjustment, extraction by heating one or several times in succession, followed by purification by means of filtration and sterilisation, in order to kill pathogenic agents;](2) or [(b) in the case of collagen, has been produced by a process that ensuring that unprocessed Category 3 material is subjected to a treatment involving washing, pH adjustment using acid or alkali followed by one or more rinses, filtration and extrusion, in order to kill pathogenic agents;]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01004701.tif.jpg)
 or [the product does not contain and is not derived from bovine, ovine or caprine materials other than those derived from animals born, continuously reared and slaughtered in a country or region classified as posing a negligible BSE risk by a decision in accordance with Article 5(2) of Regulation (EC) No 999/2001;]II.7. in the case of gelatine/collagen (2) from materials other than hides and skins:in addition as regards TSE:(2) either [in case of animal by-products intended for feeding ruminants and containing milk or milk products of ovine or caprine origin, the ovine and caprine animals from which these products are derived have been kept continuously since birth or for the last three years on a holding where no official movement restriction is imposed due to a suspicion of TSE and which has satisfied the following requirements for the last three years:(i) it has been subject to regular official veterinary checks;(ii) no classical scrapie case, as defined in point 2(g) of Annex I to Regulation (EC) No 999/2001, has been diagnosed or, following the confirmation of a classical scrapie case:all animals in which classical scrapie was confirmed have been killed and destroyed, andall goats and sheep on the holding have been killed and destroyed, except for breeding rams of the ARR/ARR genotype and breeding ewes carrying at least one ARR allele and no VRQ allele;(iii) ovine and caprine animals, with the exception of sheep of the ARR/ARR prion genotype, are introduced into the holding only if they come from a holding which complies with the requirements set out in points (i) and (ii).](2) or [in case of animal by-products intended for feeding ruminants and containing milk or milk products of ovine or caprine origin, and destined to a Member State listed in the Annex to Commission Regulation (EC) No 546/2006 (4), the ovine and caprine animals from which these products are derived have been kept continuously since birth or for the last seven years on a holding where no official movement restriction is imposed due to a suspicion of TSE and which has satisfied the following requirements for the last seven years:(i) it has been subject to regular official veterinary checks;(ii) no classical scrapie case, as defined in point 2(g) of Annex I to Regulation (EC) No 999/2001, has been diagnosed or, following the confirmation of a classical scrapie case:all animals in which classical scrapie was confirmed have been killed and destroyed, andall goats and sheep on the holding have been killed and destroyed, except for breeding rams of the ARR/ARR genotype and breeding ewes carrying at least one ARR allele and no VRQ allele;(iii) ovine and caprine animals, with the exception of sheep of the ARR/ARR prion genotype, are introduced into the holding only if they come from a holding which complies with the requirements set out in points (i) and (ii).]NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the BIP of entry into the EU.Box I.19: use the appropriate Harmonized System (HS) code under the following headings: 35.03 or 35.04.Box reference I.23: for bulk containers, the container number and the seal number (if applicable) should be included.Box reference I.25: technical use: any use other than for animal consumption.Box reference I.26 and I.27: fill in according to whether it is a transit or an import certificate.Box reference I.28: Species: select from the following: Aves, Ruminantia, Mammalia - Ruminantia, Pesca.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01004801.tif.jpg)

(j) Chapter 14(A) is replaced by the following:
‘CHAPTER 14(A)
Health certificate
For fat derivatives not intended for human consumption to be used outside the feed chain, intended for dispatch to or for transit through (2) the European Union

 and/or [- animal by-products which have been derived from animals which have been submitted to illegal treatment as defined in Article 1(2)(d) of Directive 96/22/EC or Article 2(b) of Directive 96/23/EC;](2) and/or [- animal by-products containing residues of other substances and environmental contaminants listed in Group B (3) of Annex I to Directive 96/23/EC, if such residues exceed the permitted levels laid down by Union legislation or, in the absence thereof, by legislation of the Member State of importation;]II.4.2. in case the fat derivatives are intended for use in organic fertilisers or soil improvers or other uses outside the feed chain, other than in cosmetics, pharmaceuticals and medical devices, the following Category 2 materials:(2) either [- animal by-products containing residues of authorised substances or contaminants exceeding the permitted levels referred to in Article 15(3) of Directive 96/23/EC;](2) and/or [- products of animal origin which have been declared unfit for human consumption due to the presence of foreign bodies in those products;](2) and/or [- animals and parts of animals, other than those referred to in Articles 8 and 10 of Regulation (EC) No 1069/2009, that died other than being slaughtered or killed for human consumption, including animals killed for disease control purposes;]II.4.3. the following Category 3 materials:(2) either [- carcases and parts of animals slaughtered or, in the case of game, bodies or parts of animals killed, and which are fit for human consumption in accordance with Union legislation, but are not intended for human consumption for commercial reasons;](2) and/or [- carcases and the following parts originating either from animals that have been slaughtered in a slaughterhouse and were considered fit for slaughter for human consumption following an ante-mortem inspection or bodies and the following parts of animals from game killed for human consumption in accordance with Union legislation:(i) carcases or bodies and parts of animals which are rejected as unfit for human consumption in accordance with Union legislation, but which did not show any signs of disease communicable to humans;(ii) heads of poultry;(iii) hides and skins, including trimmings and splitting thereof, horns and feet, including the phalanges and the carpus and metacarpus bones, tarsus and metatarsus bones;(iv) pig bristles;(v) feathers;](2) and/or [- blood of animals which did not show any signs of disease communicable through blood to humans or animals, obtained from animals that have been slaughtered in a slaughterhouse after having been considered fit for slaughter for human consumption following an ante-mortem inspection in accordance with Union legislation;](2) and/or [- animal by-products arising from the production of products intended for human consumption, including degreased bone, greaves and centrifuge or separator sludge from milk processing;]](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01005101.tif.jpg)
 and/or [- petfood and feedingstuffs of animal origin, or feedingstuffs containing animal by-products or derived products, which are no longer intended for feeding for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;](2) and/or [- blood, placenta, wool, feathers, hair, horns, hoof cuts and raw milk originating from live animals that did not show signs of any disease communicable through that product to humans or animals;](2) and/or [- aquatic animals, and parts of such animals, except sea mammals, which did not show any signs of diseases communicable to humans or animals;](2) and/or [- animal by-products from aquatic animals originating from plants or establishments manufacturing products for human consumption;](2) and/or [- the following material originating from animals which did not show any signs of disease communicable through that material to humans or animals:(i) shells from shellfish with soft tissue or flesh;(ii)the following originating from terrestrial animals:hatchery by-products,eggs,egg by-products, including egg shells;(iii)day-old chicks killed for commercial reasons;]II.5. in case of fat derivatives produced from animal by-products referred to in point II.4.1 and point II.4.2:(a) have been produced using the following methods:(2) either [transesterification or hydrolysis at least 200 °C, under corresponding appropriate pressure, for 20 minutes (glycerol, fatty acids and esters)](2) or [saponification with NaOH 12M (glycerol and soap):(2) either [in a batch process at 95 °C for three hours;](2) or [in a continuous process at 140 °C, 2 bars (2000 hPa) for eight minutes;]](2) or [hydrogenation at 160 °C at 12 bars (12000 hPa) pressure for 20 minutes;](b) are packaged in new containers or in containers that have been cleaned, and all precautions are taken to prevent its contamination which bear labels indicating “NOT FOR HUMAN OR ANIMAL CONSUMPTION”;II.6. in case of fat derivatives produced from animal by-products referred to in point II.4.3, the fat derivatives have been produced in accordance with one of the processing methods [1]-[2]-[3]-[4]-[5]-[6]-[7] (2) referred to in Chapter III of Annex IV to Regulation (EU) No 142/2011.NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.Box reference I.12: Place of destination: this box is to be filled in only if it is a certificate for transit commodity. The products in transit can only be stored in free zones, free warehouses and custom warehouses.Box reference I.15: Registration number (railway wagons or container and lorries), flight number (aircraft) or name (ship) is to be provided. In case of unloading and reloading, the consignor must inform the BIP of entry into the EU.Box I.19: use the appropriate Harmonized System (HS) code under the following headings: 15.16 or 15.08.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01005201.tif.jpg)

(k) Chapter 15 is replaced by the following:
‘CHAPTER 15
Health certificate
For egg products not intended for human consumption that could be used as feed material, intended for dispatch to or for transit through (2) the European Union

 and/or [- products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arise;](2) and/or [- the following material originating from terrestrial animals which did not show any signs of disease communicable through that material to humans or animals:hatchery by-products,eggs,egg by-products, including egg shells;]II.5. have been subjected to processing:(2) either [in accordance with processing method … (4) as set out in Chapter III of Annex IV to Regulation (EU) No 142/2011;](2) or [in accordance to a method and parameters which ensure that the products comply with the microbiological standards set out in Chapter I of Annex X, to Regulation (EU) No 142/2011;](2) or [in accordance with Section X, Chapters I and II of Annex III to Regulation (EC) No 853/2004;]II.6. have been examined by the competent authority taking a random sample immediately prior to dispatch and found it to comply with the following standards (5):Salmonella: absence in 25g: n = 5, c = 0, m = 0, M = 0,Enterobacteriaceae: n = 5, c = 2, m = 10, M = 300 in 1 gram;II.7. meet Union standards on residues of substances that are harmful or might alter the organoleptic characteristics of the product or make its use as feed dangerous or harmful to animal health;II.8. the end product was:(2) either [packed in new or sterilised bags,](2) or [transported in bulk in containers or other means of transport that were thoroughly cleaned and disinfected with a disinfectant approved by the competent authority before use,]and which bear labels indicating “NOT FOR HUMAN CONSUMPTION”;II.9. the end product was stored in enclosed storage;II.10. the product has undergone all precautions to avoid contamination with pathogenic agents after treatment.NotesPart I:Box reference I.6: Person responsible for the consignment in the European Union: this box is to be filled in only if it is a certificate for transit commodity; it may be filled in if the certificate is for import commodity.](./../../../resource.html?uri=celex:02013R0294-20130315.ENG.xhtml.L_2013098EN.01005501.tif.jpg)
(11) in Annex XVI, Chapter III, Section 6 is replaced by the following:
‘Section 6
Official controls regarding the feeding of wild animals and certain zoo animals with Category 1 material
The competent authority shall monitor the health status of the farmed animals in the region where feeding is carried out as referred to in Sections 2, 3 and 4 of Chapter II of Annex VI and shall carry out appropriate TSE surveillance involving regular sampling and laboratory examination for TSEs.
Those samples shall include samples taken from suspected animals and from older breeding animals.’.
( 1 ) OJ L 300, 14.11.2009, p. 1.
( 2 ) OJ L 54, 26.2.2011, p. 1.
( 3 ) EFSA Journal(2011); 9(2):1976.
( 4 ) OJ L 147, 31.5.2001, p. 1.
( 5 ) EFSA Journal 2010;8(12):1934.
( 6 ) OJ L 135, 30.5.1991, p. 40.’;







