 EUROPEAN COMMISSION
EUROPEAN COMMISSION
Brussels, 8.11.2017
SWD(2017) 353 final
COMMISSION STAFF WORKING DOCUMENT
Accompanying the document
REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
In accordance with Article 58 of Directive 2010/63/EU on the protection of animals used for scientific purposes
{COM(2017) 631 final}
Annexes
Annex 1: List of recommendations
Annex 2: Report by EURL ECVAM
This Staff Working Document is in support of the Article 58 Review Report, and provides more detailed analysis of the different consultation activities and other information sources used for the Directive review.
Executive Summary
In view of the timing of the review, and the significant differences in starting points among Member States, many involved, whether from science, welfare or regulatory backgrounds, have as yet limited experience of the new measures set out in the Directive. It is too soon after transposition to evaluate the impact of the changes brought in with the Directive, and therefore, for this review to derive definitive conclusions. It can only provide indications on progress towards the Directive’s objectives, identify any common areas of difficulty, and some examples of good practice with the application of the new requirements which could be more widely applicable.
The framework of the Directive is generally considered to be a sound foundation for the regulation of animals used in scientific research.
There are significant differences in the ways in which some aspects of the Directive have been implemented in Member States, risking the main objectives of the Directive to deliver improved science and welfare and give a level playing field for the scientific community across the EU. That this is due either to a combination of incorrect transposition or how some aspects of the Directive have been implemented within the Member State is not clear in the responses of the respondents to the User and Stakeholder surveys.
The impact of the Directive has varied among Member States. This has to a great extent been influenced by the legislative framework in place prior to transposition of Directive 2010/63/EU. In some Member States there have been few changes due to having, for example, considerable experience of project evaluation and authorisation processes, whereas others had no previous requirement, or formal structure in place for, project evaluation. It is not surprising therefore that views on the impact of the Directive vary significantly from country to country – this view was very evident in responses from the pan-European stakeholder organisations.
There are some aspects which are developing and working well, for example Animal Welfare Bodies (AWB).
The introduction of AWB is considered by the great majority of respondents to be a welcome addition, and these are already contributing positively to animal use and care practices within establishments.
Other positive effects reported include raising standards in research practice, improved Three Rs awareness, promotion of culture of care, growing recognition within the research community of the link between animal welfare and good science, and increasing transparency.
A number of areas have been identified where further progress is needed to meet the Directive objectives. Of these, project evaluation and authorisation processes are key to achieving a level playing field for operators within and between Member States. In some Member States, the introduction of formal project evaluation has significantly increased the work required to obtain an approval to use animals in research. There have also been delays to research due to the processes in place to secure some projects.
These areas should be further scrutinised by Member States, where appropriate, to ensure that the processes implemented for project evaluation and authorisation are indeed effective, efficient and resulting in consistency in the outcomes. In addition, there may be a need for the development of further guidance on implementation or interpretation in some areas, or in some cases adjustments to be considered to the national legislative framework and guidance, to deliver the desired level playing field within and among Member States.
Further harmonisation in approach is required to facilitate movement of staff and research programmes among Member States, to improve the National Committee role in promoting good practice and consistency, to improve quality of statistical reporting, for example reporting of genetically altered animals, and to improve quality and ease of access to current non-technical project summary publications, which may allay some concerns expressed by animal welfare groups on the perceived lack of transparency.
Although a common understanding of education and training requirements has been achieved to a great extent, much work is needed to make this operate in practice to facilitate free movement of personnel.
There needs to be increased efforts to improve awareness of available applicable alternatives - interpreted in its widest sense to include replacement, reduction and refinement of animal use -, and appropriate training and tools to facilitate their efficient use by all involved in the process. Even if not directly within the remit of this Directive, concerns were also raised about the need for development of more efficient processes to progress regulatory acceptance of alternative methods, and re-evaluation of validation processes to allow movement away from the need to use existing animal models as the gold standard.
When improved animal welfare standards or practices for animals used for scientific purposes are identified and evidence based, Article 2 has been interpreted by some to prevent Member States from introducing these. The delegated powers foreseen within the Directive will enable delivery of these benefits across EU and therefore promote application of improved practices throughout EU.
The full envisaged benefits of the Directive will only be realised with effective national implementation and enforcement of the legislation. Without these, the objective of a level playing field of common standards and practices will not be achieved, there will be continued imbalances in standards of science and welfare, and confusion in public opinion, as standards among Member States will continue to differ.
i. Introduction
Directive 2010/63/EU on the protection of animals used for scientific purposes was adopted to provide for more detailed and equitable rules within Member States regarding the protection of animals used for experimental and other scientific purposes in order to reduce disparities by approximating the rules applicable in that area and to ensure a proper functioning of the internal market.
To that end, it lays down rules on the following:
a)The replacement and reduction of the use of animals in procedures and the refinement of the breeding, accommodation, care and use of animals in procedures;
b)The origin, breeding, marking, care and accommodation and killing of animals;
c)The operations of breeders, suppliers and users;
d)The evaluation and authorisation of projects involving the use of animals in procedures.
Member States were required to adopt national legislation transposing the Directive by the end of 2012 and the Directive took effect on 1 January 2013.
i.i Purpose and structure of the Report
Article 58 of the Directive requires the Commission to review this Directive by 10 November 2017, taking into account advancements in the development of alternative methods not entailing the use of animals, in particular of non-human primates, and to propose amendments, where appropriate.
The review focuses on the three main objectives of the Directive, mainly to harmonise the legislation on the care and use of animals for scientific purposes to facilitate a level playing field for the operators; to ensure appropriate standards of welfare in line with Article 13 of TFEU through effective application of the Three Rs in the use, care and breeding of animals; and to improve transparency to the general public.
Although there is some overlap in these objectives, and certain elements can impact on all three objectives, these are the general headings under which the main results are presented.
i.ii Timing of the Review
Although the provisions of the Directive entered into force on 1 January 2013, it was not until spring 2015 that the last transposition was completed. An important element of the Directive, namely common standards for accommodation and care, only entered into force on 1 January 2017.
Conformity checks are ongoing, with a number of enquiries in progress. At this stage, there may be incomplete or inaccurate transpositions which will require changes to national legislation.
Member State implementation reports are not due until 2018, and the EU implementation report until 2019.
Projects started under the previous Directive can continue under transitional arrangements until the end of 2017. New authorisations are required from January 2018 at the latest, and the maximum length of a project is 5 years. Retrospective assessments of projects should be carried out after an appropriate time from the completion of a project. This may result in retrospective assessments being carried out a considerable time, e.g. 3 years, after the completion of a project. Only after January 2018 are all uses of animals covered by this Directive. The completion of 5-year projects authorised after January 2018 will take place in 2023. Subsequently, the true value of retrospective assessments can only be properly assessed after 2023, i.e. after sufficient experience of this process is attained.
For these reasons, a review at this time is based on limited experience of the new provisions in the Directive by all those involved in the use, care and breeding of animals for scientific procedures, including regulators, scientific and care staff, and on opinions expressed or evidence presented by other stakeholders.
ii. Consultation strategy and backgound information
Ii.i Stakeholder input
Surveys
Four structured questionnaires were developed to survey the experiences, interests and opinions of relevant stakeholders involved in the administration, implementation and functioning of the Directive. These were tailored to the major sectoral groups according to roles and interactions with the Directive and were distributed to:
National Contact Points: All Member State National Contact Points were invited to submit national opinions on aspects of the operation of the Directive.
Users, breeders and suppliers of animals (hereafter "user"): National Contact Points were asked to circulate the invitation to contribute to all establishments within their Member State. Each establishment, whether a user, a breeder or a supplier of animals, was invited to submit a single survey response representing the views of the establishment. The questionnaire focused on general views as well as on detailed elements of the Directive.
Other stakeholders: Interested parties representing a range of animal welfare, science/academia, industry and veterinary stakeholders were invited to submit general views on the functioning of the Directive from their members/associated parties. The majority of invitations were to pan-European organisations with interests in the care and use of animals in scientific procedures, but contributions were also sought from national organisations concerned with animal welfare.
Specific Three Rs stakeholders: As Article 58 of the Directive requires the review to be carried out paying particular attention to the availability of alternatives, an additional targeted questionnaire was prepared to seek some additional information concerning the development, validation and uptake of alternative approaches, specifically in the fields of basic, translational and applied research and in education and training. The questionnaire was sent to organisations specifically involved in these areas.
In addition to presenting the distributions of views for each of the areas, a number of quotes from the surveys are included in this Staff Working Document. The purpose for their inclusion is to provide a representative sample of typical comments received for a given question and indicating the breadth of views expressed.
Public Consultation
An open consultation meeting was held in Brussels on 31st March 2017, to which all respondents to the four questionnaires were invited, and an open invitation was placed at the EC web-site. Presentations were given on the draft findings and attendees had the opportunity to raise comments and questions. These have been analysed, considered and incorporated into the relevant sections of the report, where appropriate
ii.ii Other information sources
SCHEER Opinion
As the Article 58 Review of the Directive also required to take account, in particular, progress on the development of alternatives to the use of non-human primates, the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) was invited to review recent evidence to update the 2009 Opinion of the Scientific Committee on Health and Environmental Risks on “The need for non-human primates in biomedical research, production and testing of products and devices”.
EURL ECVAM report
Article 48 of the Directive refers to the EU Reference Laboratory, and Annex VII explains the duties of the laboratory. The EU Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) provided an update on the development and acceptance of alternatives to the use of live animals. The report is in Annex 2.
Other
Commission Communication in response to European Citizens' Initiative "Stop Vivisection" and the outcome of a subsequent Commission Scientific Conference "Non-animal approaches – the way forward".
iii. Analysis of the responses to the consultations
iii.i Limitations and interpretation of the results
The consultation had serious limitations due to the early timing of the review and these were clearly confirmed by the responses. Depending on the topic and the respondent group, the proportion of responses that considered it being 'too early to assess' reached in some cases 43 - 47% of all responses. Interestingly, the area in which a significant proportion of measures have not yet come to effect (transparency), the level of responses stating it was too early to assess whether the Directive had improved transparency was only between 5-13% depending on stakeholder group. At a closer look, this can be explained by the 36% of the animal protection groups who disagreed, or strongly disagreed that the Directive had improved transparency.
Some of the questions in the questionnaires were open to interpretation, such as when selecting an answer "no impact" arising from a specific new measure in the Directive. This could mean that effective measures were already in place, or that the measures taken had not had the desired impact. Unfortunately, not all respondents qualified their responses. In some instances "changes" were reported, without the respondent further qualifying the nature of the change.
iii.ii Background of the respondents
Member States
All 28 Member States responded to their questionnaire.
User, breeder and supplier establishments
Responses to the respective questionnaire were received from users in 25 Member States. A total of 889 responses were submitted, with distribution by Member States strongly skewed to France (34.4%), Italy (11.9%) and Germany (9.2%). Users in these three Member States submitted over 55% of all responses.
No replies were received from users in Bulgaria, Cyprus or Malta.
The table below provides responses by Member States (MS) and compares the number of replies with the number of AWBs in each Member State (as reported by Member State in their questionnaire). There are anomalies in the percentage of AWB responding with some Member State users providing up to twice the number of responses expected from the respective Member State data. On further enquiry, in a number of these cases, two or more responses were received from different facilities within a single large establishment (with one AWB).
|
MS
|
Number respondents
|
% of total respondents
|
No AWB*
|
% AWB responding
|
|
FR
|
306
|
34.42%
|
600
|
51.00%
|
|
IT
|
106
|
11.92%
|
145
|
73.10%
|
|
DE
|
82
|
9.22%
|
275
|
29.82%
|
|
SK
|
47
|
5.29%
|
23
|
204.35%
|
|
UK
|
47
|
5.29%
|
170
|
27.65%
|
|
multi**
|
37
|
4.16%
|
0
|
|
|
SE
|
33
|
3.71%
|
40
|
82.50%
|
|
BE
|
31
|
3.49%
|
300
|
10.33%
|
|
NL
|
26
|
2.92%
|
70
|
37.14%
|
|
AT
|
21
|
2.36%
|
40
|
52.50%
|
|
ES
|
21
|
2.36%
|
244
|
8.61%
|
|
PT
|
20
|
2.25%
|
20
|
100.00%
|
|
HU
|
18
|
2.02%
|
35
|
51.43%
|
|
RO
|
17
|
1.91%
|
40
|
42.50%
|
|
DK
|
15
|
1.69%
|
45
|
33.33%
|
|
CZ
|
14
|
1.57%
|
85
|
16.47%
|
|
PL
|
14
|
1.57%
|
220
|
6.36%
|
|
IE
|
9
|
1.01%
|
21
|
42.86%
|
|
EL
|
8
|
0.90%
|
45
|
17.78%
|
|
FI
|
7
|
0.79%
|
25
|
28.00%
|
|
HR
|
6
|
0.67%
|
20
|
30.00%
|
|
EE
|
5
|
0.56%
|
3
|
166.67%
|
|
LT
|
3
|
0.34%
|
4
|
75.00%
|
|
SI
|
3
|
0.34%
|
11
|
27.27%
|
|
LV
|
2
|
0.22%
|
5
|
40.00%
|
|
LU
|
1
|
0.11%
|
7
|
14.29%
|
|
BG
|
0
|
0.00%
|
13
|
0.00%
|
|
CY
|
0
|
0.00%
|
1
|
0.00%
|
|
MT
|
0
|
0.00%
|
3
|
0.00%
|
*from Member State questionnaire responses; assuming 1 AWB/establishment
** from users based in more than one Member State (including international)
Since a single Member State (France) submitted over 34% of all responses, data have been analysed with and without the replies from users in France, and for the French responses alone. Where significant differences were noted, these are reported. To put these proportions above into perspective of the total use of animals in the EU, the use in France, Germany and UK together fluctuates between 55-60% of all animals used in the EU.
Replies were received from 240 organisations in the private sector and 649 in the public sector.
A total of 169 respondents (19%) identified themselves as an small and medium-sized enterprises (SME). For France, 107 (35%) were SMEs.
Concerning areas of activity, 14 (2%) respondents were animal breeders and/or animal suppliers; some other responses also stated they bred and/or supplied animals as well but identified themselves as predominantly animal users. The vast majority, 757 (85%) represented animal users in science or academia. The breakdown of different industry sectors is below
Industry sectors where applicable
|
Sector
|
No
|
%
|
|
Chemical industry (including consumer products, biocides and plant protection products)
|
8
|
1%
|
|
Contract Research Organisation
|
38
|
4%
|
|
Food- and feed sector
|
11
|
1%
|
|
Other
|
13
|
1%
|
|
Pharmaceutical industry (including both human and animal health)
|
120
|
13%
|
|
Not specified
|
699
|
79%
|
|
Total
|
889
|
100%
|
Types of species used
The majority of the respondents used rodents, but users of all the common laboratory species responded, including users of cephalopods. A few respondents did not identify any species of animal being used at their organisation.
Stakeholder Organisations
52 stakeholder organisations responded to the questionnaire. Their distribution across sectors is indicated below

The group "other" comprised of three Three Rs centres, two animal rights organisations and a European Animal Breeding organisation.
The majority of organisations were European or international. However, to promote a balanced response, national animal welfare organisations, 16 of which participated, were also invited to contribute in addition to respondents from the user community, both at the establishment as well as at EU level. The Commission sought to reach out to all relevant stakeholder organisations at EU level.
iv. General views
Views by users and other stakeholders on three generic questions, namely on improvement of animal welfare, the quality and continuation of science, are presented here before addressing more detailed elements covered under the three key aims of the Directive.
iv.i General impact of the Directive on standards of animal welfare, care and use of animals
Respondents to the questionnaires were invited to consider whether the adoption and implementation of the Directive and the related national legislation had improved the standards of animal welfare, care and use in their country/region.
The responses indicated that the revised regulatory framework is considered beneficial, in particular in Member States which did not have a comprehensive structure in place prior to the introduction of 2010/63/EU.
User responses
|
The adoption and implementation of the Directive and the related national legislation has improved the standards of animal welfare, care and use in my establishment.
|
|
Users
|
|

|
The majority of user responses agreed that already the Directive has already had a positive impact.
Stakeholder responses
The response from the stakeholder organisations was not as positive, with 13% of the view that more time was needed to assess the impact.
|
The adoption and implementation of the Directive and the related national legislation has improved the standards of animal welfare, care and use in my country/region.
|
|
All stakeholders

|
|
Other Stakeholder Organisations
|
Animal Protection Organisations
|
|

|

|
Separating the organisations involved in research from the animal protection organisations did identify a significant difference in their views. 75% of science/research organisations agreed that the Directive was improving standards whereas only 18% of welfare organisations were of this view and around 23% who considered it too early to assess.
The main areas where improvements are expected are in the quality of authorised projects, education and training of scientists and care staff, housing and care practices, and implementation of the Three Rs. The importance of an effective AWB to deliver such benefits was emphasised by all stakeholders.
Concerns were also expressed over lack of enforcement and the restrictions placed by Article 2 on the ability of Member States to introduce improved practices – knowledge of animal welfare needs is evolving rapidly, and Member States should be encouraged to adopt improved practices.
The following quote reflects many of those received from animal protection organisations:
“The framework of the legislation is an improvement, but implementation and enforcement needs to be drastically improved for achievement of the Directive’s main goal to end the use of animals.”
iv.ii Effect of the Directive on the quality of science
The question given to the users and stakeholders concerned the impacts of the Directive on the quality of science through the application of new elements such as AWB, DV and a systematic project evaluation including harm-benefit assessment.
A number of users make the comment that improved welfare contributes to improved science, including a reduction in stress, and improved health status reducing experimental variability. Many users, however, also point out that the quality of science generated in studies using animals is affected by many other factors outside the realms of the Directive. Others reflect on some of the factors that have been improved by the additional focus introduced by the Directive obligations such as on improved experimental designs leading to more robust and reproducible science, or more data or better quality data being available from the same number of animals by more appropriate designs and planning. The AWB has also helped standardisation of methodologies within establishments.
The quality of science generated through animal studies is only partially dependent on the regulatory framework. However, the improvements in animal care and use standards and practices, including the required input to project design and evaluation, should be reflected in due course by improved quality of science, but it is too soon following transposition to provide specific evidence. The impact will again be dependent on the previous legislation, but the responses from users and scientific stakeholder organisations support the view that the introduction of e.g. AWB and a systematic project evaluation/authorisation have had a positive impact on model choice and design of procedures.
Many animal protection organisations indicated that due to the lack of transparency generally so far seen across Member States during the implementation of the Directive, it is impossible for them to measure or assess any changes in ‘quality’ deriving from the Directive.
User responses
|
The Directive has improved the quality of science in my country/region through the application of new elements such as Animal Welfare Bodies, Designated Veterinarians and a systematic project evaluation.
|
|
Users
|
|

|
40% agreed that the Directive had improved the quality of science, 18% disagreed. Of those who agreed, suggestions as to why this was the case included:
·AWB and Designated Veterinarians being mandatory in all animal facilities
·Systematic project evaluation
·Better staff training and competence
·Improved quality of monitoring of animals
·Further improvement in animal welfare and Three Rs leading to better science
Of those who disagreed, for many, the processes were in place before under previous national legislation.
Other reported issues:
·"Animal Welfare Bodies and veterinarians have inadequate experience in assessment of animal welfare or quality of project in the case of some species such as Xenopus, Danio rerio."
·Some felt that the Directive would not affect scientific quality but that this was better “controlled” by scientists during peer review during the funding processes and of manuscripts submitted for publication.
The point was made that the control systems must work together with researchers, as precluding valuable research work is not in the interests of anyone.
·"More interactions with vets and care staff, leading to benefits, such as new medication for post-surgery procedures, new methods for cleaning/maintaining animal wounds. Because animal stress is reduced and animal welfare is better, so scientific results are also better."
·"The facts that projects are now authorised based on the result of a favourable evaluation and that they are followed (from a 3Rs and animal welfare perspective) during implementation do not necessarily mean that quality of science has improved. But at least, processes are in place to make sure that the scientific approach is challenged and justified on a case-by-case basis."
·"The people performing the project evaluation lack the scientific competence necessary to improve the science in the project (many are not scientists or do not even have an academic background)"
·"Project evaluation is sometimes performed by people that do not know the study methods used which can cause difficulties to issue an opinion."
Stakeholder responses
|
The Directive has improved the quality of science in my country/region through the application of new elements such as Animal Welfare Bodies, Designated Veterinarians and a systematic project evaluation including harm-benefit assessment.
|
|
All stakeholders
|
|

|
|
Other stakeholder organisations
|
Animal protection organisations
|
|

|

|
Over a third of all respondents felt that it was too soon to be able to determine whether there had been any impacts. 36% of animal protection organisations did not agree that there had been any scientific improvements as a consequence of the different structures under the new Directive.
Animal protection stakeholder comments
·"The lack of transparency in decision making, project applications and retrospective review makes it hard to answer this question."
·"Doubtful whether the new requirements improved quality of science. Looking at the NTPs and research projects we still have the impression that projects are licensed where independent cost-benefit analysis would indicate that they should not."
·"As the regulatory system does not allow for public oversight of project licence applications, the regulatory evaluation process or systematic publication of retrospective project evaluation and harm-benefit analysis, it is difficult to comment."
·"The increased emphasis brought by the Directive on the need for education and ongoing training of those using or caring for animals, and the need to ensure their competence, is welcomed."
·"Separation of authorisation and inspection functions in our country is not helpful"
Particular concerns were expressed by an animal protection organisation in one Member State, where in their opinion no harm-benefit analysis is required prior to authorisation –
·“Authorities have to grant projects that have been formally correctly applied for.”
Another response stated
·“The cornerstone of the regulatory system, the harm-benefit assessment, is, in the absence of useful EU law setting its operational parameters, a highly discretionary exercise on a complex issue. It is astonishing that the EC believes that a level-playing field could be created in these circumstances.”
Other stakeholder comments
·"This might probably true for countries that did not have the mentioned bodies, experts or processes and still it is too early to have a clear picture if the quality has improved. or those countries that were already working as described in the Directive a change of quality cannot be seen, as it was already on a high level."
·"This question only refers to elements which have external control on research, ignoring the role of scientists themselves, arguably the most important factor for quality. Demanding more thorough external control will only be effective if scientists have knowledge and motivation to meet that demand. Good training in experimental design and analysis is essential to generate high quality science."
·"There are some areas where the quality of science will undoubtedly improve. For example, greater emphasis is being placed on good experimental design, which has scientific value, as well as welfare merits. In addition, increased sharing of good practice will improve the quality of science. However, we believe it is yet too early to appreciate the full scientific merit of the Directive."
A number of Member States had much of the Directive framework in place, and it is not expected in these countries that significant improvements relating to the Directive transposition will be seen.
In conclusion, it is clear from the responses that it is far too early following the transposition to be able to measure or assess the Directive impacts on the quality of science as it takes a number of years for e.g. publications to filter through. Furthermore, as stated, the quality of science is dependent on multiple factors, many of which are outside the scope of the Directive.
iv.iii Continuation of high-quality research in the EU using animals, where still necessary
Respondents were also invited to give views on whether or not the new Directive allows continued high quality animal research where necessary and justified.
This statement was heavily criticised by the majority of animal protection stakeholder responses as wholly unsuitable as it implied that high quality research could be derived from animal studies.
The animal protection stakeholder response also criticised the implementation of the new Directive, implying that the present regulatory and research environment also still allows badly designed or poorly carried out experiments to be funded, authorised and undertaken, which wastes animals’ lives and causes suffering that could have been avoided.
From the scientific community, the responses related to differences in implementation, rather than failings in the Directive framework. The project authorisation process has introduced in many Member States additional robust requirements to justify the use of animals and requires implementation of the Three Rs. Concerns were expressed over the time taken to obtain project authorisation, but again this varied among Member States. There are different Member State approaches to the use of simplified processes (Article 42) and the processes/justifications required for exemptions to certain articles, for example animals taken from wild, care and accommodation, and authorisations for reuse and rehoming.
Although there has been some closure and consolidation of user facilities in recent years, it is not possible to determine whether the increased bureaucracy indicated in some responses has been a contributory factor, rather the general financial situation and the increased availability of facilities in particular in Asia which are thought to be the major factors.
User responses
|
The Directive allows continued high quality research on animals where still necessary.
|
|
Users
|
|

|
Nearly 70% of users agreed that the Directive allows continued high-quality research on animals where still necessary. Only 5% disagreed.
Even amongst some who agreed, the process has limited some research at their institutes. Several stated that they felt that delays to projects have been observed and several believe that this and other aspects of the Directive reduces or, if not improved, will reduce European competitiveness. Some stated that some scientists try to avoid doing animal experiments because of the administrative burden. Another stated that they felt that the persistence of some animal experiments was only because the scientists felt that their expertise lay with using those models (and not with alternatives). There have been some problems where single housing was required for experimental reasons, and for birds where a significant increase in pen size is required, an argument is made that these large enclosure sizes are not considered to be in the welfare interests of the birds. One stated that scientific experiments on Xenopus and Dario rerio were stopped due to over interpretation of the legislation in considering procedures and reuse.
Of those who disagreed, comments included
·"It discourages people from doing their research in Europe. There is a clear risk of people doing research outside this legal framework as it becomes more and more time consuming and ineffective."
·"The directive has many aspects that allow strong enemies of experimental research with animals to find arguments against these experiments and to stop them entirely. I believe (together with many colleagues who perform research in life sciences) that in the long-term Europe will lose competitiveness in research as compared to other regions in the world. The discrepancies in the quantity of regulation in comparison to agricultural use (economically justified???) is far too high,"
Other comments
·"Thanks to the Directive, we truly believe we perform high-quality research (see publications list) by keeping animal welfare as high as possible."
·"Research projects are now written and evaluated more accurately in all the phases before authorisation."
Stakeholder responses
60% of responses agreed that the Directive allows high quality research using animals to continue, with 13% disagreeing.
|
"The Directive allows continued high quality research using animals, where still necessary, in my country/region".
|
|
All stakeholders
|
|

|
|
Other stakeholder organisations
|
Animal protection organisations
|
|

|

|
36% of animal protection stakeholders and 87% of other stakeholders considered that the Directive allowed continued high-quality research animals, where necessary, to continue.
The level of challenge to animal studies has increased, causing delays, but this is, in general, considered to have improved the quality of animal studies. The majority of responses acknowledge that the increased scrutiny towards the Three Rs, and animal welfare, accommodation and care has led to improvements in animal care and study design.
However, caution is needed that the processes are efficient, without unnecessary bureaucracy, as there are concerns that certain types of research may stop or be relocated.
The interpretation of the definition of “project” varies from a single procedure to a programme involving many different procedures. Applying for a project authorisation for each individual procedure is considered unnecessary and overly bureaucratic. The time taken for “minor” amendments was also noted as a concern, especially when the Directive requires only those amendments to be authorised that may negatively impact animal welfare.
Animal protection groups expressed concerns over the structure of the question as being biased by implying that any animal research could be of high quality. Concerns remain over the justification for animal use, study design and analysis and implementation of the Three Rs. There is little evidence to date that the Directive has impacted animal numbers.
Animal protection stakeholder comments included
·"This is a biased question. There are increasing concerns around the validity and translatability of many animal "models" and tests."
·"It is beyond dispute that it is business as usual, the numbers of animals used is rising, if anything, and much low-quality research continues to be carried out"
·"the present regulatory and research environment also still allows badly designed or poorly carried out experiments to be funded, licensed and undertaken, which wastes animals’ lives and causes suffering that could have been avoided."
Other stakeholder comments
·"The Directive allows competitive, high-quality scientific work that involves animals if no alternatives are available, without any major disruption of research, though short delays in delivering authorisations have occurred."
·"There remain significant challenges in terms of reproducibility and the quality of the design of animal experiments that the Directive has not tackled."
·"The increased scrutiny has provoked delays and cancellations in animal research, or transfers to other continents. It is difficult to evaluate the quality of research that did not take place."
·"The focus on experimental design and methodology has impacted positively. Many elements are positive but issues still exist with statistical reporting and definition of project."
Section 1 - Harmonisation of legislation
i Introduction
A key aim of the Directive was to create a level playing field for all of those using animals in research and industry, and for any others impacted by that use, through harmonisation of legislation and its objectives and outcomes. There seems to be some confusion, especially among users, over whether or not uniform operational practices could be expected as the result of the new Directive. However, Member States have the sovereignty to determine how best to achieve the objectives through national legislation, operational procedures and practices.
A number of aspects were included in the legislative framework, which are aimed at progressing the harmonisation process. These included modifications to the scope, education and training requirements, common criteria and conditions for project evaluation and authorisation, and through Article 2 the limitations on unilateral changes to adversely affect the internal market.
Although the impacts of the Directive cannot yet be fully determined or analysed, the responses from scientific and animal protection stakeholders are suggestive that the interpretation and implementation varies so significantly across, and even within, Member States, the Directive will not quickly deliver the desired level-playing field.
Many examples were provided where different practices exist.
·“There are different interpretations and level of enforcement by the sometimes disaggregated (at regional/provincial level) competent authorities with different knowledge, resources and commitment: the level of control differs significantly (response to project authorization varies a lot, many projects in some countries are pending).”
·“There still is a major lack of harmonisation especially due to different interpretation of the Directive during harmonising the local laws and regulations. Countries still do not allow execution of animal experiments if these were evaluated and accepted in foreign countries. Also the education is not always accepted from country to country, due to local laws.”
·“The Directive can be seen as the foundation for a common level playing field and indeed a certain degree of harmonisation has occurred especially in standards - but authorisation and administrative processes seem to differ which is leading to uncertainty by the applicants.”
·“The Directive has the potential to level the playing field, however problems exist around its implementation. There are signs of disparity in project evaluation and authorisation between member states; e.g. primate neurology experiments which were not authorised in one MS on severity grounds were allowed in another.”
Directives do not set out required processes or structures, unlike regulations, but there are concerns that the different structures which have evolved, in particular for project evaluation and authorisation, may not deliver common outcomes.
Although some progress is being made towards common structures, which has been acknowledged by many users, without some discussion and willingness among Member States to improve harmonisation of outcomes, it is considered unlikely that the desired level playing field will be fully realised.
Responses from some Member States acknowledged that ensuring a consistent approach to project evaluation and authorisation was still being progressed and that further guidance on the process, including the framework for and composition of evaluation review groups/bodies was still under development. National Committees are not sufficiently well established to have progressed their role in ensuring a coherent approach to project evaluation and sharing of best practice.
The requirements for project evaluation and authorisation have caused concerns over additional bureaucracy, delays and costs for scientists among users and some stakeholder organisations. Some users raised concerns over inconsistencies between evaluators/evaluations within the same Member State, and over inconsistencies between Member States on authorisations issued.
In contrast, around one third of the user respondents were of the view that the Directive had already created a level playing field. A key advantage was considered to be the harmonisation of animal care and accommodation practices. Even though these were only mandated in January 2017, the introduction of such changes is known to have commenced prior to this date.
Of those who did not indicate that the Directive was progressing harmonisation, some stated that there was divergence in the application and interpretation of the Directive at national, regional or local levels. It was suggested that different financial resources within the different Member States may be affecting development of a level playing field. It was felt by some that this was putting those who had fully implemented the Directive at a competitive disadvantage.
Others commented that there are differences in other parts of the world which can influence the EU work, including different (lower) housing requirements, which put higher constraints on EU competitiveness.
ii General views on harmonisation objective
User responses
|
"The Directive has created a level playing field by providing similar conditions for operators, irrespective of their country or region."
|
|
Users
|
|

|
One third of the users were of the view that the Directive had created a level playing field. 15% disagreed. 13% said it was too early to say.
The key advantages were harmonising of animal housing and procedures, particularly seen amongst those working in several different countries.
Of those who disagreed, some stated that there was divergence in application / interpretation of the Directive at National or local levels, and some had not seen harmonisation. There may be more standardisation in animal care than in the protocols. For some, not all the processes are available yet (simplified administrative procedure and multiple generic projects).
Comments
·"I have been working in the UK, Denmark and France. Conditions are very different between these countries"
·"Whereas the housing and care standards are very similar across different user establishments, the implementation of other aspects such as project review and authorization still differs a lot between countries and institutions."
·"In this matter, the key point is now more the potential discrepancies between the requirements enforced in EU regarding animal research vs when performed in third party countries (e.g. EMA, or North America to a lesser extent) : i.e. some protocols may be outsourced overseas."
·"Since many details of Directive 2010/63/EU are unclear and our country so far has not set up implementation rules, there are enormous disharmonies even between different regions. We further do not have the impression that other countries adopted Directive 2010/63/EU as stringently as we have."
·"Still very hard to get cross-border agreement on required training for those carrying out scientific procedures"
·"The intrinsically logical structure of the Directive came out distorted by just adapting the old law rather than renewing it. There is no level playing field and our establishments are already exporting their work to neighbouring countries."
Stakeholder responses
29% of organisations are of the view that the Directive has made progress towards a level playing field, with 32% disagreeing with this view.
|
The Directive has created a level playing field by providing similar conditions for operators, irrespective of their region or country.
|
|
All stakeholders
|
|

|
|
Other stakeholder organisations
|
Animal protection organisations
|
|

|

|
There are significantly differing views between the animal protection organisations and the other (mainly scientific) stakeholder groups - 36% vs 24% agree and 18% vs 50% disagree over progress towards a level playing field.
The main areas of concern identified by the other stakeholder groups included PE processes, size and complexity of projects, inconsistencies within different regions of individual Member State (far less across EU), different authorities being required for the same/identical projects in different Member States, and the time taken to obtain authorisations (from initial application).
There remain differing requirements for education, despite agreement on a common framework, continuing the difficulties for personnel moving between Member States.
The animal protection stakeholders acknowledged that progress is being made towards a level playing field as intended by the Directive framework, but there are differences in implementation processes and rates, and effectiveness of implementation, making it difficult to predict whether this will be fully achieved. Different authorities are imposing different controls – for example some projects not permitted in one Member State are permitted in another.
The main concern from the animal protection stakeholders is over the view that Article 2 hinders progress on animal welfare, preventing Member States adopting improved standards.
Animal protection stakeholder comments
·"The text of Directive encourages harmonisation, however a major problem exists around its proper and consistent implementation."
·"We disagree with the premise of Article 2 as it hinders progress on animal welfare and MS should be allowed to raise animal welfare standards."
·"The cornerstone of the regulatory system, the harm-benefit assessment, is, in the absence of useful EU law setting its operational parameters, a highly discretionary exercise on a complex issue. It is astonishing that the EC believes that a level-playing field could be created in these circumstances"
Other stakeholder comments
·"The Directive has created the means for a level playing field, however in practice differences exist in implementation between Member States. The inconsistency in implementation which create most uncertainty for industry are in the authorisation procedures."
·"The problem is with implementation, not with the text itself. However, a degree of harmonization was achieved through alignment of severity criteria, transparency measures, and animal welfare bodies, and an increase in husbandry and housing standards. Most divergences are in the authorization and other administrative procedures (requirements for personnel)."
·"There are still many differences in harmonisation / implementation at country\regional level. One of the key areas of concern for private and public research lies in slowness of the authorisation process."
·"There are different interpretations and level of enforcement by the sometimes disaggregated (at regional/provincial level) competent authorities with different knowledge, resources and commitment.
1.1 Project Evaluation and Authorisation (Articles 36-42, 44)
Project evaluation and authorisation are central pillars of the new regulatory system, and consistency and efficiency in process and outcomes are essential to deliver a level playing field for the scientific community and consistently deliver the desired welfare and scientific benefits.
The requirements for project applications are set out in Article 37 and Annex VI. The requirements for verification of the content of the applications and considerations for project evaluation are laid out in Article 38. The requirements for project authorisations issued by the competent authority are described in Articles 40 and 41.
Both the project evaluation and project authorisation must be carried out by a competent authority (Article 36) and with a degree of detail appropriate for the type of project (Article 38(1)). Furthermore, the project evaluation should be performed in an impartial manner and it may integrate the opinion of independent parties (Article 38(4)).
Specific guidance has been produced to assist in the development of these processes. The greater majority of Member States have disseminated the EU Guidance on Project Evaluation, although some only recently. However, it was not clear from the responses if project evaluators have received it or if they are using it.
1.1.1 Project evaluation (Article 36 and 38)
In many Member States, a project evaluation and authorisation processes were in place under previous legislation, but the detailed requirements have changed under the new Directive.
There are now clear requirements for what is expected to be included in the application for authorisation (Articles 37, 38 and Annex VI). The project evaluators verify that constraints set by the Directive are complied with, for example restrictions on use of non-human primates and use of endangered species, evaluate its objectives and compliance with the Three Rs and the expected harms. Finally, with all necessary information, the evaluators need to determine whether on balance the benefits are likely to be achieved and that they outweigh the expected harms. Projects may not be authorised unless this is the case.
Across the 28 Member States a number of differing structures have been developed to meet these requirements. In some Member States, a single competent authority/committee considers applications from the entire country, perform project evaluation and, where appropriate, project authorisation. In others, there are regional committees, or committees within user establishments, often integrated with the AWB. There are differing challenges, dependent on the structure, to meet the various requirements set out in the Directive for project evaluation and project authorisation, including, in particular, impartiality, proportionality and consistency.
With project evaluation at a national level, additional information may be required on the quality of the facilities and availability of experienced staff within the establishment in which the work will be performed. This information is needed to assess the likelihood of success as part of the harm-benefit analysis and thus requires input from a local perspective. However, dealing with applications at the local level raises questions over impartiality. At a local level, there will also necessarily be a greater number of project evaluation committees which poses additional challenges to ensure a consistent outcome, one of the key aspects of creating a level playing field. The fewer the number of committees/evaluators, the easier it is to achieve consistency – however, currently the number of committees/evaluators range from 1 to around 125 (within a single Member State). The EU Guidance details some of the pros and cons of the different approaches.
Both users and stakeholder organisations have identified the varied implementation strategies as a significant risk to the attainment of a level playing field. European stakeholder organisations noted significant differences in the contributions from individual Member State constituent organisations (e.g. AFSTAL/LASA to FELASA).
Comments included
·"We do note however that the Directive has placed an additional burden on companies during their assessment of a project, and that EU guidance has been lacking or is insufficiently known to users or in cases not applied by authorities. Moreover, this resource is not accessible and/or available in all official languages. A concrete example, informs us that the template is not always possible to complete with the relevant information and in an accessible manner. Overall it is our assessment that the Directive will need to be applied more widely across EU Member States if it is to have a more significant impact, and hereby improve animal welfare on a larger scale. For this reason, there are elements relating to evaluation and authorization that are too early to definitively assess."
·"The multiplication of project reviewing entities in some countries (National Committee+local committees) cause bureaucracy and delays."
·"The requirements to explain more clearly the harms to animals and information on the 3Rs complemented with the individual animal severity assessment have already had an impact on planning and executing studies and on consideration for animal welfare. However, the system needs to be worked out and time is required to settle down properly and not being considered as purely administrative burden."
Detailed information on the project evaluation and retrospective assessment processes were not provided by Member States for this review, but these will be submitted by Member States in 2018 to form part of the Commission implementation report due by November 2019.
Half of the users considered that the processes of project evaluation and authorisation were effective and efficient. However, users and stakeholder organisations have reported the existence of inefficiencies or ineffectiveness of the project evaluation and authorisation processes in many, if not all countries. It should be noted that the timing of the review is such that many scientists have yet to submit a project application and are still using authorisations issued under previous legislation.
For some users, particularly in some Member States, the level of scrutiny and the delays caused have been detrimental to scientific output. Such delays do not occur in all Member States but processes in others may require some adjustment to allow progress to be made.
It is clear from the user responses that for some applicants, the understanding of the requirements for the application submission, and of the processes for project evaluation and authorisation are not ideal. There were also concerns raised about duplication in the processes - in some circumstances, review by up to three separate committees - and in the content required to be submitted in project applications. Information that it is in excess of that required by the Directive seemed to be requested by some evaluators, and there were reported inconsistencies between what the establishment internally asks and what the competent authority requires.
Some of the delays and inconsistencies were reported to be due to insufficient or inexperienced staff who are undertaking project evaluation. Independence of the evaluation process was questioned when evaluation was carried out within the establishment..
Multiple generic projects and the simple administrative processes (both measures designed to simplify processes and reduce bureaucracy) have not been utilised extensively, indeed many user responses suggested that neither was available nor indeed understood. However, where a simplified process is available and known about, two-thirds of the users stated that there was an improvement in administrative savings or processing times with regards to these project types after the implementation of the Directive.
These responses did however highlight the different approaches taken by Member States towards the nature, size and complexity of projects. This seems to vary essentially from a project containing a single procedure involving a few animals of one species to a project for a five-year programme of work involving multiple procedures and species and many thousands of animals. Although both approaches to authorisations are acceptable, such differences in approach are raising concerns and difficulties when studies or projects are required in more than one Member State or when a project is being transferred from one Member State to another.
Amendments to projects already authorised were discussed only by a few consultation respondents, probably due to limited experience of such measures. Users requested greater efficiency in evaluation and approval of amendments. The requirement by a few Member States to require changes to projects before researchers can implement improvements to the application of the Three Rs causes frustration within the scientific and welfare community and delays implementation of welfare improvements. This may also exceed the requirements of Article 44.
Member State responses
When project evaluation and authorisation processes were introduced under the new Directive, were the previous processes critically reviewed to optimise the efficiency and effectiveness of administrative processes?
|
Yes
|
18/28
|
|
No
|
6/28
|
|
Not applicable
|
4/28
|
The majority of Member States indicated that during the implementation of the new Directive, the opportunity was taken to optimise the efficiency and effectiveness of the administrative processes around the project authorisation processes.
The revised processes included revised project application forms and processes, and simplified handling of minor amendments. Electronic submission and documentation have improved efficiency in some Member States.
Some concern was expressed by one Member State on the costs of implementing the project evaluation and authorisation process.
Has the task of project evaluation be assigned to a Competent Authority other than a public authority in your Member State?
There are significant differences among Member States with regard to the project evaluation and authorisation processes. The systems seem to vary from evaluation and authorisation at local ethical committees to a single national committee looking at all proposals within the Member State. The number of committees ranges from 1-125. Both users and stakeholder organisations have identified the varied implementation strategies as a significant risk to the development of a level playing field.
User responses
|
Are the processes of project evaluation and authorisation effective and efficient? Please consider also processes required for amendments and renewals?
|
|

|
46% of user responses indicated satisfaction over the project evaluation and authorisation processes. The majority of concerns were over the time taken to obtain project authorisation.
Despite guidance on applications, applicants found it difficult to submit the necessary information to the competent authority. There remain some teething problems in Member States where electronic submissions have been introduced.
Some concerns were expressed over the lack of (numbers and experience) staff at the competent authority to deal with applications.
Inconsistency within the project evaluation process was highlighted, in particular where multiple competent authorities and review groups were involved within Member States, and between Member States with different processes.
·"With the new requirements, the process is less efficient and has increased the administrative burden on users."
·"Provide a reduced project application to facilitate Pilot studies using only few animals – full project application is unduly onerous for a project which may not be required if initial pilot fails."
·"The main impact is very high administrative load (and cost). In our view systematic project evaluation is unnecessary and redundant in many aspects with other procedures. In our view, what should be evaluated once until significant modification occurs are the protocols and procedures. Projects that are submitted for funding should indicate whether protocols are approved. The local animal welfare committee should have the autonomy to approve projects."
·"More complex situation and additional burden when evaluation at the procedure level, which already existed in our establishment before the Directive, changed to evaluation at the project level"
EU Guidance on project evaluation
Member State responses
Has the developed EU guidance been disseminated and used by those carrying out project evaluations?
Where no, this was due to a delayed dissemination until national language versions were made available.
Has the developed EU guidance been of benefit to those carrying out project evaluations?
|
Yes
|
16/28
|
|
Too early
|
6/28
|
|
No opinion
|
1/28
|
|
No response submitted
|
5/28
|
Generally well-received by project evaluators to assist the process and to promote a harmonised, consistent approach but some are still evaluating the benefits of the current documents.
Has any training been established for Project Evaluators as a result of the developed EU guidance on Project Evaluation and on Education and Training Framework (including training module for project evaluators)?
Where training has been provided, benefits were noted in terms of the analysis undertaken regarding animal welfare, scientific value, statistical design, severity assessment and consistency of approach.
Stakeholder responses stressed the importance of trained evaluators to ensure an informed and consistent process.
User responses
Are you aware of the guidance developed in the EU by Member States and stakeholders to facilitate the common understanding and implementation of the Directive?
|
|
All users
|
France
|
Rest of EU
|
|
|
No
|
%
|
No
|
%
|
No
|
%
|
|
No
|
273
|
31%
|
144
|
47%
|
129
|
22%
|
|
Yes
|
616
|
69%
|
162
|
53%
|
454
|
78%
|
|
Total responses
|
889
|
100%
|
306
|
100%
|
583
|
100%
|
Further dissemination of the developed guidance would be beneficial.
Some additional comments on guidance is summarised below:
·There may be scope to improve the guidance as some find them difficult to understand. Comments were received stating that the guidance was too long, whereas others requested more content.
·The role and tasks of the Designated Veterinarian may not be sufficiently clear in some countries, such that excessive costs are seen to be incurred.
·More species-specific information is requested e.g. for fish.
·Improvements in consistency in consideration of the same procedure in different countries were requested.
|
Has the developed EU guidance been helpful to those preparing project proposals?
|
|

|
The guidance was generally considered helpful. A number of users were unaware of the guidance, and others did not have access to translated versions. Some comments on concerns included:
·"Examples of PE would be helpful – in particular in determining the level of detail and proportionality of the evaluation processes."
·"Clarification is needed to interpret the conditions for reuse in a uniform manner."
1.1.2 Multiple generic projects (Article 40)
Article 40 allows Member States to authorise multiple generic projects carried out by the same user if such projects are to satisfy regulatory requirements or if such projects use animals for production or diagnostic purposes with established methods.
Member State responses
Is authorisation of multiple generic projects in Article 40(4) allowed in your Member State?
Have preliminary benefits been observed in terms of any administrative savings or processing timelines for respective competent authorities from multiple generic projects?
It was stated to be too early to assess as only a few projects will have been approved under this article. Some indication of a reduction in administration was identified by a few, but others were of the opposite view and suggested the level of administration required by applicant/establishment is not reduced. Some Member States indicated that the possibility was already available under previous legislation. In one Member State, an additional request for regular progress reports and summary of animal use has been introduced for such projects.
User responses
|
If an authorisation of multiple generic projects is allowed in your country (Article 40), is it used by your establishment?
|
|

|
Most report that this is not allowed. Examples where it is used include maintenance of parasite cycles, regulatory toxicology and breeding of genetically altered animals.
|
Have preliminary benefits been observed from authorisation of multiple generic projects in terms of any administrative savings or processing timelines for your establishment?
|
|

|
Only few comments were received, but one example indicated significantly reduced administration where it has replaced individual authorisations for regulatory toxicology work (1 versus 100 projects).
1.1.3 Simplified administrative procedure (Article 42)
Article 42 allows Member States to introduce a simplified administrative procedure for projects containing procedures classified as non-recovery, mild or moderate and not using non-human primates, and that are necessary to satisfy regulatory requirements or which use animals for production or diagnostic purposes with established methods.
Member State responses
Is simplified administrative procedure in Article 42 allowed in your Member State?
In response to whether preliminary benefits had been observed in terms of any administrative savings or processing timelines for respective competent authorities from simplified administrative procedure, only few benefits had been perceived, including the waiving of the non-technical project summary.
User responses
|
If a simplified administrative procedure (Article 42) is allowed in your country (projects to satisfy regulatory requirements with no severe procedures and not using non-human primates), is it used by your establishment?
|
|

|
|
Have preliminary benefits been observed from simplified administrative procedure in terms of any administrative savings or processing timelines for your establishment?
|
|

|
There may be some misinterpretation of these questions. Some responses appear to be discussing changes in administrative procedures such as moving to electronic systems. Some respondents from countries without simplified systems reported that they used them. Simplified procedures are not always available where repeat and standardised studies are required for regulatory reasons. An individual submission for each is still required by some Member States.
In conclusion, the term "simplified administrative procedure" does not seem to be clearly understood. Half of the Member States have not adopted this measure and some users in countries without the possibility for a simplified administrative procedure think that they use these.
1.1.4 Authorisation decisions (Article 41)
Article 41 sets deadlines within which both project evaluation and project authorisation processes should be completed and communicated to the applicant. However, these timelines must be counted from the receipt of the complete and correct application.
Half of the users replied that the decisions on projects were communicated within the required maximum timelines. However, there were several responses stating that the process takes too long, outside the required 40/55 days, with some taking up to several months. It was reported that to deliver a “complete and correct” application can involve lengthy negotiations with the regulator, before the 40-55 days begin. Therefore, clarification on how the days were calculated was requested by some.
In some Member States, a “stop/start” clock system is used to determine the time taken to consider the application – that is, at each stage of the process when a draft application is under consideration by the evaluators the clock is running: when it is returned to the applicant the clock stops. The different accounting systems can make significant differences as to whether or not the Member State achieves the targets set in Article 41, but does not necessarily reflect the application to authorisation time for the scientist. For some, times for obtaining authorisations are holding up staff and / or science. Some problems were reported over some electronic submission systems at the time of the consultation.
A number of Member States have introduced financial charges for projects. Concerns were raised that, despite paying for the service, the time-lines set out for authorisation decisions in Article 41 were not met.
User responses
|
Are authorisation decisions (Article 41) taken and communicated to project applicants within the timeframes (40 working days with a possibility to extend by 15 working days for complex projects)?
|
|

|
Of those who responded, almost half considered that the authorisation was not communicated within the required timelines. Further examination of this question revealed that there was a significant difference between the responses from users in France and those from other Member States, with 74% of responses from France indicating that authorisation dates were not communicated within the 40/55 days set out in the Directive, compared with 32% elsewhere.
|
|
All users
|
France
|
Rest of EU
|
|
|
No
|
%
|
No
|
%
|
No
|
%
|
|
No
|
327
|
47%
|
190
|
74%
|
137
|
32%
|
|
Yes
|
364
|
53%
|
68
|
26%
|
296
|
68%
|
|
Total responses
|
691
|
100%
|
258
|
100%
|
433
|
100%
|
A significant amount of frustration was conveyed in the comments, a small selection of which are included below:
·"We are a CRO, our sponsors contact us when they need and do not want to wait first 40 (+15) days prior to initiation of a study. They may find help in other countries outside EU."
·"Clarify what is needed for ethical evaluation. lot of variation in evaluation from one ethical committee to another."
·"Dedicate specific resources for ethical evaluation."
·"Ask the authorizing body!! They don't work in time but ask money for it!"
·"On the whole most applications are returned within 40 working days. However application/interpretation of how to use the 40 working days has led to significant delays with the processing of some licences."
·"No. However, this depends on the definition as to when the 40 days start. The total loop hole on when the “40 days starts" is ridiculous. (The definition of complete and correct application). What’s the point of sending letters saying it, when applicants don't get their project even looked at for 4-6 months. When the CA is recruiting/training new Evaluators it should have been more efficient and recruit a few spare as you'll be in the same position soon for sure. The burden the current evaluators are under is immense."
·"Improve national project evaluation process, reduce response times, increase body of inspector or have a EU / Brussels based analysis of projects system"
Increased resource for project evaluation is requested. Improved training by competent authority for applicants and evaluators to explain requirements would be helpful.
Consistency needs to be improved as significant regional variation was reported impacting on ability to meet scientific/sponsor deadlines.
1.1.5 Role of National Committees (Article 49)
Recital 48 and Article 49 lays out the purpose and the tasks of the National Committee with regard to project evaluation i.e. to ensure a coherent approach to, and share best practice on project evaluation.
Less than one quarter of the users considered that the National Committee had been effective in promoting a coherent approach, perhaps understandably as many are not yet well established. As the majority are only in the early stages of development, there appears to have been little activity to date on sharing best practice on project evaluation. This is an aspect of their work which, if effective, would improve confidence in the project evaluation process.
Member State responses
|
Has the National Committee (Article 49) been effective in ensuring a harmonised approach to project evaluation and harm-benefit assessment by different competent authorities (when more than one) throughout the country?
|
|
Yes
|
7/28
|
|
No
|
3/28
|
|
No opinion / NA
|
8/28
|
|
Too early to assess
|
10/28
|
Among the contributions made by Member States are promoting standardisation of the approach to project evaluation with an agreed template and producing guidance on the functions of project evaluation committees. Examples were also provided of joint membership of, or observer status at project evaluation committee(s). Advice also provided to remind project holders of obligations to apply the Three Rs throughout the lifetime of project.
User responses
Users were invited to consider the impact of the introduction of National Committee in promoting a consistent approach to project evaluation, and on their effectiveness in supporting AWB.
|
Has the National Committee (Article 49) been effective in promoting a coherent approach to project evaluation and level playing field?
|
|

|
There were significant variations in responses, again reflecting the structures in place under earlier national legislation and the speed of progress with the implementation of the 2010 Directive.
Inconsistencies among project evaluators within the same Member State were cited as concerns. For example
·"Different regions not applying always the same criteria. However, they work on it."
·"Too many discrepancies among ethical committees."
Recommendations
ØThe Commission services and Member States should engage in discussions to improve guidance and provide further examples for the scientific community on what constitutes a "project".
ØMember States should review if additional administrative gains could be attained for authorities and operators from a wider use of multiple generic project authorisation and simplified administrative procedures.
ØWhere lacking, Member States should provide clear guidance on the required content for a project application, review that the requested elements directly relate to the performance of the harm-benefit assessment in line with Article 38, and that the level of detail is appropriate for the type of project.
ØMember States should engage with relevant stakeholders to review their respective project evaluation and authorisation processes to identify any duplication and to establish measures of simplification aimed at efficient, effective and timely processing of applications.
ØTraining for both project applicants and project evaluators would seem beneficial. Joint efforts by the Commission services, Member States and other stakeholders should be made to create opportunities for such training.
ØUrgent focus is needed by National Committees on their key task to establish a coherent approach to project evaluation in particular in Member States with multiple competent authorities tasked with project evaluation. The Commission services, Member States and National Committees should engage in discussions to develop appropriate tools for this purpose.
1.2 Changes in Scope of Directive (Articles 1 and 5)
Under the previous Directive 86/609/EC and transposed Member State legislation, there were countries who extended legislative protection to certain other specified types of animals, animals at various stages of development and types of work using animals. These inclusions were reviewed during the development of 2010/63/EU, and the scope revised to include those which were justified on scientific and welfare grounds, to promote harmonisation and afford additional welfare protection.
1.2.1 Inclusion of cephalopods (Article 1)
Only few users and four Member States reported use of cephalopods. In one Member State, cephalopods were protected already under the previous legislation. For scientists in other Member States, it was reported that the administration has slightly increased.
The European Cephalopod Research Association (EuroCeph) submitted a response providing an update on issues relating to cephalopod research. EuroCeph noted that in their experience regulation is having a positive effect in EU and abroad by creating a culture of care for this taxon. The degree of development of knowledge on the adequate conditions for the maintenance and care of cephalopods in captive conditions is still relatively low, and for many species in its infancy.
Neither Annex III (Care and accommodation) nor IV (Methods of killing) contains specific guidance on cephalopods. Since the adoption of the Directive EuroCeph has invested significant efforts to fill this gap. Once sufficient evidence is available, the necessary amendments to the respective annexes should be made to ensure EU wide application.
1.2.2 Foetal forms of mammals in the last third of normal development (Article 1)
Foetal forms were already protected in many Member States, but not in all. Where this was a new requirement, responses from eight Member States cited increased administration with little evidence to date of improved welfare or science.
Member State responses
Has the inclusion of foetal forms of mammalian species under the scope of the Directive had an impact in terms of administration, quality of science and animal welfare?
|
Impact on administration
|
|
Yes
|
8/28
|
|
No
|
10/28
|
|
No view/NA
|
4/28
|
|
Too early to assess
|
6/28
|
|
Impact on quality of science
|
|
Yes
|
5/28
|
|
No
|
6/28
|
|
No view/NA
|
7/28
|
|
Too early to assess
|
10/28
|
|
Impact on animal welfare
|
|
Yes
|
8/28
|
|
No
|
5/28
|
|
No view/NA
|
6/28
|
|
Too early to assess
|
9/28
|
User responses
Is your organisation using foetal forms of mammalian species?
|
|
No
|
%
|
|
No
|
641
|
72%
|
|
Yes
|
248
|
28%
|
|
Total responses
|
889
|
100%
|
Has the inclusion of foetal forms of mammalian species under the scope of the Directive had an impact in terms of administration, quality of science and animal welfare?
|
Impact on administration
|
No
|
%
|
|
No
|
88
|
35%
|
|
Yes
|
117
|
47%
|
|
Too early
|
26
|
10%
|
|
No view/NA
|
17
|
7%
|
|
Total responses
|
248
|
100%
|
|
Impact on quality of science
|
No
|
%
|
|
No
|
153
|
62%
|
|
Yes
|
37
|
15%
|
|
Too early
|
43
|
17%
|
|
No view/NA
|
15
|
6%
|
|
Total responses
|
248
|
100%
|
|
Impact on animal welfare
|
No
|
%
|
|
No
|
134
|
54%
|
|
Yes
|
63
|
25%
|
|
Too early
|
35
|
14%
|
|
No view/NA
|
16
|
6%
|
|
Total responses
|
248
|
100%
|
Most comments reflected on increased administration. A few suggested that greater thought, planning and oversight had improved science and welfare. However, in general it was not considered to have improved science.
The addition of killing methods specific for foetal forms in Annex IV was requested.
1.2.3 Animals used for the purposes of education and training (Article 5)
Article 5 which sets out the purposes for which procedures can be performed in the EU includes animals used in higher education, or training for the acquisition, maintenance or improvement of vocational skills.
Using animals in scientific procedures for educational purposes in schools was controversial and new provisions exclude lower education establishment from using them. Project authorisation is now required for use of animals in higher education and training in vocational skills. Similar provisions were already in place in many Member States, but not in all.
Changes were generally, but not entirely, considered beneficial, in terms of refinement of and reduction in animal use. One Member State expressed disappointment that in their view the quality of teaching in schools has been reduced.
Member State responses
Has the inclusion of animals used for education and training under the scope of the Directive had an impact in terms of administration, quality of teaching and animal welfare?
|
Impact on administration
|
|
Yes
|
8/28
|
|
No
|
13/28
|
|
No view/NA
|
4/28
|
|
Too early to assess
|
3/28
|
|
Impact on quality of teaching
|
|
Yes
|
6/28
|
|
No
|
14/28
|
|
No view/NA
|
4/28
|
|
Too early to assess
|
4/28
|
|
Impact on animal welfare
|
|
Yes
|
10/28
|
|
No
|
12/28
|
|
No view/NA
|
4/28
|
|
Too early to assess
|
2/28
|
User responses
Is your organisation using animals for the purposes of education and training?
|
|
No
|
%
|
|
No
|
496
|
56%
|
|
Yes
|
393
|
44%
|
Has the inclusion of animals used for education and training under the scope of the Directive had an impact in terms of administration, quality of teaching and animal welfare?
|
Impact on administration
|
No
|
%
|
|
No
|
133
|
34%
|
|
Yes
|
202
|
51%
|
|
Too early
|
31
|
8%
|
|
No opinion/NA
|
27
|
7%
|
|
Total responses
|
393
|
100%
|
|
Impact on quality of teaching
|
No
|
%
|
|
No
|
166
|
42%
|
|
Yes
|
178
|
45%
|
|
Too early
|
28
|
7%
|
|
No opinion/NA
|
21
|
5%
|
|
Total responses
|
393
|
100%
|
|
Impact on animal welfare
|
No
|
%
|
|
No
|
188
|
48%
|
|
Yes
|
161
|
41%
|
|
Too early
|
29
|
7%
|
|
No opinion/NA
|
15
|
4%
|
|
Total responses
|
393
|
100%
|
Where use of animals for educational and training purposes was not previously included in national legislation, then comments indicate an increase in administrative burden but also acknowledges greater consideration for such use thus benefiting positively animal welfare. Some impact was noted on reduction by greater use of individual animal tissues, for example by sharing among research groups.
One response stated that more animals were required to train people as local authorities ask for specific courses in addition to (adequate) training in university courses.
Another response suggested this change has had a very negative consequence:
·“one training for experimental surgery using only alternative methods (like inert models, ...) has been approved in my country and as a consequence all other training applications which still used animals have been rejected. In my opinion using plastic models or animals for the training of surgery certainly does not give the same training quality. This is a threat for the welfare of animals/patients undergoing subsequent surgery by the diplomates.”
Other comments included:
· “formal recording of training and competency in a central database has been a very effective means of verifying the competency of researchers and ensuring compliance”
·“The creation of a High-Tech simulation platform allowed to replace many exercises in vivo and develop more practical curriculum, thereby increasing the quality of teaching.”
·“One new aspect is the retrospective assessment of individual animal severity. This has the potential to be used for learning and further implementation of 3Rs generally.”
·“we re-evaluated the number of animals needed to master techniques. Students work in pairs and make optimal use of the animals to learn the most techniques possible per week and we harvest skin (for suturing techniques) and many organs that serve as base materials for other courses (e.g. histology)."
1.2.4 Animals used for the purposes of routine production (Article 5)
The use of animals in routine production, such as blood harvest, was already covered in the vast majority of Member States under previous legislation. Most Member State responses state no impact since the introduction of the Directive.
User Responses
Is your establishment using animals for the purposes of routine production such as for blood based products?
|
|
No
|
%
|
|
No
|
792
|
89%
|
|
Yes
|
97
|
11%
|
|
Total responses
|
889
|
100%
|
Has the inclusion of animals used for routine production under the scope of the Directive had an impact in terms of administration, quality of science and animal welfare?
|
Impact on administration
|
No
|
%
|
|
No
|
42
|
43%
|
|
Yes
|
40
|
41%
|
|
Too early
|
5
|
5%
|
|
No opinion/NA
|
10
|
10%
|
|
Total responses
|
97
|
100%
|
|
Impact on quality of science
|
No
|
%
|
|
No
|
56
|
58%
|
|
Yes
|
27
|
28%
|
|
Too early
|
6
|
6%
|
|
No opinion/NA
|
8
|
8%
|
|
Total responses
|
97
|
100%
|
|
Impact on animal welfare
|
No
|
%
|
|
No
|
51
|
53%
|
|
Yes
|
35
|
36%
|
|
Too early
|
5
|
5%
|
|
No opinion/NA
|
6
|
6%
|
|
Total responses
|
97
|
100%
|
The majority of users were already used to previous legislation and did not see any impact.
Where introduced in a Member State for the first time, there were some reports that timely authorisation processes do not always occur and concerns were expressed on delays getting authorisation in commercial environment - highlighting the issue stated earlier on differences in size and complexity of “projects”, from individual procedure to complex 5 year programme with multiple procedures. There is scope for a wider use of multiple generic projects and/or a simplified administrative procedure for these types of projects.
Some expressed benefits of greater consideration for animal welfare, with standardised accommodation and care and more refined procedures in the area of routine production.
Recommendations
ØFurther guidance should be developed to improve clarity on the minimum threshold of severity needed to bring a procedure under the scope of the Directive.
ØThe European Commission should propose amendments to Annexes III and IV for cephalopods once sufficient evidence is available.
ØThe European Commission should consider incorporating appropriate killing methods for foetal forms of mammalian species in Annex IV and review whether methods already contained in the Annex are still in line with the latest scientific knowledge.
1.3 Education and training of staff (Article 23)
Requirements for education, training and competence of staff are described in Article 23 and Annex V of the Directive. As a new element, the Directive has paid particular attention to acquisition, demonstration and maintenance of competence.
Free movement of personnel is one of the key aims of the new Directive but detailed requirements for education and training are the competence of the Member States. However, some level of harmonisation is considered essential by users and stakeholders if a level playing field allowing free movement of personnel is to be achieved.
The majority of Member States have established a formal authorisation process for personnel, with defined education, training and competence requirements. In some the responsibility for ensuring appropriate education and training lies with the establishment.
The majority view from the users (63%) was that ensuring and maintaining competence of staff was being satisfactorily addressed, but differences in expectations of training requirements between Member States have been reported, such that duplication of training is still required in some cases. There is currently little clarity on the expectations for continued professional development (CPD).
The agreed EU Education and Training Framework has been published to promote harmonisation through acceptance of training according to a common modular framework and specified learning outcomes. Despite this, there are obviously still concerns over recognition of training delivered elsewhere. This was highlighted at the public consultation meeting, where comments were received that scientists were still having to repeat training when moving between Member States and that much work was still needed to facilitate free movement.
A few difficulties are also being encountered in relation to access to training, including availability of training courses, in particular for the less common species. Training was also suggested for specific functions such as members of AWB as well as further guidance on assessment of competence.
There is an important role for the Education and Training Platform in Laboratory Animal Science (ETPLAS) in increasing awareness of availability and quality of training courses by engaging with all relevant stakeholders to progress with commonly acceptable quality standards. ETPLAS was formed as a result of the recommendation contained in the EU Education and Training Framework assembling the three key players and facilitators, namely the relevant Member State authorities, course providers and course accreditors. The activities of the platform are still evolving, but progress has been limited due to lack of resources and active engagement by all three parties.
Recommendations
ØEfforts should be made by all relevant stakeholders to improve availability and access to, and variety of, training courses essential for obtaining the requisite competences in different knowledge areas, techniques and species.
ØThe three partners of ETPLAS (Member State representatives, course providers and accreditors) should increase collaboration and engagement in order to progress with the development and agreement of common quality standards aimed at free movement of competent staff.
ØETPLAS should take a more active role and step up its efforts to establish itself as a central repository for information on LAS (Laboratory Animal Science) training and quality standards in EU.
1.4 Harmonisation of welfare standards and stricter animal welfare measures (Annexes III and IV, Article 2)
Annex III defines standards for care and accommodation of animals which are now fully mandated in all Member States since the beginning of 2017. Annex IV defines appropriate methods of killing.
Article 2 created the possibility in certain circumstances for Member States to maintain more stringent animal welfare standards in force before the new Directive was adopted. This affected mainly the retention of standards of accommodation and care higher than those contained in Annex III, which was intended to set common standards across EU, and form part of the framework for creating a level playing field. So, from the outset, there were always going to be some minor differences among Member States.
Whilst similar standards were already in place in some countries previously, Directive 2010/63/EU introduced for the first time clear, mandatory standards across all Member States, in some cases resulting in much improved welfare practices than contained in the previous guidance.
Over half the user respondents of the consultation thought that the Directive had improved animal welfare by application of improved housing and care practices, including for example inclusion of enrichment requirements and the need for trained and competent care staff. For some, daily monitoring of animals, including at weekends and holidays has been introduced which was generally seen as highly beneficial. There is increased awareness of the need for careful consideration of animal welfare, with appropriate defined and legally enforceable standards.
For many Member States, with implementation of Annex III, the enclosure sizes for certain species has changed, requiring significant investment in new or alterations to buildings and enclosures. Although this was raised as a significant issue in the Impact Assessment that accompanied the Commission proposal for the new Directive, few issues were raised in the responses concerning the investments needed, possibly as the welfare benefits were acknowledged and an additional four years had been given until January 2017 to meet these new standards. Concerns were raised that the size of certain of the bird enclosures was incompatible with good welfare in certain circumstances. These concerns should be considered as part of future adaptations of Annex III (see below).
Annex IV contains a list of approved standard methods of killing, for which no specific project authorisation is necessary, although competence in the persons performing the task is obligatory. Concerns were expressed that some of the methods for certain species were now “proven” to be unsuitable and should be deleted.
As well as Annex IV methods, Member States may authorise additional methods to be used without project authorisation if the competent authority is satisfied that there is sufficient evidence that the method is as humane as those in Annex IV for that species. Member States must provide an annual report to the European Commission on additional methods of killing approved in each calendar year under Article 6(4)(a). Annex IV is foreseen to be amended through delegated powers in due course (Article 50) to include such additional methods, and remove any which are deemed unsuitable.
There were significant concerns expressed by animal protection stakeholders that Article 2 would prohibit uptake of improved practices. The Directive does not prevent the application of improved care practices as this is firmly embedded in the Directive requiring that the Three Rs are continuously applied (for example Articles 4, 13, 27). When it is intended to propose changes to Annex III standards, these should be based upon sound reproducible evidence.
With the delegated powers embedded in the Directive, benefits of any such changes will be able to be accrued throughout EU and not impact only on animals in a single Member State, if such evidence-based cases can be made for changes to Annex III.
Recommendations
ØMember States should provide evidence-based cases to the Commission services where amendments to Annexes III and IV are considered appropriate.
ØWith the proposal to include standards for, inter alia, cephalopods in Annexes III and IV, the European Commission should consider other amendments on the basis of exemptions granted under Article 6(4)(a) and other evidence brought forward.
Section 2 – Animal Welfare and the Three Rs
i Introduction
The Directive has introduced a number of elements that should contribute to systematic application of the Three Rs, improved animal welfare, the uptake and use of existing alternatives approaches (in its largest sense covering replacement, reduction and refinement) and to further accelerate the development, validation and regulatory acceptance of new alternative approaches.
Beyond the provisions establishing uniform welfare standards such as binding care and accommodation conditions or methods of killing, the systematic, case by case application of project evaluation is expected to deliver one of the most significant impacts in ensuring compliance with the Three Rs. This allied to the activities of the AWB and the requirements for training and competencies are expected to derive significant welfare improvements in animal care and use.
This section will first discuss the findings on a series of provisions all aimed at improving welfare of animals and proper balancing of the Three Rs. The section will then evaluate the impacts of the project evaluation process on achieving its objectives, including how existing alternative approaches are taken up and integrated in the projects.
Finally, some comments are incorporated on how the development, validation and regulatory acceptance of new alternative approaches are impacted by the provisions of this Directive.
2.1 Animal Welfare Bodies, AWB (Articles 26 and 27)
Articles 26 and 27 lay out the requirements for one of the important new aspects of the Directive, the AWB. It has a central role in ensuring continuous application of the Three Rs in all care and use of animals within the establishment.
The requirement for AWB has been welcomed by Member States, users and stakeholder organisations.
There are significant positive indicators of the benefits of the introduction of AWB, in particular the heightened awareness within establishments of welfare needs, in particular with regard to refinements.
The size and complexity of AWB vary significantly, in some cases dependent on the nature and size of establishment, the previous requirements in place before the Directive, and on the specific Member State transposition of Articles 26 and 27.
In a number of Member States, the Designated Veterinarian is included as a required member of AWB. This has been received positively.
There were many examples in all scientific sectors and in many Member States, of positive benefits to both animal welfare, with refinement of procedures, and improved experimental design following discussion of projects within the AWB.
The interactions in AWBs among scientists, care staff and veterinarian are viewed very positively.
Where the AWBs are properly resourced, with staff having appropriate skills, (including in all the relevant species), and where the decisions made by it are supported by establishment management, they can deliver the requirements and aspirations of the Directive. This requires balanced representation from science and welfare interests. Significant advantages have been reported with inclusion of specific expertise on experimental design. External (sometimes lay) input is also often considered beneficial, especially in considering the non-technical project summaries as part of the support given by the AWB during the development of a project proposal.
Specific skills on alternatives and literature searching are rarely included in the composition of AWB, but some have suggested that their inclusion would have significant benefit.
In some Member States, the role of AWB is reported as unclear, in particular where preliminary evaluation of projects is performed within the establishment. As the required tasks of AWBs and project evaluators are different (Articles 27 and 38 respectively), it is very important that each is aware of what they must do, and that the competencies of personnel involved are appropriate for these separate processes.
AWBs have a number of different tasks within the establishment to support good animal welfare practices, and sufficient resource must be available to meet these, in particular where a considerable amount of time is taken up with project development.
It is vital that all of the core tasks of the AWBs are performed and not constrained to assisting in the development of projects.
However, where there is effective completion of all their designated tasks, this will positively contribute to the local culture and processes such that the time required for project development is likely to be reduced.
Users reported that the AWB has had a positive impact in improving the culture of care, for example by increasing numbers and improving quality of staff, including by better training, by improving communications between them and improving teamwork directed towards optimising animal welfare and ensuring robust scientific output.
Feedback from Member States on inspections supported the view that AWBs are developing well, and impacting positively on animal welfare within establishments.
It is considered essential to ensure AWBs are properly resourced and decisions supported by management to effectively deliver the requirements of AWB. The composition of AWB is generally wider than set out in the Directive, often with the Designated Veterinarian as a full member, and including individuals with specific expertise on Three Rs and experimental design.. There needs to be a balanced representation from science and welfare interests.
2.1.1 AWB and impact on the Three Rs and animal welfare
Stakeholder responses
|
Animal Welfare Bodies have improved the implementation of the Three Rs and welfare of animals in my country/region.
|
|
All stakeholders
|
|

|
|
Other stakeholder organisations
|
Animal protection organisations
|
|

|

|
The overall responses from stakeholder organisations were positive, but significant differences were again noted when the views from scientific and animal protection organisations were separated.
Animal protection stakeholder comments
General concerns were expressed over lack of visibility or clarity over roles of AWB, and therefore it was difficult to form a view on how these are working. The minimum composition as set out in the Directive is considered inadequate, and the Designated Veterinarian should be a legal requirement. However, AWB with appropriate training and support can contribute positively to animal welfare.
·"It is neither publicly known whether all relevant institutions have already established AWBs, nor how they are composed nor whether they fulfil their legal function."
·"The work (if any) of animal welfare bodies is hidden so there is no way to evaluate the outcome."
·"There are no obligations made on official training for the 3Rs and bio-statistics"
Other stakeholder comments
The responses acknowledged benefits of AWBs, but highlighted the need to ensure appropriate composition, expertise and resourcing. AWBs need to be empowered by the establishment management.
·"Beyond compliance with processes, welfare is put at the centre of operations and welfare considerations are brought to the daily operational level."
·"The mere existence of AWBs is an indication of progress, as formerly they were a lot less widespread from a European-wide perspective"
·"In countries where AWB were already established, improvements will vary, but they have had a positive impact. They have proved useful for: 1) Disseminating best practice and addressing concerns 2) Improving cross stakeholder dialogue between scientists, veterinary staff, animal welfare staff 3) The monitoring of compliance in the establishment"
·"It is unquestionable that the AWBs have increased the awareness of scientists to focus more on animal welfare. Beyond compliance, welfare considerations are brought directly to the “ground”/everyday operations"
·"Where it has been reported that AWB members are non-scientists they have sometimes failed to advise researchers correctly negatively affecting research."
User responses
|
Have the Animal Welfare Bodies contributed, through tasks defined in Article 27, to an improved welfare of animals in general and facilitated the uptake of the Three Rs within your establishment (whether breeder, supplier or user)?
|
|

|
The majority of users were of the view that AWB had contributed to improved welfare in their establishment. Many of those answering negatively indicated that a similar framework had been in place prior to the new Directive.
A number of specific examples of where AWB had contributed to improved welfare or uptake of the Three Rs were submitted. These include
·Improved housing including provision of nesting materials; refinement of animal housing, especially with less well-known species; provision of “group” advice on renovations; specific health status monitoring.
·Training and habituation of animals to procedures; improved handling; development of a socialisation plan including specific exercise regimens; improvement of breeding programmes.
·Adaptation in sampling including micro-sampling; improved surgical techniques and peri-operative care; refined endpoints; use of “alert” cards on cages to require action; additional monitoring; better record keeping; establishing post-operative follow-up score sheets; improved methods of anaesthesia and euthanasia.
·Improved training in aspects of care and recognition of pain and distress including enforcing daily observation of animals; improved supervision of junior scientists.
·Improved communication between care and scientific staff; valued input by Designated Veterinarian – in some cases a mandated member of the AWB.
·"Fewer animals used due to statistical review or exchange of ideas about how more can be obtained from fewer animals."
·"There is “more exchange of best practices. Problems are identified, discussed and solutions are proposed.”
·"Follow up on work done has encouraged (further) improvements especially when unexpected events happen (with welfare consequences) when AWB reviews complete report to try to learn lessons for the future."
·“AWB members are also split in sub groups to address specific areas e.g. the 3Rs / animal care and accommodation, harm / benefit analysis, management systems, training. Champions of the function groups lead in addressing specific areas, - This approach allows in depth reviews of each area and implementation of actions identified.”
·“recognises local good practice through awards, sponsors 3Rs lectures, poster events”
·“Quarterly newsletter now produced; 1:1 discussions held with project licence holders about 3Rs”
·“Alternative species seminars”
Of those who said it was not beneficial, for many this was because a similar process was already in place and in some cases the respondents said that the person in charge of the unit was already doing those tasks. For a few they saw it as additional bureaucracy / cost without benefit. Many of these places seem to be small, and with a small number of well-defined programmes, for example provision of blood products.
Have the Animal Welfare Bodies been effective in improving participation and communication between different members of staff (e.g. scientific/technical/veterinary)?
|
|
All users
|
France
|
Rest of EU
|
|
|
No
|
%
|
No
|
%
|
No
|
%
|
|
No
|
118
|
13%
|
38
|
12%
|
80
|
14%
|
|
Yes
|
610
|
69%
|
223
|
73%
|
387
|
66%
|
|
Too early
|
76
|
9%
|
27
|
9%
|
67
|
8%
|
|
No opinion/NA
|
85
|
10%
|
18
|
6%
|
49
|
11%
|
|
Total responses
|
889
|
100%
|
306
|
100%
|
583
|
100%
|
On the specific question concerning the impact of AWB on improving communication, the French users seemed to hold a slightly more positive view. However, some of those who said that no impact had been noted, often commented on similar structures having been in place already previously.
On positive impact on communication, users stated:
·"Encourages “peer to peer" discussion and communication”
·“more consideration and respect, from the users, for the lab animals used”
·“Animal Welfare Body in our institution has enabled more communication (formal and informal) between different participants in animal experiments and animal welfare which enables better awareness about various issues in connection with animals in experiments”
2.1.2 AWB and impact on the quality of science
Member State responses
Have Animal Welfare Bodies been effective in improving the quality of science within establishments?
|
Yes
|
12/28
|
|
No
|
1/28
|
|
No opinion
|
3/28
|
|
Too early to assess
|
12/28
|
There are indications in the Member State responses that AWB are impacting positively on the quality of animal research being conducted within establishments. It was noted that the structure promotes improved and positive interactions and exchanges of information among the animal care and scientific staff. Input from the Designated Veterinarian was considered very helpful.
A number of responses noted the improved quality of project applications following input by AWB, in particular in refinements and animal care, contributing to improved science. This helped the efficiency of the project evaluation process.
User responses
|
Have the Animal Welfare Bodies been effective in improving the quality of science (e.g. through contributing into project proposals and monitoring of their outcomes) within your establishment?
|
|

|
There were some differences in views among French users compared to the rest of the EU with the latter seeing the benefits to science more positively, from 24% to 39%.
|
|
All users
|
France
|
Rest of EU
|
|
|
No
|
%
|
No
|
%
|
No
|
%
|
|
Yes
|
271
|
33%
|
70
|
24%
|
201
|
39%
|
|
No
|
200
|
25%
|
77
|
26%
|
123
|
24%
|
|
Too early
|
224
|
28%
|
100
|
34%
|
124
|
24%
|
|
No opinion/NA
|
119
|
15%
|
50
|
17%
|
69
|
13%
|
|
Total responses
|
814
|
100%
|
297
|
100%
|
517
|
100%
|
Of those who thought the AWB had contributed to the scientific quality, the cited examples included:
·Improved project applications following AWB check; improved literature review reducing duplication of experiments; scientific contribution from members of the AWB (peer review) that improves the quality of project proposals; better definition of projects, avoiding unplanned, non-standardised experiments; determining whether hypothesis will truly be answered by study design, with the expectation of clear experimental results.
·Optimising experimental design; statistics, randomising and blinding; improving replication and improving reproducibility; model homogeneity including defined standard procedures, with no interference of pain or animal stress.
·Stopping experiments when confounding factors influence science; reduce the severity of models to better evaluate the activity of drug candidates; avoid using animals that are injured; avoid interactions between animal health status/welfare and the readouts of the studies; better record keeping and standardised methodologies within establishment.
·Internal sharing of tissues.
·"developed a special cage for irradiation, which secures the correct irradiation dose for the animals, thereby increasing animal welfare and improving experimental results"
Of those who responded that the AWB had not affected quality of science, some stated that they do not consider scientific quality, some leaving this to the project evaluation by the competent authority, and others to experts not included in AWB. Some believe that the personnel on the AWB do not have the necessary scientific skills especially in particular areas. Some felt that this should not be a role for the AWB, but others in the establishment.
Examples of potential detriment to science:
·"Scientific requirement, e.g. individual animal housing when appropriate, is being over-ruled even where animals are provided with significant interaction time and playtime. This results in certain types of projects being unworkable (topical efficacy) due to individual, subjective and personal considerations. Directive and its guidance should be encouraging good science and welfare within EU rather than try to force scientific use of animals, to areas outside EU."
2.1.3 Obstacles in delivering tasks of the AWB
User responses
|
Have obstacles been encountered in delivering the tasks of the Animal Welfare Body in Article 27?
|
|

|
More than half of the user responses responded that no difficulties had been encountered when delivering the tasks of the AWB.
Training, resources and having sufficient authority were the main issues of concern raised. AWB members need to be trained for their specific tasks, including effective knowledge of the species involved and understanding of the requirements of the scientific research. Skills on experimental design were considered very helpful.
Expertise in alternative methods is reported as less than ideal in some establishments.
Feedback on obstacles included
·Duplication of work between project evaluation and AWB (and user committees or other parts of institution) was reported by some.
·Lack of clarity on expectations of content of applications for project evaluation makes it difficult for AWB to contribute effectively and efficiently to support project applicants.
·Inadequate resourcing.
·No guidance on addressing conflicts of interest (including some with independence from the institution).
· “There are a lot of established scientists in the animal welfare body, thinking "we are doing a good job for decades". For animal caretakers it is hard to be heard.”
·"Domination by a single person, or poor chairperson, not in the interests of optimal functioning and balanced outcomes taking views of all."
2.1.4 Guidance on AWB
EU Guidance on the composition and functioning of the AWB has been agreed and disseminated. In response to the question on the need for further guidance, many of the suggestions would seem to be already addressed in the existing EU guidance.
User responses
Elements that were highlighted include
·Improve status of and ensure appropriate support and empowerment for the AWB within an establishment.
·Need for the development of training for members and opportunities to meet members from different establishments.
·Improve role of National Committee (and National Contact Points) in communication and dissemination of best practices.
·Better dissemination of present guidance and increase awareness of other relevant publications.
Has the developed EU guidance on Animal Welfare Bodies been helpful?
|
|
All users
|
France
|
Rest of EU
|
|
|
No
|
%
|
No
|
%
|
No
|
%
|
|
No
|
107
|
13%
|
44
|
14%
|
63
|
12%
|
|
Yes
|
380
|
46%
|
108
|
35%
|
272
|
52%
|
|
Too early
|
106
|
13%
|
37
|
12%
|
69
|
13%
|
|
Not aware
|
119
|
14%
|
63
|
21%
|
56
|
11%
|
|
No opinion
|
119
|
14%
|
54
|
18%
|
65
|
12%
|
|
Total responses
|
831
|
100%
|
306
|
100%
|
525
|
100%
|
Difference between French and other users seems to be due awareness of the guidance and availability of translated versions.
Recommendations
ØEstablishments and Member States (through inspection) should ensure that all core tasks of the AWB are being fulfilled.
ØMember States should clarify roles and responsibilities of the AWB and project evaluation, in particular where there may be some integration or overlap with following the development of projects, including application of the Three Rs and project evaluation process.
ØSenior management of the establishment should ensure that the AWB has sufficient resources and empowerment to carry out the required tasks.
ØEstablishments could consider the addition of a Designated Veterinarian as a full member of the AWB.
2.2 National Committees (Article 49)
Article 49 of the Directive describes the requirements for the establishment of National Committees for the protection of animals used for scientific purposes in each Member State. National Committees should facilitate a coherent approach to project evaluation and promote the Three Rs as well as playing an important role in the exchange of good practice within the Member State and at the level of the Union.
Although a few Member States had similar Committees to those required under Article 49, the majority of Member States needed to set these up from scratch, and currently many National Committees are still establishing their role and have yet to make an impact at national level. A few Member States have yet to establish their National Committee, and only 18/28 were active at the time of the review.
Many users, in selected Member States, were unaware of the existence or functions of a National Committee.
However, there are National Committees already flourishing with the development of guidance material and development of networks with and sharing practices among AWB.
There has been one meeting, hosted by the Commission, of National Committee chairs to initiate communications and consider ways sharing of best practices, but at the time only 17 National Committee representatives were able to attend, and effective channels of communication and information dissemination have yet to evolve.
The structure, membership, responsibilities and activities of National Committees vary significantly among Member States, and concerns were raised over the resources made available to perform their functions.
Member States and users were invited to consider the impact of the introduction of National Committee in promoting a consistent approach to project evaluation (covered under Section 1 of this report), and on their effectiveness in supporting AWB.
Member State responses
|
Has the National Committee carried out activities to share/disseminate best practice on animal welfare and use, and to advise Competent Authorities?
|
|
No
|
10/28
|
|
Yes
|
18/28
|
The distribution of responses again reflects the relative experience of the National Committees.
Advice has been offered to competent authorities and AWB on a range of topics including, for example, training, annual seminars for AWB members, web-sites with information on and links to Three Rs resources, breeding and managing of surplus genetically altered animals, recognition, prevention and management of pain and on approval of statutory training programmes.
Are there areas of difficulty being experienced? How can these be improved?
One suggestion was made that advice developed by National Committees in different Member States should be endorsed at EU level to strengthen its role.
However, more experience is required with the new structures before informed views can be drawn.
User responses
|
Has the National Committee (Article 49) been effective in reaching out to the Animal Welfare Bodies in your establishment to facilitate their role and provide advice on matters dealing with the acquisition, breeding, accommodation, care and use of animals, and ensure sharing of best practice?
|
|

|
Although many are still in the early stages of development, progress is being made on communication with AWB, for example a web-based platform to share material and information, and training/information days have been held in a number of Member States.
It is important that all National Committee members are knowledgeable of their role, and as necessary, receive appropriate training.
AWB Regional Hubs developed through National Committee in UK was mentioned as positive, as it facilitates information exchange and identification of good practice.
Improved sharing of information and best practice is requested, however, many National Committees are still evolving. Suggestions were made for a central, easily accessible repository for this purpose.
Stakeholder responses
|
National Committees have helped establishments to improve the implementation of the Three Rs and animal welfare in my country/region.
|
|
All stakeholders
|
|

|
|
Other stakeholder organisations
|
Animal protection organisations
|
|

|

|
There was general agreement among the stakeholder organisations that at present it is too early to form a view on the impact of the National Committees. Animal protection organisations were concerned over the lack of transparency on the role, function and activities of National Committees. Lack of involvement of animal protection organisations was a common concern.
·"Eurogroup surveyed the 28 MS in 2015. While some NCs are active and having an effect, many are not yet fulfilling their required functions, and others have not yet even been formed. Some NCs lack the expertise required, and there is often little link or liaison with AWBs and a lack of transparency or involvement of stakeholders."
·"As a national organization it is hard to answer that question because we don't know it."
·"Efficient National Committees are rarely seen. In most cases, countries nominate them just "on the paper" but have little or no activity."
·"Not all countries have had the opportunity to build up their experience yet - this may still take a few years but in the meantime a systemic dialogue between the NCs may help to create common understanding common practice which should help overall implementation and harmonisation "
·"In several countries clarity of roles and responsibilities of national committees is still to be achieved."
Recommendations
ØMember States should facilitate and resource National Committees where this is not yet established, or where it is not fully functional, to ensure that its role and tasks are fulfilled as these tasks play key roles in the attainment of the overall objectives of the Directive.
ØIn preparation for the EU implementation report under Article 57, Member States should consider whether and how National Committees are:
2.3 Training and education and requirements for personnel (Articles 23 and 24)
2.3.1 Education, training and competence requirements on staff
Animal welfare can be significantly improved when staff dealing with animals are well-trained and competent. In its Article 23, the Directive requires that staff carrying out procedures on animals, caring for, and killing animals are adequately educated and trained, and provided with supervision until competence has been attained and demonstrated.
The agreed EU Education and Training Framework promotes “learning outcome”-focused, modular-based training to facilitate tailor made provision of training to meet the specific needs and existing skill/knowledge set of the trainees.
There was previously much variability in the training required before animal procedures were undertaken. Where formal high quality training was not previously implemented there were significant benefits reported to animal welfare and design of experiments following the introduction of improved measures of training and supervision. Some have reported that better training has led to better welfare, better recognition of pain and better understanding of animal behaviours and needs under different circumstances.
Some AWBs have also contributed to this improvement. The requirement of the Directive for an establishment to have a person responsible for training and competence (Article 24) has brought this issue to the fore. In some establishments, this person is a member of the AWB. Generally, the oversight of the person for training and competence was valued, but it seemed from some user responses that such a person had yet to have been appointed or to have had an overt, visible role. Feedback from Member States on inspections indicated that common education and training practices and competence assessment are still under development, but the Article 24 training person was considered to be helpful.
However, many organisations with a specific interest in alternatives as well as welfare organisations stated that training in non-animal alternatives, and in searching for them was not as good as it could be.
Many users remain unaware of the EU Guidance and other guidance documents available from the Member States, or the respective National Committee. There is clearly room for improved communication of these guidance documents.
Member State responses
The Directive requires competence in those persons performing a number of functions (Article 23). How are the competence requirements ensured in the Member State (authorisation/other means)?
|
Authorisation
|
19/28
|
|
Other means
|
9/28
|
The majority of Member States have a formal authorisation process in place, with defined educational requirements, in others the responsibility for ensuring appropriate education and training lies with the establishment.
User responses
|
Are there any difficulties in ensuring and maintaining competence in these staff?
|
|

|
The majority view from the users (63%) was that ensuring and maintaining competence of staff was being satisfactorily addressed.
However, the difficulties encountered by others included
·Availability (including timeliness, language) of species specific training courses (amphibia, farm animals, fish, birds, working on animals in the wild), including in particular, the less commonly used species; cost of appropriate training. A catalogue of available training courses would be very helpful
·Training in practical methods using non-human primates is prohibited in some Member States – N.B. this can be achieved when done under supervision and using procedures required as part of another authorised scientific project (not for educational or training purposes).
·Training requirements in some Member States are not clear for users.
·Difficulties in maintaining competences when people do not perform a procedure for a long time; no clarification of ongoing training (Continued Professional Development) requirements; ensuring that staff working outside the establishment (e.g. work in the wild) maintain and demonstrate competence; fixed frequency of retraining is providing problems for some (implied that this has little/no benefit).
·Maintaining sufficient competent staff.
·Difficulties in ensuring competence in killing of some animals e.g. wild animals which are not killed as part of the procedure; assessing competence of unusual / complex procedures not done by others.
·Separation of training by function has provided difficulties for some; in one case, formal training for animal care staff is considered to be less organised and not guaranteed.
·Training required for persons coming from outside the EU, and lack of recognition for 'on the job' learning.
·Difficulties in compelling researchers to change ways of thinking and practices which have been in use for many years
·Time resource of trainers and person responsible for training and competence.
·The position and the legal responsibilities of the person responsible for training and competence is reported to be not always as clear as it could be.
|
Have any changes in attitudes toward animals been noted as a result of the increased focus on competence?
|
|

|
A marginal positive response to this question by users.
Examples of good practice included:
·“Establishing a culture of care within the organisation.”
·“An ethos of continuous improvement has been started.”
·“Better training has led to better welfare, better recognition of pain, better understanding of animal behaviours and requirements under different circumstances”
·“Working towards better definitions and application of endpoints.”
2.3.2 Other named, responsible persons in Articles 24 and 27
There has been a requirement for establishments to appoint other named persons, including a Designated Veterinarian (Article 27). Whilst this position was already established in several Member States, in others, users reported significant improvements due to the formal appointment of this role. The Designated Veterinarian's input to AWB discussions was considered valuable, and a number of Member States mandate the Designated Veterinarian as a permanent member of the AWB. The Designated Veterinarians had a significant impact in developing the training for and, assisting in the training of, other staff.
There seems to be some difficulty with the recognition and implementation of the role for the person responsible for information (Article 24(1)(b)), in particular with their input on scientific considerations of animal models and their use. This role was rarely mentioned suggesting little impact, but the need for co-ordinated and focussed information dissemination of many types (not just information on species) was considered essential by many users, animal protection organisations and those with a specific interest in alternatives. Development of this role including further guidance for it may be of value to contribute to the improvement in availability of relevant, up to date information promoting replacement, refinement and reduction, as well as species specific information.
Member State responses
Are the persons identified in Article 24 (persons responsible for overseeing welfare, ensuring access to information, and education and training) being effective in their roles? Are they contributing to the implementation of the Three Rs?
|
Yes
|
15/28
|
|
No
|
-/28
|
|
No opinion
|
1/28
|
|
Too early to assess
|
12/28
|
Generally positive response, but too early to draw firm conclusions.
Introduction of these key responsibilities seems to have prompted improved communication within establishments, among scientists, care staff and the AWB.
Concerning the person overseeing welfare and care of animals, in many cases such an individual was already in place previously thus no great change had been noted. When new, their co-ordination of technical input to refinement is considered in particular to be valuable.
· “Training officer role has had a massive impact on refinement in terms of ensuring competency in the performance of procedures, therefore reducing suffering.”
The person responsible for provision of information seems to have had an impact on improving communication within establishments. However, there is some confusion over this role, in particular with input on scientific considerations.
·“They are effective in their roles and contributing to the implementation of the Three Rs by e.g. giving advice to the staff on matters related to the welfare of animals, preparing the animal experiment rules of the institution, controlling the implementation of the animal experiment rules of the institution, organising the education and training of the personnel, approving the experiments prior to project evaluation, etc.”
·“Staff devised and tested a novel way of obtaining saliva samples from pigs which removed the need for restraint and which was easy to train the pigs to use. It has been scientifically validated and replaced the need for some authorised procedures. In another establishment, A24 staff set up a series of seminars and organised a 3Rs day for staff to present posters of 3Rs improvements. The staff are embedding the refinements identified and the event has been made an annual event.”
Additional guidance has been developed by some Member States to facilitate these roles.
User responses
|
Are the persons identified in Article 24 (persons responsible for overseeing welfare, ensuring access to information, and education, training and competence) being effective in their roles and contributing to the implementation of the Three Rs and improved animal welfare?
|
|

|
81% of user responses indicated that the persons identified in Article 24 are being effective.
Of those who did not think that these persons were effective, some stated that the systems were already in place before the new Directive, and others stated that they needed more training in aspects of science, or Three Rs and animal welfare.
·Resources including Handbooks for Article 24 persons and others have been produced in some Member States by a number of organisations (e.g. LASA, RSPCA, UFAW, NC3Rs, IAT).
·Advanced training programmes are in place in some Member States for the specialisation of veterinarians in laboratory animal science, and for the role of animal welfare officer. Training courses are also available for persons responsible for information.
There seems to be some misunderstanding or lack of knowledge of the role and responsibilities of the Article 24 person.
Examples of good practice:
·Improvements in specific techniques and husbandry and care described
·Better definition / monitoring / application of welfare endpoints
·"Introduction of training for all staff in their specific roles / Three Rs / animal welfare"
·"The organisation has prioritized funding for training of the staff."
·"Participation in all educational courses by the person responsible for overseeing welfare and care, which leads to a good contact with new scientists and the care staff, reducing any reluctance to ask for help and support regarding animal welfare."
·"Regular meetings for staff with the aim to improve knowledge and to give ongoing education"
·"Early discussion of experimental design" and "Involvement of Article 24 persons in construction of SOPs, and including welfare / husbandry details"
·"Creation of internal website where all information on SOPs, best practices etc. can be found."
·"Newsletters are distributed regularly"
·"Implementation of Continuous Professional Development plan, in some cases including specific courses annually"
·"Development of web based training materials"
Recommendations
ØWhere not yet available, Member States should publish minimum requirements for education and training, and for obtaining, demonstrating and maintaining competence, and increase efforts to disseminate EU and other guidance on education and training to scientific users to indicate their expectations for trained and competent staff. National guidance should be shared with the relevant stakeholder organisations and other Member States.
ØMember States should ensure clarity of Article 24 roles, in particular those of the training and information persons, to ensure effective implementation and also to increase awareness of their role and the support they can provide within establishments.
2.4 Reuse (Article 16)
Article 16 of the Directive lays out the condition for reuse of animals. Under specified conditions relating to the severity experienced by an animal in previous procedures, reuse may be permitted.
It is too early to determine whether there has been any significant impact as a consequence of these new requirements. Also, the baseline for statistical reporting has changed and thus a detection of change in numbers is not possible at present.
Member State responses
Clarification was requested from Member States on the impacts of cumulative severity or reduction of animal numbers in connection with the reuse.
In many Member States, reuse was permissible under earlier legislation, but the new Directive sets out new obligations. However, there is yet too little experience to determine whether there has been any significant impact as a consequence of these new requirements.
Although reuse often reflects a sensible use of animals, for example following mild procedures with little effect on the animals, a number of comments received called for a considered balance of reduction and refinement i.e. use more animals of lower severity versus higher welfare harms to individuals undergoing reuse.
Reuse is more common in routine production of, for example, blood products.
In response to whether difficulties had been encountered with the provisions of Article 16, Member States requested additional practical examples on reuse/continued use and clarification on reuse of surgically prepared animals. Also, additional guidance was requested on the assessment of “cumulative” severity.
Other reported issues concerned clarity of “exceptional circumstances” in Article 16(2) – this is considered to cause unnecessary restriction on reuse.
User responses
|
Have the new controls over reuse provided the correct balance between individual animal welfare and a reduction in animal numbers used?
|
|

|
The highest response (41%) was from users who commented that their animals were always killed at the end of study to obtain tissues for analysis and reuse was not therefore a consideration.
Of the remaining responses, 20% were of the view that the new controls provided a reasonable balance, 11% disagreed and 15% thought it was too early to give a view.
As with Member State responses, further clarity on reuse/continued use was requested. In particular, use/reuse/continued use of genetically altered animals should be made clearer. Difficulties were encountered with genetically altered animals that had gone through invasive genotyping as such animals could no longer be reused in “severe” procedures. Other similar examples included, a single blood sample from dog or non-human primate would preclude use later in a severe procedure.
Assessment of severity in long term use/reuse of telemetry animals was considered challenging.
Recommendations
ØThe Commission services and Member States should develop additional guidance on reuse.
2.5 Avoidance of unnecessary duplication (Article 46 and Annex VI)
The project evaluation and authorisation processes are the main methods of control imposed by Member States to ensure compliance with Article 46 and Annex VI which lays a requirement to avoid unnecessary duplication.
Member States control this either by requesting information on actions taken to avoid unnecessary duplication or by legal declarations. A single authority, responsible for project evaluation for the entire Member State, has a good overview and is better positioned to detect unnecessary duplication than when there are multiple authorities.
Improved communication, coordination and dissemination of information from key regulators is requested when new data become available – e.g. European Medicines Agency (EMA), European Chemicals Agency (ECHA). It is important that all efforts are made by users to actively access and consider current, already available information.
Prompt publication and updating of non-technical project summaries, and a central searchable EU tool to access these would improve availability of information on authorised projects. However, there may be language issues to overcome.
Member State responses
· “Improved publication of all procedures using animals (also consider publication of Retrospective Assessments and Project Evaluations.”
·“Searchable European database of all NTSs – would increase awareness and further improve transparency”
·“National database of authorised animal studies.”
User responses
Across all sectors, the quality of experimental design and execution is seen as essential to produce good results first time, and some AWB help scientists to achieve this; for example working to Good Manufacturing Practice (GMP) or Good Laboratory Practice (GLP), Standard Operating Procedures, meticulous record keeping.
Some use internal databases to check for duplication. Some use EU wide databases and one included patent review. Others were clearly unaware of these resources.
It is important to remember that there are cases where duplication is legitimate:
·“Duplication of published work may make sense if 1) those results conflict own in vitro findings, 2) the experimental conditions in vivo are insufficiently revealed 3) the study appears dubious for other reasons. Publishers should demand a thorough description of the animal experiments to be published.”
Some aspects are dealt with differently in different sectors:
Academic – requirement for extensive literature review around their specific field was reported by many as key to avoiding duplication. The project submission should document the novelty / scientific innovation of the work. In some Member States, applicants must submit statements that there is no pre-existing duplicated research. In some cases, proposals are evaluated by specialists, so reducing the risk of duplication of procedures. In other cases, specialists are not available.
Some were of the view that there may be duplication in genetically altered animal production and breeding, but there is also evidence of good practice, such as sharing of lines, as well as significant sharing of tissues to avoid duplication.
Commercial – tests are carried out to satisfy regulatory requirements such as those in the European Pharmacopoeia, in some cases including batch testing (necessary duplication). Seeking regulatory and expert advice was seen by some as important in this area including communication with sponsors and specific regulatory authorities. Monitoring regulatory developments was reported as important. Some search for commercially available sources of product before undertaking animal use.
Comments
·“We're a Contract Research Organisation (CRO), so we do what our clients order from us. We do not know which drug candidates they're developing, so if two clients would have the same drug candidate in testing, we would not know that. However, this seems highly unlikely.”
·“We need to trust in our customer, that a planned project was indeed not performed previously, since we are unable to review their data, if they are not already published.”
Good practice
·“Regulatory authorities may request a repeat of in-vivo studies. In these circumstances, we always request formal documentation from the client as to why a repeat study is required.”
·“Weekly exchange of information between MSs (Official Medical Control Laboratories - OMCLs) on future/intended testing (e.g. Official Control Authority Batch Release) and acceptance of results of testing performed by OMCL of other MS”
Problems included
·“Projects authorised in one MS are not recognised in other MS which is an obstacle to free exchange in EU environment. This situation unfortunately drives additionally to avoidable duplication of procedures”
Some clarification is needed to differentiate duplication (the same study design to answer the same scientific aim) and replication (the same study design to test the reproducibility of results) and the reasons for batch testing as part of the regulatory process.
A suggestion was that non-technical project summaries should be made available across all Member States in a searchable database (see more in section 3.1).
Recommendations
ØMember States should re-enforce the awareness of the need for researchers and project evaluators to ensure that no unnecessary duplication takes place, in particular, in the development of new genetically altered animal lines.
2.6 Setting free and rehoming (Article 19)
The provisions of Article 19 allow animals used or intended for use to be rehomed, or returned to a suitable husbandry system or to be set free to a suitable habitat subject to health and welfare safeguards.
Rehoming is new to many Member States, with establishments now aware of the possibility that animals may be rehomed at the end of procedures. Around 50% of Member States have changed policies on rehoming due to changes in the Directive.
Although many Member States have alerted establishments to this practice, it seems that only very few animals are affected. There are little quantitative data on numbers and there is no legal obligation for collection or collation of numbers of animals set free or rehomed.
Users reported that rehoming is not suitable for the majority of animals and species used in procedures as, for example tissues/samples are required on completion of studies.
Although additional guidance was requested by some respondents, some guidance has already been prepared by Member States and interested organisations.
Member State responses
Did the policy on re-homing in your Member State change with the new Directive (e.g. not done previously, now actively promoted?
|
Yes
|
14/28
|
|
No
|
7/28
|
|
Not applicable
|
7/28
|
Have the number and type of animals being rehomed changed with the introduction of the Directive?
|
Yes
|
7/28
|
|
No
|
9/28
|
|
No opinion/NA
|
12/28
|
Rehoming was reported for only a few dogs and even fewer rabbits.
A number of Member States indicated that farm animals were being set free or "rehomed" where such animals were being returned to farms for agricultural practices. The title of the Directive provision is confusing for some users and stakeholders.
User responses
|
Has the Directive resulted in a new or amended policy on rehoming in your establishment?
|
|

|
|
Has the new / amended policy been effective in facilitating suitable animals to be rehomed?
|
|

|
The user responses indicate that very few have been affected by the changes to the legislation regarding rehoming. There are also difficulties reported in identifying “homes” or places for adoption, and long term costs can be an issue e.g. non-human primates. Additional guidance was requested.
Responses varied as many clearly had existing policies which either have not significantly changed or been made clearer, although, in a few cases reported as being apparently more difficult. Where no policy existed previously there were reports of some increased numbers being rehomed, mostly for larger species with many stating that rehoming rats and mice was difficult. For many others rehoming is not appropriate because of the nature of the science, and the need for tissues at the end of the procedure, or legal constraints e.g. legislation on genetically modified organisms.
Recommendations
ØWhere appropriate, Member States should share relevant guidance material on rehoming, as well as make use of guidance developed by other Member States/stakeholder organisations.
2.7 Sharing organs and tissues (Article 18)
Sharing tissues should reduce the numbers of animals used and therefore have welfare impacts by the reduced use of animals. Article 18 of the Directive calls for Member States to facilitate, establishment of programmes for the sharing of organs and tissues of animals killed.
For some users, tissue sharing has been available for a long time, e.g. EUPRIM-Net, AniMatch, ShARM, European Xenopus Resource Centre. These initiatives which have been set up across Europe for the sharing of tissues, appear not to be sufficiently well known throughout the scientific community.
In some Member States, a legislative requirement is included that requires establishments set up a tissue sharing framework and its impact is assessed during Inspections. Few systems are coordinated by Member States, but one Member State reported that it has a National Telematics Data Bank. National Three R centres are taking an interest in this issue.
Users responded that the requirements in the Directive have heightened the need and importance to do this to make best use of animals and reduce numbers. AWB in some establishments have taken the lead to develop an effective communication strategy, and exchanges between establishments are also in place.
Many who used tissue sharing stated that this had reduced the numbers of animals used overall.
Concerns were raised by some over the difficulties in moving tissues between Member States (due to health and safety issues).
Member State responses
Few systems are coordinated by Member States, but the practise is encouraged and promoted at establishments.
Included in legislative requirement in some Member States is a requirement for establishments to institute a tissue sharing framework and its impact is assessed during Inspections.
Have these measures been effective in reducing the number of animals needed to meet demands for tissues and organs?
|
Yes
|
5/28
|
|
No
|
-/28
|
|
No opinion/NA
|
10/28
|
|
Too early to assess
|
9/28
|
|
No answers
|
4/28
|
It is too early to draw any conclusions on the impact on numbers but some indications that active funding to promote measures at national or EU level would be helpful.
Good practice was noted requiring tissue users in establishments to confirm to internal AWB that efforts have been made to obtain tissues by sharing.
User responses
What measures have been taken at establishment level to promote the sharing of organs and tissues? Have these measures been effective in reducing the number of animals needed to meet demands for tissues and organs?
Tissue sharing was an established practice in some establishments before the new Directive requirements, generally for the larger species, for example dogs, non-human primates and farm species, driven primarily by cost.
The requirements in the Directive have heightened the need and importance to do this to make best use of animals and reduce numbers. AWB in some establishments have taken the lead to develop an effective communication strategy.
Good practice
·Good communication between researchers and animal care staff within the establishment is reported as essential. Suggestions and recommendations may be made by the AWB.
·Including a question on sharing of tissues as a standard question in the submission for ethical consent approval is required by at least one establishment.
·Cryopreservation of well characterised tissues including tumours was reported.
·"Announcing planned animal killing, in one establishment by an internal calendar assists planning."
·"The creation of a common experimental histology facility where the person in charge can centralize samples and co-ordinate the needs of different users."
·"Central management for Xenopus and Zebrafish embryo production allows reduction in the numbers"
·"Including as part of the routine health monitoring and pathology program. Various tissues are sampled and in some cases stored frozen and available free of charge to the scientific community, internally including geographically remote sites and externally in some cases."
·"After some surgery courses on animals, organs are collected for other use, e.g. eyes, skin and gut for ex vivo surgical training, or basic research."
Less good practice
·Information on the availability of genetically altered animal lines is very variable between researchers.
·"The researchers work too much alone / too individualistically to share"
·"There still remains some fear from researchers about sharing information about their work"
Difficulties
·"Tissues are not always prepared in the same / an appropriate way."
·"Tissues deteriorate rapidly without appropriate collection, preservation and storage."
·"Some authorisations seem to require specific statements of sharing, or other legislation maybe limiting sharing e.g. transport or safety legislation."
·"Complex bureaucratic procedures for the transport of organs and tissues, including import / export (health/safety requirements)."
·"Sharing is not always possible due to use of infective agents or radioactivity, or different health status of animals affecting relevant tissues."
·"Tissues are not always available at the right time."
·"Appropriate communication outside establishment is not in place."
·"Costs of running sharing schemes."
2.8 Use of existing alternative approaches and implementation of the Three Rs
2.8.1 The role of project evaluation in the use of alternative approaches and implementation of the Three Rs
With regard to the Three Rs and animal welfare, half of the user respondents felt that the introduction of project evaluation, with advice provided during the evaluation, had had a positive impact on animal welfare. Examples included the improved use of analgesia, more clearly defined endpoints, and more refined protocols (reduced harm to animals). The use of literature searches including Three Rs elements were reported to be more overt, and included the identification and subsequent application of various specific refinement guidelines.
Indirect improvement to animal welfare included a reduction in numbers used, due to better project designs or by replacement of animals using alternative methods. Several commented that the Directive had created an increased focus on the Three Rs, and improved justification for the procedures used, which is suggestive that there could be a reduction in animal use realised in due course. For many users, similar processes were in place before the Directive, and so no significant changes or improvements have been seen since implementation.
A number of aspects of the new Directive are aimed specifically at improving animal welfare and requiring effective implementation of the Three Rs in the use, care and breeding animals for scientific purposes. Guidance developed at EU for Severity Assessment Framework has been especially useful in promoting the concept of continuous refinement; the consideration of severity and the ways in which it can be minimised within the initial study design, through the study-specific day-to-day monitoring of animals during the project, to the “actual” severity assessment upon completion of the study. A number of successful workshops on promoting consistent interpretation and application of the severity framework have been in held, coordinated by FELASA, with the support of Member State authorities, involving scientists, veterinarians, care staff, regulators and inspectors.
There is a positive response among users, Member States and scientific stakeholder groups that the Directive has improved the application of the Three Rs, in particular in the area of project evaluation and authorisation. The level of impact varies among Member States but the positive impact is greater in those countries which previously did not have a project evaluation and authorisation processes in place.
In contrast, animal protection organisations generally disagree that the increased focus on the Three Rs and project evaluation processes benefitted animal welfare.
Member State responses
Many elements within the Directive require consideration of the Three Rs. Have these been effective?
|
Yes
|
17/28
|
|
No
|
1/28
|
|
No opinion
|
-/28
|
|
Too early to assess
|
10/28
|
Has the requirement to comply with the Three Rs had an impact on animal procedures? Has it improved the design of procedures and/or projects? Has it improved animal welfare?
|
Yes
|
18/28
|
|
No
|
2/28
|
|
No opinion
|
2/28
|
|
Too early to assess
|
6/28
|
The main positives indicated by Member State submissions include the improved designs of projects, more careful consideration and application of the Three Rs, obligatory independent evaluation process, severity classification and implementation of humane end-points and contributions by AWB at each establishment.
·"Although too early to assess, the establishment of animal welfare bodies is foreseen to have a positive impact on the general awareness of the 3Rs. In addition, the requirement of the non-technical summaries highlights the need to apply to the 3Rs."
·“There is a need for improved communication, coordination and dissemination of information - key regulators should better publicise when alternatives accepted – e.g. EMA ; ECVAM “
User responses
|
Has there been an impact from the introduction of the requirement for systematic project evaluation on animal welfare in your establishment?
|
|

|
Of those who answered, the impact of the project evaluation and authorisation processes in France seems to have been greater than in other countries.
|
|
All users
|
France
|
Rest of EU
|
|
|
No
|
%
|
No
|
%
|
No
|
%
|
|
No
|
207
|
25%
|
67
|
22%
|
140
|
26%
|
|
Yes
|
417
|
50%
|
189
|
63%
|
228
|
43%
|
|
Too early
|
117
|
14%
|
30
|
10%
|
87
|
16%
|
|
No opinion/NA
|
92
|
11%
|
14
|
5%
|
78
|
15%
|
|
Total responses
|
833
|
100%
|
300
|
100%
|
533
|
100%
|
The impact can be interpreted as positive in terms of improved experimental design and consideration of Three Rs or negative as increased bureaucratic burden. Analysis of responses relies on the comments.
Project evaluation has had a greater impact in Member States which did not have a requirement previously.
Many who stated that the Directive had no impact judged that the previous legislation in place had already applied the Three Rs, unlike some who stated that it had served to reinforce this.
Of those suggesting that the Directive had had an effect on the Three Rs, some considered that the requirement for project evaluation with better definition of expected effects and endpoints, along with discussion of these issues with others (animal care staff, AWB, project evaluators) had refined procedures. Several felt there was better use of analgesia, or better controls over reuse. Better definition of what the AWB should achieve through the additional EU guidance was thought to have assisted.
For some, there had been an improvement in animal housing conditions including enrichment, group housing and the use of positive reinforcement.
Others felt that there was better experimental design as a result of the project evaluation process, including consultation with a statistician and consideration of appropriate control groups.
Some have been able to introduce alternative methods and some respondents stated that there has been increased sharing of tissues.
Many suggested that better education of staff with assurance of competence is improving the Three Rs. The requirement for a Designated Veterinarian contributed to reinforcement of good practices in surgery, anaesthesia, analgesia and euthanasia.
There are also external drivers and constraints on the application of the Three Rs. Several respondents stated that Three Rs were not possible to apply as there was a regulatory requirement for the testing performed in a particular way. There were also a few comments that publications require in vivo models or numbers of animals which were higher than those determined locally.
Comments
·"The requirement of a project authorization encourages researchers to think about the design of their experiments: the reflection on the number of animals and the statistical approach upstream."
·"Our national authorities issued in 2014 a new template for project evaluation, in which more attention is paid to 3Rs. At the same time, the ethical committees were expanded to a minimum of 7 members (with at least two external members), with expertise or competence in ethics, alternative methods, animal health, animal welfare, research techniques, study design and statistical analysis. This led to a more balanced review."
·"Systematic project evaluation was already in place prior to the directive, and the three R’s were already used at the basis of the project evaluation. What has improved over the years is that the forms that were used for the project evaluation have evolved to pay more explicit attention to the three R’s. Also, the improved focus on correct statistics has had its impact, even though this has not always led to a reduction in the number of animals."
·"An important administrative change in a country where the project authorization via an ethical committee was not in the law but on a voluntary base. The project authorization was very positive for research studies. It has obliged us to better define the study design and limit some experiments."
·"Project evaluation has led to the development of user groups and the sharing of good/best practice. This has led to significant refinements in some cases, better record keeping and monitoring regimes and also shared resources."
·"We don't see an improvement due to the requirement for project evaluation compared to our SOPs beforehand."
·"Much of the guidance is directed at rodents, with little information on the less common species such as farm animals and fish. There are challenges in dealing with unusual species."
·"Additional guidance requested on conflict between reduction and refinement regarding severity and severity thresholds including GA phenotypes."
·"The introduction of these requirements has increased the time necessary to the preparation of protocols but had the merit of ensuring a much deeper reflection of many technical aspects. More internal debate to find better solutions to improve the quality of the project."
·"Better statistical evaluation of the minimum animal number required for the studyBetter attention to animal care and to reduce animal suffering"
·"Because of systematic project evaluation, researchers need to design the entire projects before the beginning of the projects. Consequently, the number of animals can be optimized and reduced and attention can be paid to animals used in several procedures."
Stakeholder organisations
|
Systematic project evaluation and project authorisation has improved the implementation of the Three Rs and welfare of animal in my country/region.
|
|
All stakeholders
|
|

|
|
Other stakeholder organisations
|
Animal protection organisations
|
|

|

|
Other stakeholder comments
Scientific stakeholder groups acknowledge the need for robust processes to ensure effective application of Three Rs within a project. Impact varies significantly dependent on previous Member State legislation.
·"Project authorisation, where not in place previously, is expected to have a positive impact on the implementation of the 3Rs. The requirements to explain more clearly the harms to animals and information on the 3Rs in applications have already had an impact on planning and executing studies."
·"Systematic Project evaluation and authorization process led the Licence Holders to revise the way to present and explain their project and the experimental protocols, increasing the awareness that the use of animals for experimental purposes is allowed only if no other alternatives are available"
·"This is too early to say how much is improved in practice. The awareness of Refinement and animal welfare has increased a lot- this is very positive thing. No real effect seen in projects on implementation of replacement methods to decrease animal tests".
·"The requirements to explain more clearly the harms to animals and information on the 3Rs complemented with the individual animal severity assessment have already had an impact on planning and executing studies and on consideration for animal welfare. However, the system needs to be worked out and time is required to settle down properly and not being considered as purely administrative burden."
Animal protection stakeholder comments
In contrast to the generally positive views of Member States, users and other stakeholder groups, the animal protection groups expressed significant concerns over the impact of project evaluation and authorisation on Three Rs implementation. The main concerns relate to the transparency of the process, and that all projects are authorised. One response suggested that in one Member State no harm-benefit was included within the national legislation.
·"We need better transparency of projects in order to make an informed opinion. We are concerned that a great number of severe experiments are still being licensed. Also, It is unclear how 3Rs are being considered and what priority is given to animal welfare."
·"Project evaluations tend to use the same arguments and hollow phrases to justify animal use from one application to another. Very little in-depth analysis is shown in any individual project to argument its case. Authorization is always granted."
·Related to one Member State "According to law the authorities don’t have the possibility to balance the harm-benefits. They can only check applications formally but not reject an experiment for ethical reasons. The result is that even most severe and frivolous experiments are being authorized."
·"There is a lack of transparency on the evaluation & authorisation process. It is generally unclear how 3Rs are being considered and what priority is given to animal welfare."
·"All the projects get authorized which reveals the system lacking real judgement as the outcome is already set in the beginning."
2.8.2 Use of existing alternative approaches
The term ‘alternatives’ in this context includes all assays, tests, methods, techniques, tools, strategies and approaches etc. that contribute to the practical implementation of the Three Rs, that is to
·obtain the required information without the use of live animals;
·use fewer animals whilst obtaining the same level of information;
·improve the way procedures are carried out so as to cause less pain, distress or suffering, or improve the welfare of the animals
The Directive makes a firm legal obligation on scientists and establishments that animal procedures may only be carried out when there are no non-animal methods available to achieve the scientific objective. Alternative approaches are promoted in a number of provisions in the Directive, for example Articles 4, 13, 27, 38 and 49, promoting focus on the application of the Three Rs at project, establishment and national level. Whereas Article 13 makes a specific obligation to use recognised alternatives in the regulatory testing context, Article 4 ensures that the legal obligation to use alternative approaches covers all areas of animal use.
In many Member States, specific information is requested during the project application process, for example information on searches, choice of model and design of study. These are reviewed by project evaluators. Declarations and conditions of authorisation are also used to confirm that the Three Rs will be subject to continuous review during the period of authorisation.
A few Member States highlighted the importance of inspection programmes to verify compliance with the Three Rs and the continued application of new alternatives throughout the life of the project (necessitating that inspectors are trained and current on developments in Three Rs and alternatives opportunities). As part of the project submission and evaluation process, the Directive requires that scientists consider and document whether their work can be done without the use of live animals, such as applicants checking alternatives’ websites prior to project application.
Of the scientists who responded, a third agreed that the Directive has increased the focus, activities and resources aimed at alternatives. Examples of where alternatives had been adopted included the use of cell lines as a part of many work programmes. Some establishments stated that they include training in cell culture methods for all scientists.
Animal protection organisations requested simple effective mechanisms to promote uptake and communication/dissemination of alternatives, in particular, in fields other than regulatory toxicology.
Member State responses
How do competent authorities ensure alternatives are used wherever possible (Article 4 and 13), including adaptation to technical progress during the life cycle of a project?
Member States have adopted a number of measures to ensure that alternatives are used where available and appropriate for the scientific study. The project evaluation and authorisation processes are considered the main pillars to ensure that alternatives are given due consideration. Although the applicant is expected to know the availability of alternatives, the evaluation process should engage sufficient expertise to confirm that indeed there are no alternatives possible within the proposed programme of work.
Authorised projects often explicitly state the requirement that alternatives be adopted as these became available.
AWB have oversight of the Three Rs within establishments and are expected to advise project authorisation holders of relevant new developments in alternatives.
Retrospective assessment is expected to provide a further opportunity to consider the Three Rs, but impact is too early to assess.
User responses
Users echoed the need for better communication, easier searching methods, and better availability of information on alternatives. The role of the information person (Article 24(1)(b)) is still evolving, but assistance in searching and keeping establishments up-to-date on alternative methods would help to fill a gap recognised in a number of user responses. AWB have oversight of the Three Rs within establishments and are expected to advise project authorisation holders of relevant new developments in alternatives (Article 27(d)). This can be achieved, for example, through individual communications, newsletters or Three R seminars. However, some AWB do not seem as yet to have developed information dissemination strategies within their establishment.
Many users were of the view that Member States had some obligation to inform users of developments in alternatives, in particular as project authorisation holders will be held responsible should these not be applied appropriately.
Many agreed that it was difficult to assess the impact of these activities to promote alternative strategies. There has been no apparent reduction in animal use to date, but it may take some time for reductions due to introduction of alternatives to become apparent. Some specific cases by responses from a few individual users contradicted this perception, identifying examples where significant reductions had been effected by the use of alternative methods.
Users indicated that studies on some aspects of biology such as conscience and vigilance states, reproduction, and developmental biology continue to need in vivo experimentation and alternative methods are unlikely to be available in the foreseeable future.
It is also the case at this time, that the European Pharmacopoeia / chemicals registrations / other regulations require the use of animal studies and therefore changes in other legislative areas are required before reliance on alternatives in these fields can be achieved.
|
The Directive has increased the focus, activities and resources aimed at the development, validation and uptake of alternative approaches.
|
|

|
30% of the user responses suggested a positive impact of the Directive on an increased focus on alternatives. 24% neither agreed nor disagreed, 10% disagreed and around 34% had either no opinion or of the view that it was too early to tell. The Directive is one factor driving alternatives, but scientific, ethical and economic factors are also important elements. Scientists want to do the best science and only use animals when necessary. In some research fields there have been more advances than in others, but there will be a continued need for animals for some years to come. Regulatory requirements still demand animal testing, and alternative validations can take a long time.
Some requested an easier searching method, better availability of information on alternatives and experts to help find them relevant to the field of work. The mandatory training now required will improve knowledge on alternative strategies.
·“Many experiments are now made on cell lines, and some establishments include training in cell culture methods for all scientists.”
·“There have been development and validation (in some cases) of non-invasive methods in ecotoxicology, development of PCR methods in replacement, 3D co-cultures for toxicity studies.”
·“Teaching animals are supplemented and, if possible, replaced by simulator-based training”
·“Project applicants encouraged to check alternatives website prior to AWB review.”
·“There is much evidence of alternative development within ECVAM of the number of methods in validation phase.”
Obstacles to using alternatives included
·Lack of knowledge/awareness of alternatives.
·“Scientist who work with non-animal methods do not get in contact with the animal facility. Also, knowledge about non-animal methods is lacking in staff advising scientists using animal models.”
·“Generally, there are not much alternative approaches available in our field of work(eg systematic neuroscience).”
·“Our experiments are regulated by the European Pharmacopoeia.”
· “The use of animals is most of the time more expensive than alternative methodologies. And using animals when it can be done other way is distasteful. Economic forces and ethical considerations were driving the development of alternative approaches before the new Directive.”
·“The Directive has further increased the focus on 3Rs, which were however already embedded in our institute's culture. A practical problem is the fact that most resources or databases are for regulatory testing or educational purposes. There is little available for more fundamental, basic research.”
Stakeholder responses
|
The Directive has increased the focus, activities and resources aimed at the development, validation and update of alternative approaches.
|
|
All stakeholders
|
|

|
|
Other stakeholder organisations
|
Animal protection organisations
|
|

|
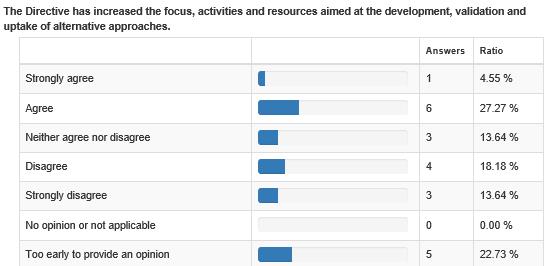
|
As many organisations agree as disagree on the impact of the Directive on alternatives.
A higher proportion of animal protection organisations are of the view that the Directive has increased the focus on alternatives.
There should be improved communication on acceptance of alternatives, ensuring that establishments are informed.
It was felt that there was significant scope for the replacement of animals used for education and training purposes, with many alternatives available, but these do not seem always to be taken up. Reasons for this were similar to those given for alternatives in other areas, but it was felt that solutions, especially in the area of education, might be more readily available than in other fields, and that a themed review, and specific support/guidance, and/or targeted inspection could be beneficial.
Animal protection stakeholder comments
·"The increase in non-animal technologies has not translated into a reduction in animal use."
·"MS are not supporting the development of alternatives (Article 47). ECVAM and the Commission and are not validating and implementing alternatives in a timely manner."
·"Profile of 3Rs has increased in EU, but this is not leading to substantial enough changes in practices, impacts or a fast take up of new approaches. It is unfortunate that Art. 13 only prohibits animal use when an alternative method 'is recognised under the legislation of the Union’ (only likely to apply to reg. toxicology). There is little evidence EURL ECVAM is more effective/ better funded now than under Dir. 86/609/EEC."
·"More needs to be done in general to make sure the legal obligation to use alternatives is implemented and to shift the emphasis from using animal models as the gold standard against which alternatives have to be validated."
·"The competent authority should notify the scientific community of the acceptance of the alternative methods"
Other stakeholder comments
·"Alternatives to animal research was already on the focus within national legislations and regulations, but the Directive strengthens their position and contributed that the involved entities gained a higher attention and better funding opportunities."
·"The Directive per se has clearly increased focus on 3Rs, but with limitation to establishment / project level. Nevertheless, individual initiatives to develop and validate alternative approaches are too limited. Huge efforts are still needed in terms of scouting, supporting, funding, inter-connecting and valuing such initiatives. It is a multi-player challenge where EU and national authorities, 3Rs centres need to increase connections and communication, with the whole research community."
·"Centres for alternatives have been/are in place in many member states, but international validation of those is painfully slow and the focus on development, validation and uptake of alternative approaches seems not to have been implemented."
·"Few reports of new alternatives arising as a result of the Directive per se. Instead advances in alternatives have been driven by usual process of scientific curiosity and research"
·"Lack of resources aimed at development, validation and uptake of alternative approaches were reasons for those siting disagreement"
Responses from the organisations with a specific interest in alternatives
The responses from the organisations with a specific interest in alternative methods identified a number of key issues hindering the uptake of alternatives. These can be grouped into categories of lack of knowledge, poor communication/dissemination of information, acceptability, and cost.
Lack of knowledge / availability of information
There is scope for improvement of the knowledge and dissemination of what information there is available on non-animal and other alternative methods, and their applicability and validity, in order to improve the breadth of options for scientists to consider. A single searchable European-wide database with better training on its use and updating of resources to upskill searching strategies were indicated as desirable outcomes. There is evidence of this knowledge transfer occurring, but it needs to be extended, perhaps moving from a passive status e.g. by publishing on Three Rs websites to a much more active communication programme of for example, thematic reviews and following through by assisting in the implementation process, and development of training resources on alternatives applicable for various levels of knowledge from school through to field -specific science areas.
Acceptability
Issues of acceptability are a significant hurdle. This can be from scientists who prefer to use traditional and well accepted ways using animals to answer their scientific questions. There are also expectations of peers, reviewers and editors, who may be reluctant to accept alternative methods. If an alternative method becomes available it may be difficult to correlate historical data with the new method. Regulatory requirements will continue to require the use of animals, until specified alternatives or other strategies for obtaining the necessary information are validated and clearly applicable in that domain. Getting a new method validated and accepted across different regulatory authorities and geographical regions can be challenging, and fear of not having the alternative test accepted as valid in these cases forms a significant hurdle to their early adoption. It is important that all relevant stakeholders are involved in validation processes to ensure that the tests are broadly acceptable or that the limits to the applicability domains are clear.
Cost
The complexity of some of the alternative methods can require significant investment, both in equipment, training and in-house validation, and ready access to consultants with high levels of knowledge in these techniques is not good currently. There may be scope for reviewing existing resources and opportunities for networks to share in a more co-ordinated manner, especially in the education and training sector. There were several requests for the funding bodies to be sympathetic to the development of methods and to training in alternative methods, and indications that some such resources are already available.
Future Progress Required
Respondents indicated a significant role for AWB, project evaluators and competent authorities to challenge the necessity of the use of some animal models when alternatives exist. Whilst there is an onus on the scientists to show why the alternative method cannot be used, including in non-technical project summaries, there is scope for the alternative “developers” to define the uses, limitations and current status of acceptability, as is being done on the EURL ECVAM Tracking System for Alternative Test Methods (TSAR) database.
EURL ECVAM has a valuable role in co-ordinating validations and maintaining databases of information on existing alternatives. There is scope to extend these activities and to provide advice on these issues, and co-ordinate other work in these areas including that done by the national Three Rs centres. Several databases are available including DataBase service on Alternative Methods (DB-ALM) providing information on alternative methods and TSAR showing progress from proposal through validation through to adoption and inclusion in into the regulatory framework.
EURL ECVAM have also produced a user-friendly Search Guide to inform and support users to find high quality information on alternative methods and are investigating sharing opportunities to accelerate progress in Three Rs in basic research, toxicological testing and for education and training purposes.
The National Committees can have a role in collecting, collating and disseminating progress in the area of alternatives, and international collaboration would be valuable to increase global awareness
Three R developments in the use of non-human primates
More focus has been directed at the use of non-human primates under the Directive, and only where well-justified on scientific grounds and no other alternative exists, is the use of non-human primates authorised.
Drawing from the SCHEER Opinion and the responses received, some progress has been made in reducing non-human primate use, by, for example the development and validation of new methods for vaccine testing e.g. polio vaccine.
Responses indicated that although some progress has been made, there are still areas where the use of non-human primates remains necessary. The main areas identified were in regulatory safety testing, neuroscience and certain infectious and neurodegenerative diseases.
Member State responses
Use of NHPs Has the use of non-human primates increased or decreased under the new Directive?
|
Increased
|
1/28
|
|
No change
|
11/28
|
|
Decreased
|
2/28
|
|
Not applicable
|
15/28
|
Has there been any use of endangered non-human primates under the new Directive?
User responses
Can you provide any specific examples of areas where the use of non-human primates has been replaced by non-animal methods or by other species? What were the reasons for these changes? Was the choice of species influenced by the project evaluation process required by the Directive?
Examples of the responses included
·“The lab currently using NHPs is now also developing and evaluating imaging studies in humans as a replacement.”
·“In our institution we investigate the complexity of memory consolidation and networks in the brain. This can seldom be replaced with alternative methods.”
·“In non-preclinical experiments, NHP are replaced as much as we can by pigs. The choice of the pig was not influents by the process required by the directive, because this is our own consideration since a long time.”
·“In terms of interspecies in-vitro metabolism, when two non-rodent species are closer to humans, the primate is not selected for toxicological investigations. The minipig has been added to the list of non-rodent species to be tested in-vitro in order to select it for further in vivo experiments if required. The reasons behind this choice were not influenced by the project evaluation process, but more by ethical considerations and ease of implementation.”
·“A small a number of transgenic mouse models have been developed for candidate selection and regulatory toxicology.”
· “The use of non-human primates cannot be replaced by non-animal methods in cognitive neuroscience.”
·"Most if not all our activities are based on regulatory requirements (ICH guidelines) and no change has observed with the new Directive. Whenever non-human primates are necessary for scientific purposes (biological target or metabolism specificities), they are still used in the same way. More scrutiny is applied during project evaluation for the species selection."
·"NHP are used only when no other species are usable (justification based on metabolism data, pharmacological data, toxicological response which showed that other species are not enough close to Human response). In some cases, in regulatory toxicity development, NHP only is acceptable by the authorities. The use of not adequate species doesn't allow to be in accordance with regulatory requirements"
·"Non-human primates are mainly used to assess the safety of biotechnology derived drugs. No alternative currently exist to comply with international regulatory guidelines (ie ICH) asking data from the "most predictive animal species"
·“Use of NHPs is driven by complex questions of vaccine and therapeutic efficacy: thus animals are only used where there are no alternatives. We have for many years developed and refined in vitro methods such as continuous culture that serve as early screening models for bacterial latency and potential antibiotic treatments. However there is still a need to test novel therapies and vaccines in a relevant disease model prior to clinical trials. Choice of species is influenced by prior knowledge “
·“NHP's were used in neurophysiology research for many years and these studies are stepwise being replaced by studies in rodents. This change had started before the implementation of the directive.”
·“NHPs are only used in the absence of feasible alternatives, and sparsely. In our institute, this is restricted to the use for research on infectious diseases that are a threat to public health, including emerging diseases.”
Recommendations
ØContinue efforts to ensure promotion and sharing of alternative approaches and dissemination of information at national level.
ØDevelop a high-level strategy to encourage a shift of attitudes and priorities to make significant progress towards the implementation of non-animal methods.
ØEnsure training remains current in the field of alternatives and in the tools available to search for them.
ØEnsure that appropriate consideration is given to the use of alternatives in particular in the field of education, and that project evaluators are up-to-date in the advancement of alternatives in this field.
Users
ØAlways consider alternative methods and approaches, including thinking laterally to revise original hypotheses to try to replace animal use.
ØEnsure robust searches are carried out using all available, up-to-date resources to find potential alternatives in their field including in education and training. Demonstrate in the project applications why alternative methods available in the field will not suffice to fulfil the scientific objectives.
Organisations with specific interest in alternatives
ØDevelop online courses in alternative methods for specific areas of science, toxicology, and education and training.
ØDevelop search tools for alternatives, especially in the non-regulatory use of animals.
ØIn existing and new databases, alternatives should be accessible by discipline e.g. neuroscience; immunology etc.
ØDevelop improved communication and cooperation among relevant "alternative stakeholders" on the availability and the potential for sharing relevant high-quality teaching resources at costs which could be acceptable to trainers / trainees.
Training providers
ØEnsure, in cooperation with alternatives organisations, that training for scientists remains current and prioritises the importance of experimental design and implementation of the Three Rs.
Regulatory authorities
ØRegulatory agencies, in collaboration with Member State authorities and the user community, should consider how the dissemination of information on newly adopted alternative methods could be improved to reach all relevant players in a timely fashion.
2.9 Development, validation and regulatory acceptance of new alternative approaches
The Directive provides obligations to both the European Commission and Member States to contribute to the development and validation of alternatives asset out in Article 47. However, the type and nature of these contributions are not detailed in the Directive. Even if the validation or regulatory acceptance processes in different sectors are not in the direct remit of the Directive, it provides some general tools and infrastructures aimed at facilitating and accelerating the development, validation and promotion of alternatives. Furthermore, it requires the Member States to promote alternatives at national level, whilst the European Commission is required to promote acceptance and uptake at international level.
More precisely, Article 47 requires Member States to appoint laboratories for carrying out validation studies (European Union Network of Laboratories for the Validation of Alternative Methods - EU-NETVAL) and to nominate a single point of contact to provide advice on the regulatory relevance and suitability of new alternative approaches proposed for validation (Preliminary Assessment of Regulatory Relevance PARERE Network).
Article 48 created a legal basis for the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM), which coordinates the validation of alternative approaches for both regulatory testing and basic and applied research.
Animal protection organisations stated frustration at the lack of funding and slow progress towards validation and acceptance of alternative methods. However, responses indicated that there had been investment and activities to move this field forwards.
There were requests by the users that EURL ECVAM continue to broaden the remit from predominantly regulatory toxicology area to be more balanced across other science areas.
Structures and Processes Developed
The EURL ECVAM report (in Annex 2) describes the structures underlying the enlarged scope of EURL ECVAM. EU-NETVAL was set up as a laboratory network to support validation studies. Article 47(5) required a single point of contact to provide advice on the regulatory relevance and sustainability of alternative approaches proposed for validation. This network, the Preliminary Assessment of REgulatory Relevance (PARERE), is comprised of regulators nominated by Member States and representatives from EU regulatory agencies, with the aim of expediting regulatory acceptance of alternative methods. In line with Annex VII(e) the ECVAM Stakeholder Forum (ESTAF) was established to maintain dialogue with industrial and research organisations and society as a whole, with outcomes released on the EURL ECVAM website. Scientific expertise is provided by the ECVAM Scientific Advisory Committee (ESAC), which provides independent peer reviews of validation studies.
The work of these networks is crucial to facilitate smooth progress from initial method development, through validation into regulatory uptake. The networks have started to provide new resources, improve coordination of tasks, and dissemination of information on new upcoming alternative approaches. However, especially in the case of EU-NETVAL, the current work is somewhat hindered by limited funding available for its members. Some Member States have provided specific funds for these activities and see this as one of the practical ways to respond to Article 47(1) requirement to contribute to the development and validation of alternative approaches, however, this is not yet common practice. It is still very early days to draw even preliminary conclusions as to their actual impact in these processes.
Research funding through EU research programmes
The European Commission has actively supported research on all aspects of the Three Rs through its successive Framework Programmes for Research and Innovation (FPs), including the current seven-year programme Horizon 2020 (H2020: 2014 to 2020). During the last decade, the European Commission funding in this field of research has remained stable and significant. During the period 2012-2016, sixty-nine research projects were running at various stages of implementation, with EUR 350 million from the European Commission programmes. These research projects have focussed mainly on alternatives to animals. They included innovative tools for safety testing of chemicals, nanomaterials and food mixtures, quality control of vaccines, the creation of databases, tissue cultures with human induced pluripotent stem cells, bioinformatics and modelling. As part of this effort, thirteen projects were co-financed within the context of public-private partnerships with Cosmetics Europe (the seven projects from the SEURAT-1 cluster) or the European Federation of Pharmaceutical Industries and Associations (the six projects from the Innovative Medicines Initiative: IMI). The additional resources provided by the industry to these projects were estimated to represent more than EUR 100 million. Overall, these projects developed a range of various novel in silico and in vitro approaches, from innovative modelling tools to multiple organs-on-a-chip, which could allow a significant replacement and reduction of test animals in biomedical sciences and safety testing.
There is always a lag in the regulatory implementation of new alternative methods developed by any research project, including projects with the European Commission funding. This is usually due to the long time needed between the development of the methods, their validation, and their regulatory acceptance. Therefore, regulatory impact starts to be observed from FP6 (2002-2006) projects for less complex toxicological endpoints, such as skin sensitization for instance. Additional regulatory impacts are expected to come out of FP7 (2007-2013) and H2020 (2014-2020) projects, including in the areas of more complex toxicological endpoints, such as repeated dose systemic toxicity, developmental and reproductive toxicity, and carcinogenesis.
Progress on Alternatives
Summarising from the EURL ECVAM report, significant progress has been made in the EU on alternatives since 2010. New amendments to Annexes of EC Regulation 1907/2006 on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) have been made regarding skin corrosion/irritation, eye irritation, and skin sensitisation. At the International Conference on Harmonisation (ICH) an in vitro test on phototoxicity has been included in ICH S10. Strategies have been developed in areas of acute mammalian systemic toxicity, genotoxicity, bioavailability, endocrine disruption, including work towards Integrated Approaches to Testing and Assessment (IATA) in some of these areas. Addition to OECD Guideline 236 (zebrafish embryo acute toxicity test) and Guidance Document 126 (Short guidance on the use of the threshold approach for acute fish toxicity testing) have been progressed to work towards replacement of acute fish testing. ECHA have concluded that OECD Guideline 236 can be used in a weight of evidence approach.
There has also been significant progress in the quality control of pharmaceuticals, including an agreement to delete the general safety test/abnormal toxicity test from batch safety testing, since more adequate quality control measures are in place.
As described in the SCHEER Opinion on the use of non-human primates, progress has been made in replacement strategies for treatment and prevention of infectious diseases with the development and use of controlled human challenge models for typhoid, Plasmodium falciparum malaria and transmission studies with specific influenza strains, and in the development and safety testing of new medicines. Furthermore, non-human primates are no longer considered acceptable organ donors for practical and ethical reasons.
SCHEER Opinion, however, underlined that where alternatives do not exist, appropriate use of non-human primates remains essential in some areas of biomedical and biological research and for the safety assessment of pharmaceuticals.
Due to a wide number of factors influencing the speed of progress in obtaining new alternative approaches to replace the use of non-human primates, it is currently not feasible to set up a timeline to phase out their use. A number of the recommendations contained in the SCHEER Opinion have been incorporated in this review report. However, as these are applicable beyond non-human primate use, these have been worded to give more general applicability.
In the light of the general progress made with alternatives, the indications are that the revised framework and structures within the Directive support the delivery of the policy objectives, and that the provisions contained in it remain fully valid.
Member State contribution to the development and validation of alternatives approaches
As stated, the Directive requires that the Member States contribute to the development and validation of alternative approaches. 14 out of 28 Member States have submitted voluntary reports under Article 47 detailing the approaches taken to the efforts made towards the development, validation and promotion of alternative methods.
Many Member States have increased their activities, for example, increasing research funding, development of Three Rs centres, supporting educational seminars/workshops, publishing links to information on the Three Rs, and contributing to EU-NETVAL and PARERE networks, and EURL ECVAM activities..
Although it is still premature to assess the impact, increased activity in this area has been confirmed. In many Member States, there is an increased political awareness of the importance of the Three Rs. Examples of how Three Rs are promoted by the Member States include:
·At national level, requiring and ensuring biostatistical input at AWB and project evaluation process;
·Annual meetings and training events with the scientific community to promote the Three Rs;
·Examples of specific work to eliminate certain animal use e.g. shellfish bioassay;
·Educational programmes promoting alternatives in non-EU countries;
·Establishment of national Three Rs centres.
In conclusion, despite being still in the early stages after implementation of the Directive, there has been increased attention given by Member States and users towards progressing alternatives. However, it is evident that there is still some way to go to improve the access to and dissemination of information on existing alternatives, increase Member State contributions to the development and validation of new alternatives, and to ensure comprehensive uptake by the scientific community.
Recommendations
ØThe Commission services should request regular updates to the SCHEER Opinion to closely monitor progress in the development and uptake of alternatives replacing the use of non-human primates.
ØMember States, where not yet done, should consider ways in which the activities of their respective EU-NETVAL laboratories could be better supported.
Section 3 – Improving transparency
Transparency is essential to develop a trust in the systems of ethical and socially acceptable care and use of animals in science as the basis for a continued research using animals in the EU until such time their use can be replaced by non-animal alternatives. The Directive introduced a number of elements aimed at improving transparency.
The majority of Member States and users were of the view that the requirements in the Directive for publication of non-technical project summaries and annual statistical data have positively contributed to transparency, although the full impact has yet to be realised.
Among the scientific stakeholder responses, there was a similar response, but around 40% of the animal protection organisations disagreed or strongly disagreed that the Directive had yet improved transparency. The main concerns expressed regarded the accessibility and quality of non-technical project summaries, the lack of detailed statistics in the new format from certain Member States, and the lack of transparency of the project evaluation process.
Two key areas of change regarding transparency are required by the Directive, the obligation on Member States to publish non-technical project summaries (Article 43) and more detailed annual statistical information (Article 54 and Commission Implementing Decision 2012/707/EU) on the use of animals in procedures, including information on the actual severity of procedures and on the origin and species of non-human primates used in procedures. Member States are also required to ensure that the project evaluation process is transparent (Article 38).
Article 57 requires that the Commission shall submit an implementation report and a summary report of the statistical information provided by Member States by 10 November 2019.
The Directive furthermore requires in Article 38(4), that the project evaluation is performed in an impartial manner and the process needs to be transparent. Finally, inspections under Article 34 will play an increasing role in improving transparency and public accountability in all use and care of animals for scientific purposes.
User responses
|
The Directive has improved the transparency of animal use for scientific purposes in my country/region?
|
|
Users
|
|

|
The majority of users agreed that the requirements in the Directive for publishing non-technical project summaries and reporting statistical information, including on severity of procedures experienced by the animals, have improved transparency. However, these publications are reported not always to be easily found, and the content can be quite/too technical.
The Directive is one factor among a number of initiatives to improve understanding of the use of animals in research to general public.
Comments
·"It is important to explain the importance of laboratory animals in research. Explanation and transparency are critical for a better understanding of this research in Europe."
·"Organisations such as Understanding Animal Research (UAR) and European Animal Research Association (EARA) are making significant progress in this area and have been widely supported by the scientific community eg Concordat on Openness."
The majority of Member States and users were of the view that the requirements in the Directive for publication of non-technical project summaries and annual statistics have positively contributed to transparency, although the full impact has yet to be realised.
Stakeholder responses
|
All stakeholders
|
|

|
|
Other stakeholder organisations
|
Animal protection organisations
|
|

|

|
Just under half of the stakeholder organisations (25) agree that the Directive has had a positive effect, 11 disagree with this view. While nine of the animal protection stakeholder organisations agree that the Directive has improved the transparency of animal use, eight others either disagree or strongly disagree with this view.
Greater transparency is requested, in particular with the detail and balance of non-technical project summaries. Few Member States seem yet to have published the process of project evaluation.
Many animal protection organisations expressed concerns over the lack of transparency in the new processes introduced under the Directive, and over the quality of non-technical project summaries and inadequacies in the annual statistical reporting by some Member States.
Two Member States make public the majority of the content of authorised projects (excluding personal information and Intellectual Property) and a number of animal protection organisations request improved access to the details of projects.
The scientific community need to continue and improve efforts to explain why at this stage the use of animals in scientific procedures is still necessary, and what efforts are being made to replace animal use.
Accessibility
Collecting statistical data and publishing non-technical project summaries will improve transparency only if the information is readily accessible. Accessibility of information appears from many users' and science stakeholders’ comments to require further attention. Respondents pointed out that accessibility comprises of at least two issues – to know where to find information and to know how to interpret the data. Until these are addressed there is scope for improvement to truly increase transparency.
3.1 Non-Technical Project Summaries (Article 43)
Summary
The non-technical project summaries are considered a key tool to improve transparency in the area of the use of animals for scientific purposes. Article 43 of the Directive calls for information on the objectives and benefits of the project, numbers and types of animals (species and life-stages) of animals to be used, and the predicted harms to the animals which are expected to occur as a result of the procedures applied. It is also necessary that the non-technical project summaries include information on compliance with the Three Rs.
Non-technical project summaries were published by two Member States already before the new Directive. Six have not yet managed to publish them, but over half of Member States agree that there has been an increase in transparency as a result of publication. More than half of scientific users felt that transparency had improved as a result of publication of non-technical project summaries, a view agreed by most scientific stakeholders.
Significant differences in quality have been noted by animal protection groups. In particular, concerns were raised about a lack of appropriate balance; emphasising generic, sometimes unrealistic benefits without sufficient information on harms. The views expressed were that authorities should ensure accuracy and balance of published non-technical project summaries. Non-technical project summaries should be timely, easily accessible and searchable which is not yet the case in many Member States. The development of a central EU database, with open access and search facilities, was highlighted as a potential tool to provide a pan-European view of the scientific use of animals.
Member State responses
Has the publication of Non-technical Project Summaries improved transparency in your Member State?
|
Yes
|
17/28
|
|
No
|
1/28
|
|
No opinion
|
6/28
|
|
Too early to assess
|
4/28
|
The timing of publication after project authorisation varies considerably, from immediately post-authorisation to 30 months. Eight Member States publish within six months of authorisation.
A recommendation for a format for presentation and content of non-technical project summaries was agreed at a National Contact Point meeting and has been adopted by a number of Member States. The Directive leaves it to the Member States to decide whether non-technical project summaries should be updated with the results of retrospective assessment of projects. Not all Member States have opted to provide this update.
User responses
·"Non-technical project summaries facilitate the understanding for and increase the availability of facts for the general public and policy makers, and are now published (or will be) by all countries."
·"There is more discussion internally within an establishment."
·"Difficult to find the non-technical summaries and statistics makes us think that transparency is not optimal."
·"The Directive has improved the level of information which is available to the public, for example on actual severity. With regards to NTS the public can see the types of projects approved, however, due to confidentiality issues there is little detail on the benefits."
·"The non-technical project summaries include the danger that information becomes state of the art which leaves "burnt ground" for intellectual property protection."
·"The NTS's did not really improve the transparency to the general public (except for activists). Our institute is working together with European Animal Research Association (EARA) to improve transparency. It is up to each institute to ensure transparency of the research."
In addition, some said that there was not much information in the non-technical project summaries.
Stakeholder responses
Publication times should be improved and the project evaluation process should ensure that the non-technical project summary is an accurate representation of the project.
Comments
·"Non-technical summaries are too often poorly (and too technically) written, one-sided and give minimal insight."
·"There are significant shortcomings in the quality and tone of the NTSs."
·"The NTSs are written by the scientists without any “neutral” editing, thus animal suffering is often downgraded, while the alleged benefits are exaggerated. The NTSs should be provided by the authorities and include details of the harm-benefit analysis."
·"Many read like PR documents, extolling alleged benefits and downplaying or ignoring suffering."
·"Harms to animals are not described detailed enough in common language that people could have a change to judge the nature of the experiment."
Recommendations
ØTraining for scientists (EU Education and Training Framework Module 11) should include training on requirements and expectations of non-technical project summaries.
ØMember States should ensure that non-technical project summaries are published in a timely manner.
ØCompetent authorities, through the project evaluation and authorisation processes, should ensure that non-technical project summaries are accurate, fairly represent harms and be realistic about the expected benefits to improve the quality of non-technical project summaries.
ØThe Commission services, Member States and stakeholders should explore possibilities of a central repository of (or provide easy, searchable access to) all non-technical project summaries at EU level taking into account the legal requirements and linguistic limitations.
3.2 Statistical data (Article 54)
Statistical reporting was comprehensively revised after the adoption of the new Directive. The reporting requirements are detailed in the Commission Implementing Decisions 2012/70/EU. Member States were required to publish statistical data on the basis of the new requirements for the first time in 2015. Although, all Member States have published their national data, most did not include all the new data elements as set out in the Commission Implementing Decision.
It is too early to determine the impact of the new reporting requirements on improving transparency, but for the first time in EU information is provided inter alia on the actual severity experienced by each animal used. The origin and species of non-human primates is also reported. Further work is necessary by all involved to ensure coherent reporting across the EU in time for the first EU report in 2019, and every three years thereafter.
The scientific community needs to continue and improve efforts to explain to wider audiences why, at this stage, the use of animals in scientific procedures is still necessary, and what efforts are being made to replace animal use. Statistical reports need to be explained and contextualised to improve communication with the public about what these numbers and categories mean. The importance of consistent and understandable terminology was highlighted.
Member State responses
Is the new information collected under Commission Implementing Decision 2012/707/EU helpful in presenting improved data on animal use to the general public in your Member State?
|
Yes
|
19/28
|
|
No
|
1/28
|
|
No opinion
|
2/28
|
|
Too early to assess
|
6/28
|
The reporting system has been very helpful, but it has been challenging to ensure that all users understand and implement the changed requirements, for example reporting animals at the end of procedure and reporting actual severity for each animal.
User responses
·Some pointed out that changes in reporting, leading to changes (particularly increases) in numbers of animals used need to be clearly explained during the publication process, especially if this is not actually an increase but just a change in the way reporting occurs.
·Some said that the accessibility (know where to find it and know how to interpret the data) of the information needs to be addressed before it can be said to be an increase in transparency.
·"The average person has no idea about what genetic status, categories and (assumed) severities means."
Stakeholder responses
·"Statistical reports need to be explained and contextualised to improve communication with the public."
Recommendations
ØMember States, when publishing statistical data, as set out in the Commission Implementing Decision 2012/707/EU, should use the accompanying narrative to communicate about animal use in the wider context and explain key findings and trends in a manner that is easily understood by the general public.
3.3 Project Evaluation (Articles 36 and 38)
In Article 38(4), the Directive requires that the project evaluation is performed in an impartial manner and the process needs to be transparent. Specific guidance has been produced at EU level to assist in the development of these processes. There was a high awareness of the guidance within the user community, although availability, including in some languages has been reported to be an issue for developing efficient and effective processes, and would consequently be a hindrance to transparency at the time of the consultation. The greater majority of Member States have disseminated the EU Guidance on project evaluations, although some only recently.
Some Member States have published their processes and the related requirements for project evaluation and authorisation, and shared their information as part of this consultation but these are not yet universally available throughout EU. It is not clear in many cases whether these processes have been communicated only to project evaluators, or whether they are more widely available to other stakeholders, which would improve the transparency of the Directive expectations. Animal protection organisations requested improved transparency on the processes used in project evaluation and authorisation. This would provide greater public confidence that the Three Rs are being applied.
The National Committee should have a role in ensuring a harmonised approach to project evaluation by different competent authorities (when more than one) throughout the country. It would be likely that this would include a described transparent approach to project evaluation including harm-benefit assessment. Only seven National Committees had done this at the time of the consultation. Users in many Member States requested clarification of requirements for project evaluation to improve efficiency of the process.
Mutual acceptance of project evaluations was requested by some users and scientific stakeholders to facilitate transfer of projects or collaborative multi-site projects involving more than one Member State. If such processes are published and accessible, this might assist in determining whether a country’s process complied with the requirements of another Member State.
Just over half of the Member States stated that the Guidance on project evaluation and retrospective assessment was helpful, and several had used it to develop processes applicable within their own Member State. Few if any comments from users directly related to project evaluation Guidance, but there was clearly some scope for improvement of dissemination of individual Member State processes to the scientific community. The Guidance document on Severity Classification is also widely used in project evaluation, although further examples of, in particular, studies using genetically altered animals have been requested by some users and Member States to assist in the transparency and consistency of decision-making on assignment of severity. Some Member States, and stakeholder organisations are already developing these.
Some Member States and users requested more information on the availability of the Three Rs and it is not clear whether they were aware of the Working Document on the Availability of Information on the Three Rs.
Recommendations
ØMember States should publish easily accessible, transparent requirements for project evaluation, the related operational processes and responsibilities, and provide clarity on the criteria for the type of omissions that result in rendering an application incomplete/incorrect, and how these are processed (including timelines).
3.4 Inspections (Article 34)
Requirements for inspections are detailed in Article 34. Details of inspections, including the proportion of announced and unannounced visits, and details of non-compliance form part of Member State implementation reports due in 2018, consequently insufficient information is available yet for a comprehensive analysis. When available, these will increase the transparency of the inspection process.
In half of the Member States there has been no change in inspection frequency, with 13 Member States indicating an increase. The majority of users have not yet noticed any change in inspection frequency, but almost a third have reported an increase.
The EU guidance has generally been found to be helpful, and the common EU risk criteria are being used by the majority of Member States. A number of Member States have developed additional guidance to assist inspectors. Some Member States reported specific training initiatives directed to inspectors, a practice that would benefit from a wider implementation, especially in countries where inspections under this Directive form only a minor part of the role of the inspectors.
Section 4 - Additional comments
During the consultation process, stakeholders were invited to provide additional comments relevant to the review of the Directive. Below is a summary of the main comments, not discussed elsewhere in the Staff Working Document.
Member State comments
16 Member States provided additional comments. Among the issues raised by individual Member States included request for clarification over the upper limit of pain and suffering, and concerns over procedures causing intense/severe pain and suffering.
User comments
·"A much more supporting attitude is necessary by some authorities, as it is perceived that otherwise “this important sector of research in life sciences will be deteriorating in Europe completely”."
·"Improve communication between institutions, and between regulators and users (direct not indirect via others)"
·"The Directive has necessitated closure of some animal units as they did not comply with the requirements, but it has helped in the design of the replacement facilities."
·"It is especially important that a central part of the Directive is the case-by-case evaluation of projects. In our opinion, this is the only way to deal with a complex, ethically challenging topic such as animal experiments. The benefit to humans through the further development of science and the welfare of animals are both very important goals, and balancing them in a responsible manner will not be achieved through blanket assessments. Therefore the principle to have each individual project evaluated is the only way of doing the difficulty of the issue justice. The Directive is doing just that, providing a good balance between the goals of scientific progress and animal welfare.”
Stakeholder comments
Animal protection stakeholder comments
Some animal protection organisations provided additional contributions, which have been considered and incorporated as applicable in the relevant sections of this document.
Some elements put forward concerned issues that are outside the scope of this Directive such as processes for regulatory acceptance of (alternative) test methods detailed in different sector legislation e.g. for chemicals or pharmaceuticals.
A number of contributions called for the use of thematic reviews, especially on the use of non-human primates and alternatives.
Other comments included:
·"Further improve transparency with publication of Projects and Retrospective Assessments."
·"Require robust, effective enforcement by well-trained Inspectors."
·"There needs to be stronger enforcement of regulation to use alternatives and infrastructure put in place to ensure that non-animal method developments are more widely disseminated."
Other stakeholder comments
·“For scientists in many MS, there have not been significant changes to the requirements”.
·"Acknowledgement that use of animals will be necessary for the foreseeable future – there will not be alternatives to address every scientific question or need."
·"The industry expected a level playing field with no gold plating of standards. It is important that the Directive is fully implemented by all EU members as quickly as possible and policed properly going forward to ensure that this happens."
·"The positives impact includes raising standards in research practice, Three Rs awareness, promotion of culture of care, growing recognition within the research community of the link between animal welfare and good science, and increasing transparency. Much is subjective and it would be useful if the EC could think about how funding might be directed towards building a stronger evidence base in this area"
·"To achieve the goals, the EC has to react quickly and firmly against countries that do not implement the basics of the Directive, as this will create imbalances for research within the union and confusion in public opinion as standards in different MS will differ so much."
List of abbreviations
AFSTAL - l'Association Française des Sciences et Techniques de l'Animal de Laboratoire
AWB
- Animal Welfare Body
CA
- Competent Authority
CPD
- continued professional development
CRO
- contract research organisation
DB-ALM - Database on Alternative Methods
DV
- Designated Veterinarian
EARA
- European Animal Research Association
EU
- European Union
EC
- European Commission
ECHA - European Chemicals Agency
EMA
- European Medicines Agency
ESAC
- EURL ECVAM Scientific Advisory Committee
ESTAF - EURL ECVAM Stakeholder Forum
ETPLAS - Education and Training Platform in Laboratory Animal Science
EURL ECVAM - EU Reference Laboratory for
Alternatives to Animal Testing
EU-NETVAL - European Union Network of Laboratories for the Validation of Alternative
Methods
E&T
- education and training
FELASA - Federation for Laboratory Animal Science Associations
GA
- genetically altered
IAT
- Institute of Animal Technology
IATA
- Integrated Approaches to Testing and Assessment
ICH
- the International Council for Harmonisation of Technical Requirements for
Pharmaceuticals for Human Use
IMI
- Innovative Medicines Initiative
LASA - Laboratory Animal Science Association
MS
- Member State
NC
- National Committee
NCP
- National Contact Point – Member State authority responsible for the implementation
of the Directive
NC3Rs - National Centre for the Replacement, Refinement & Reduction of Animals in
Research
NHP
- non-human primate
NTS
- Non-technical Project Summary
OECD - the Organisation for Economic Co-operation and Development
PA
- Project Authorisation
PARERE - Preliminary Assessment of Regulatory Relevance Network
PE
- Project Evaluation
RSPCA - Royal Society for the Prevention of Cruelty to Animals
SCHEER - Scientific Committee on Health, Environmental and Emerging Risks
TFEU
- Treaty on the Functioning of the European Union
TSAR
- Tracking System for Alternative Test Methods
UAR
- Understanding Animal Research
UFAW - Universities Federation for Animal Welfare
3R
- Three Rs (Replacement, Reduction and Refinement of animal use and care)
Annexes
1. List of recommendations
2. Report by EURL ECVAM
Annex 1: List of recommendations
1. Harmonisation of legislation
1.1 Project evaluation
1.The Commission services and Member States should engage in discussions to improve guidance and provide further examples for the scientific community on what constitutes a "project".
2.Member States should review if additional administrative gains could be attained for authorities and operators from a wider use of multiple generic project authorisation and simplified administrative procedures.
3.Where lacking, Member States should provide clear guidance on the required content for a project application, review that the requested elements directly relate to the performance of the harm-benefit assessment in line with Article 38, and that the level of detail is appropriate for the type of project.
4.Member States should engage with relevant stakeholders to review their respective project evaluation and authorisation processes to identify any duplication and to establish measures of simplification aimed at efficient, effective and timely processing of applications.
5.Training for both project applicants and project evaluators would seem beneficial. Joint efforts by the Commission services, Member States and other stakeholders should be made to create opportunities for such training.
6.Urgent focus is needed by National Committees on their key task to establish a coherent approach to project evaluation in particular in Member States with multiple competent authorities tasked with project evaluation. The Commission services, Member States and National Committees should engage in discussions to develop appropriate tools for this purpose.
1.2 Changes in Scope of Directive
7.Further guidance should be developed to improve clarity on the minimum threshold of severity needed to bring a procedure under the scope of the Directive.
8.The European Commission should propose amendments to Annexes III and IV for cephalopods once sufficient evidence is available.
9.The European Commission should consider incorporating appropriate killing methods for foetal forms of mammalian species in Annex IV and review whether methods already contained in the Annex are still in line with the latest scientific knowledge.
1.3 Education and training of staff
10.Efforts should be made by all relevant stakeholders to improve availability and access to, and variety of, training courses essential for obtaining the requisite competences in different knowledge areas, techniques and species.
11.The three partners of ETPLAS (Member State representatives, course providers and accreditors) should increase collaboration and engagement in order to progress with the development and agreement of common quality standards aimed at free movement of competent staff.
12.ETPLAS should take a more active role and step up its efforts to establish itself as a central repository for information on LAS (Laboratory Animal Science) training and quality standards in EU.
1.4 Harmonisation of welfare standards
13.Member States should provide evidence-based cases to the Commission services where amendments to Annexes III and IV are considered appropriate.
14.With the proposal to include standards for, inter alia, cephalopods in Annexes III and IV, the European Commission should consider other amendments on the basis of exemptions granted under Article 6(4)(a) and other evidence brought forward.
Section 2 – Animal Welfare and the Three Rs
2.1 Animal Welfare Bodies
15.Establishments and Member States (through inspection) should ensure that all core tasks of the AWB are being fulfilled.
16.Member States should clarify roles and responsibilities of the AWB and project evaluation, in particular where there may be some integration or overlap with following the development of projects, including application of the Three Rs and project evaluation process.
17.Senior management of the establishment should ensure that the AWB has sufficient resources and empowerment to carry out the required tasks.
18.Establishments could consider the addition of a Designated Veterinarian as a full member of the AWB.
2.2 National Committees
19.Member States should facilitate and resource National Committees where this is not yet established, or where it is not fully functional, to ensure that its role and tasks are fulfilled as these tasks play key roles in the attainment of the overall objectives of the Directive.
20.In preparation for the EU implementation report under Article 57, Member States should look closer at whether and how National Committees are:
2.3 Training and education and requirements for personnel
21.Where not yet available, Member States should publish minimum requirements for education and training, and for obtaining, demonstrating and maintaining competence, and increase efforts to disseminate EU and other guidance on education and training to scientific users to indicate their expectations for trained and competent staff. National guidance should be shared with the relevant stakeholder organisations and other Member States.
22.Member States should ensure clarity of Article 24 roles, in particular those of the training and information persons, to ensure effective implementation and also to increase awareness of their role and the support they can provide within establishments.
2.4 Reuse
23.The Commission services and Member States should develop additional guidance on reuse.
2.5 Avoidance of unnecessary duplication
24.Member States should re-enforce the awareness of the need for researchers and project evaluators to ensure that no unnecessary duplication takes place, in particular, in the development of new genetically altered animal lines.
2.6 Setting free and rehoming
25.Where appropriate, Member States should share relevant guidance material on rehoming, as well as make use of guidance developed by other Member States/stakeholder organisations.
2.8 Use of existing alternative approaches and implementation of the Three Rs
26.Continue efforts to ensure promotion and sharing of alternative approaches and dissemination of information at national level.
27.Develop a high-level strategy to encourage a shift of attitudes and priorities to make significant progress towards the implementation of non-animal methods.
28.Ensure training remains current in the field of alternatives and in the tools available to search for them.
29.Ensure that appropriate consideration is given to the use of alternatives in particular in the field of education, and that project evaluators are up-to-date in the advancement of alternatives in this field.
Users
30.Always consider alternative methods and approaches, including thinking laterally to revise original hypotheses to try to replace animal use.
31.Ensure robust searches are carried out using all available, up-to-date resources to find potential alternatives in their field including in education and training. Demonstrate in the project applications why alternative methods available in the field will not suffice to fulfil the scientific objectives.
Organisations with specific interest in alternatives
32.Develop online courses in alternative methods for specific areas of science, toxicology, and education and training.
33.Develop search tools for alternatives, especially in the non-regulatory use of animals.
34.In existing and new databases, alternatives should be accessible by discipline e.g. neuroscience; immunology etc.
35.Develop improved communication and cooperation among relevant "alternative stakeholders" on the availability and the potential for sharing relevant high-quality teaching resources at costs which could be acceptable to trainers / trainees.
Training providers
36.Ensure, in cooperation with alternatives organisations, that training for scientists remains current and prioritises the importance of experimental design and implementation of the Three Rs.
Regulatory authorities
37.Regulatory agencies, in collaboration with Member State authorities and the user community, should consider how the dissemination of information on newly adopted alternative methods could be improved to reach all relevant players in a timely fashion.
2.9 Development, validation and regulatory acceptance of new alternative approaches
38.The Commission services should request regular updates to the SCHEER Opinion to closely monitor progress in the development and uptake of alternatives replacing the use of non-human primates.
39.Member States, where not yet done, should consider ways in which the activities of their respective EU-NETVAL laboratories could be better supported.
Section 3 – Improving transparency
3.1 Non-Technical Project Summaries
40.Training for scientists (EU Education and Training Framework Module 11) should include training on requirements and expectations of non-technical project summaries.
41.Member States should ensure that non-technical project summaries are published in a timely manner.
42.Competent authorities, through the project evaluation and authorisation processes, should ensure that non-technical project summaries are accurate, fairly represent harms and be realistic about the expected benefits to improve the quality of non-technical project summaries.
43.The Commission services, Member States and stakeholders should explore possibilities of a central repository of (or provide easy, searchable access to) all non-technical project summaries at EU level taking into account the legal requirements and linguistic limitations.
3.2 Statistical data
44.Member States, when publishing statistical data, as set out in the Commission Implementing Decision 2012/707/EU, should use the accompanying narrative to communicate about animal use in the wider context and explain key findings and trends in a manner that is easily understood by the general public.
3.3 Project Evaluation
45.Member States should publish easily accessible, transparent requirements for project evaluation, the related operational processes and responsibilities, and provide clarity on the criteria for the type of omissions that result in rendering an application incomplete/incorrect, and how these are processed (including timelines).
Annex 2: Report by EURL ECVAM
EURL ECVAM's contribution to the review of Directive 2010/63/EU on the protection of animals used for scientific purposes (status of 13/02/2017)
Background
Directive 2010/63/EU formally established the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) and defined its duties in Article 48 and Annex VII.
Its key responsibilities are to (a) coordinate and promote the development and use of alternatives to procedures in the areas of basic and applied research and regulatory testing; (b) coordinate the validation of alternative approaches at Union level; (c) act as a focal point for the exchange of information on the development of alternative approaches; (d) set up, maintain and manage public databases and information systems on alternative approaches and their state of development, and; (e) promote dialogue between legislators, regulators, and all relevant stakeholders, in particular, industry, biomedical scientists, consumer organisations and animal-welfare groups, with a view to the development, validation, regulatory acceptance, international recognition, and application of alternative approaches.
The directive also mentions in the same Annex that the Union Reference Laboratory should participate in the validation of alternative approaches.
These duties are in line with the former Commission Communication to the Council and the European Parliament on the establishment of a European Centre for the Validation of Alternative Methods.
The Directive mandates the application of scientifically valid alternative approaches and establishes mechanisms to speed up their development, validation and uptake.
For example, Article 47 requires that "The Commission and the Member States shall contribute to the development and validation of alternative approaches which could provide the same or higher levels of information as those obtained in procedures using animals, but which do not involve the use of animals or use fewer animals or which entail less painful procedures, and they shall take such other steps as they consider appropriate to encourage research in this field." This provision is known as the "Three Rs", i.e. replacement, reduction and refinement of animal use in scientific procedures. Recital 12 of the Directive stipulates that "the use of animals for scientific or educational purposes should [therefore] only be considered where a non-animal alternative is unavailable. Use of animals for scientific procedures in other areas under the competence of the Union should be prohibited."
Policy context
EURL ECVAM primarily focuses on regulatory safety testing (with emphasis on chemicals over the last 4 years) required under various EU legislations such as Regulation 1223/2009 on cosmetic products, Regulation (EC) No 1907/2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), Regulation (EC) No 1272/2008 on Classification, Labelling and Packaging of substances and mixtures (CLP), Regulation (EC) No 1107/2009 concerning the placing of plant protection products on the market, Regulation 283/2013 on data requirements for active substances, Regulation 284/2013 on data requirements for plant protection products, Regulation 528/29012 concerning the making available on the market and use of biocidal products, Directive 2001/83/EC (and amendments) on the Community code relating to medicinal products for human use, Directive 2001/82/EC (and amendments) on the Community code relating to medicinal products for veterinary use, Regulation 1829/2003 on genetically modified food and feed, the Community Strategy on combined exposures 'Mixtures' and the Community Strategy on Endocrine Disrupters.
The above cited EU Directives, Regulations and Community Strategies are, together with the horizontal Directive 2010/63/EU and Regulation 440/2008 on test methods, the regulatory drivers for EURL ECVAM's work on alternatives. All these pieces of legislation either refer to alternative approaches and/or allow them to be used in hazard, risk and safety assessments.
Vertical regulations with considerable impact on the Three Rs were the Cosmetics Regulation and REACH. The Cosmetics Regulation completely bans animal testing and the marketing in the EU of cosmetics tested on animals altogether since 2013, while REACH requires since 2008, that animal tests are used only as a very last resort when no other, validated and approved non-animal tests are available. Moreover, recent updates of the REACH annexes for more advanced endpoints makes the use of validated and accepted non-animal tests the default information requirement for assessing whether chemicals have the potential to cause these hazards, irrespective of the tonnage level of production.
In the area of human and veterinary medicinal products, non-clinical testing and quality control often requires the use of animals to comply with Directives 2001/83/EC and 2001/82/EC and their associated guidelines and/or pharmacopoeia monographs. Ethical and animal welfare considerations demand that animal use is limited, if not avoided, as much as possible. In this respect, Directive 2010/63/EU on the protection of animals used for scientific purposes, which is fully applicable to regulatory testing of human and veterinary medicinal products, promotes the application of the principle of the Three Rs when considering choice of methods to be used (EMA, 2016).
EMA recently established The Joint Committee for Medicinal Products for Veterinary Use/Committee for Medicinal Products for Human Use Working Group on the Application of the Three Rs in Regulatory Testing of Medicinal Products (J3RsWG) replacing the formerly EMA expert group JEG 3Rs (2010 – 2016). The J3RsWG provides advice and recommendations to the Committee for Medicinal Products for Veterinary Use (CVMP) and Committee for Medicinal Products for Human Use (CHMP) on all matters relating to the use of animals and the application of the Three Rs principles in the testing of medicines for regulatory purposes.
New structures provided by the Directive and enlarged scope for EURL ECVAM
Other provisions of Directive 2010/63/EU had an impact on EURL ECVAM's work. For instance, as a response to Article 47(2) which requires that Member States assist the Commission in identifying and nominating suitable specialised and qualified laboratories to carry out validation studies, EURL ECVAM set up a European Union Network of Laboratories for the Validation of Alternative Methods (EU-NETVAL) in 2014. This network, coordinated by EURL ECVAM, currently comprises 37 members (from 15 EU Member States and EFTA countries) which were selected through open calls and against pre-defined eligibility criteria and endorsed by the National Contact Points of the Member States for the implementation of Directive 2010/63/EU.
EU-NETVAL has a potential to significantly increase the European Union's validation capacity of in vitro methods by generating in vitro method information that is reliable, relevant and based on current best quality and scientific practices and provides a laboratory network knowledgeable on the routine implementation of good in vitro method practices for regulatory use in human safety assessment. EU-NETVAL members support validation studies through the execution of one or more specific tasks and also contribute to the development of guidance documents and training materials supporting good in vitro method development and practices. However, the capacity for EU-NETVAL members to participate actively is largely dependent on funding made available by the Member States to their respective EU-NETVAL members. Some Member States have clearly seen this as one of the practical ways to respond to Article 47(1) requirement to contribute to the development and validation of alternative approaches. It is hoped that more Member States will follow suit to enable EU-NETVAL to reach its full potential.
Similarly, Article 47(5) requires that Member States nominate a single point of contact to provide advice on the regulatory relevance and suitability of alternative approaches proposed for validation. The Preliminary Assessment of REgulatory Relevance (PARERE) Network was established by EURL ECVAM in 2011. The network is composed of regulators nominated by the EU Member States, representatives from EU regulatory agencies such as the European Medicines Agency (EMA), the European Chemicals Agency (ECHA) and the European Food Safety Authority (EFSA), and relevant Commission services. In order to expedite the process of regulatory acceptance of alternative methods, it was considered that regulators should be involved as early as possible in providing a preliminary view on the potential regulatory relevance of methods submitted to EURL ECVAM for validation. PARERE has additional tasks which are described on the EURL ECVAM website. Finally Article 47 (6) asks the Commission to take appropriate action with a view to obtaining international acceptance of alternative approaches validated in the Union. Besides involving regulators early on in the evaluation process of new tests and approaches, EURL ECVAM also reinforced its support to the OECD test guideline programme by leading the drafting of several new OECD Test Guidelines or Guidance Documents and the review process by the OECD member countries. In addition, EURL ECVAM supports the EU National Coordinator and participates at the annual meeting of the Working Group of National Coordinators of the OECD Test Guideline Programme and at many expert meetings on specific human health or environmental effects. The Mutual Acceptance of Data Agreement (MAD) is the main instrument at the OECD to ensure a globally harmonised approach to the testing and assessment of chemicals. This reduces costs and saves thousands of animals every year. The OECD is also the default route for taking up new test methods into the EU Test Method Regulation No 440/2008. International cooperation is also taking place through the International Cooperation on Alternative Test Methods (ICATM). ICATM includes governmental organisations from the EU, US, Japan, Canada, South Korea, Brazil and China who are working together to promote enhanced international cooperation and coordination on the scientific development, validation and regulatory use of alternative approaches.
In line with Annex VII (e), EURL ECVAM also established in 2011 the ECVAM Stakeholder Forum (ESTAF) to maintain dialogue with the Stakeholder community involving industrial associations, research organisations and civil society. Through ESTAF, EURL ECVAM maintains close dialogue with and between stakeholders concerning new activities, trends, scientific and technical issues, forward-looking aspects of test method development, optimisation, validation and use. Information on the membership and roles of ESTAF and outcomes of meetings can be retrieved from the EURL ECVAM website.
In 2010, the ECVAM Scientific Advisory Committee (ESAC) has been reformed and restructured in line with other scientific committees of the European Commission to ensure a clear separation of the provision of independent scientific advice from any vested interests. Consequently, the ESAC has been renewed in 2010 (with a mandate of 3 years) and again in 2013 and only includes senior scientists selected on the basis of their scientific expertise and who are required to act independently and on the basis of scientific considerations. The selection of candidates for the next renewal is underway. ESAC's main role is to conduct independent peer reviews of validation studies of alternative test methods, assessing their scientific validity for a given purpose. Since 2010, ESAC scientifically peer reviewed 16 methods and validation studies in the areas of skin sensitisation, serious eye damage/eye irritation, skin irritation, acute toxicity, carcinogenicity and acute aquatic toxicity. Notably, over the years, the number of validation studies which were carried out externally (i.e. not coordinated or carried out by EURL ECVAM) and submitted to EURL ECVAM for evaluation and ESAC peer review has considerably increased. The ESAC's advice to EURL ECVAM is formally provided as ESAC working group reports and ESAC Opinions at the end of the peer review process. ESAC's advice serves as the basis for the development of EURL ECVAM Recommendations that summarise EURL ECVAM's view on the validity of a test method and advise on its possible regulatory applicability, limitations and proper scientific use. EURL ECVAM Recommendations identify knowledge gaps and define follow-up actions. Developed in close dialogue with regulators (PARERE), stakeholders (ESTAF) and international partners (within the framework of ICATM), EURL ECVAM Recommendations prepare and support the international recognition and regulatory use of alternative methods as well as their application by end users (see figure 1).
Progress made in the EU on alternatives since 2010
Since 2010, considerable progress has been made in the EU in the development, validation, regulatory acceptance and international adoption of alternative approaches in the areas of skin irritation/corrosion, serious eye damage/eye irritation, skin sensitisation and phototoxicity for the human health-related effects, and aquatic toxicity testing for environmental effects. In these areas the underpinning science is more advanced and mature alternative methods and knowledge on how to optimally combine them in integrated approaches are available (Zuang et al., 2013; Zuang et al., 2014; Zuang et al., 2015; Zuang et al., 2016). Notably, the development and validation of promising methods in these areas and their international adoption (e.g. through inclusion into OECD Test Guidelines) led to changes in EU legislation.
Human health effects
New amendments to the REACH Annexes VII and VIII regarding skin corrosion/irritation (point 8.1 of Annexes VII and VIII), serious eye damage/eye irritation (point 8.2 of Annexes VII and VIII) and skin sensitisation (point 8.3 of Annex VII) entered into force in 2016 (EU, 2016a&b). The adopted in chemico and in vitro methods are now the default requirement and in vivo studies can only be conducted in exceptional cases, i.e. when the non-animal test methods are not applicable, or if the test results are not adequate for classification and risk assessment.
At the International Conference on the Harmonisation of Technical Requirements for Registration of Pharmaceutical Products for Human Use (ICH), the 3T3 Neutral Red Uptake Phototoxicity Test adopted as OECD TG 432 in 2004 was included in ICH S10 on photosafety evaluation of pharmaceuticals in 2014. The guideline considered the recommendations made at the joint ECVAM-EFPIA workshop of 2010 (Ceridono et al. 2012) to better define how data based on OECD TG 432 can be used for risk assessment of pharmaceuticals.
For acute systemic toxicity, reduction in the animal use could be achieved through the amendment of REACH annex VIII (point 8.5). An acute dermal toxicity study can now be waived for those substances which are non-toxic via the oral route. This amendment was possible due to the scientific evidence indicating that substances demonstrated to be of low acute toxicity by the oral route are also of low toxicity by the dermal route and, therefore, that dermal testing for acute systemic toxicity of such substances adds nothing to the hazard characterisation.
EURL ECVAM published its strategy to replace, reduce and refine the use of animals in the assessment of acute mammalian systemic toxicity which highlights additional options for achieving Three Rs impact, like for instance the better use of existing alternative methods such as mechanistically relevant in vitro assays, as well as existing information on repeated dose toxicity, and collecting and organising mechanistic knowledge related to this health effect in order to improve the design and validation of predictive models and approaches.
In the area of genotoxicity, progress has been made on the overall improvement of the current testing strategy for better hazard assessment with the use of fewer or no animals to satisfy the information requirements of various pieces of EU legislation. As outlined in the EURL ECVAM Strategy to avoid and reduce animal use in genotoxicity testing this includes enhancing the performance of the in vitro testing battery so that fewer in vivo follow-up tests are necessary and guiding more intelligent in vivo follow-up testing to reduce and optimise the use of animals. In this context, a considerable number of activities have been carried out in the EU and worldwide with the aim of optimising strategies for genotoxicity testing and harmonising the genotoxicity safety assessment across sectors. For instance, the OECD has recently updated almost all the genotoxicity test guidelines and is currently investing in a retrospective analysis of available miniaturised tests for gene mutation in bacteria. Additionally, the OECD is taking up the discussion on innovative, more mechanistically-based in vitro genotoxicity methods. EURL ECVAM recently provided the scientific and regulatory community with a curated genotoxicity and carcinogenicity database which, together with a recommended list of genotoxic and non-genotoxic chemicals for assessing the performance of new in vitro genotoxicity test methods (Kirkland et al, 2016) have become powerful tools for data analysis and in vitro genotoxicity tests development and improvement. It is worth noting that changes to the in vitro testing battery (Kirkland et al., 2011) have been adopted in the safety assessment of substances in food and feed by EFSA (EFSA, 2011), as well as for cosmetics ingredients in the EU (SCCS, 2015).
Exposure to a chemical does not automatically mean that all of the dose will be bioavailable and therefore able to cause a specific toxicity. Information on the human toxicokinetics (the biological fate of a substance in the human body) plays thus an important role in human safety assessment. Current EU test methods and OECD test guidelines are mostly based on animal procedures. However the integration of new technologies such as in vitro methods and computer models allows the prediction of absorption, distribution, metabolism and excretion (ADME), the four underlying processes driving toxicokinetic behaviour. In general however, the lack of standardisation of these methods is hampering their regulatory acceptance and use. Efforts are thus focused on the characterisation and description of human in vitro absorption, distribution, metabolism and excretion (ADME) methods and on good modelling practice. Human hepatic metabolic clearance (HHMC) represents in many cases the main driving process of kinetics to determine the concentration-time profile of a chemical in a biological system and is an indispensable information source to support the chemical risk assessment. A call for submission of in vitro human hepatic metabolic clearance methods triggered the submission of 15 HHMC methods that aim to measure in vitro the rate at which a test chemical is metabolised by a human liver-based test system. A new project aiming at developing a Guidance Document for the characterisation and description of in vitro hepatic metabolic clearance methods was recently submitted to the OECD. This Guidance Document is focused on the use of in vitro methods to measure hepatic metabolic clearance as a proxy to information derived from in vivo metabolism studies.
Most of the in vitro methods which were developed and submitted to EURL ECVAM for validation and/or peer review from 2010 to 2016 were in the areas of (in decreasing order) skin sensitisation, skin and eye irritation (including several similar methods to already validated and adopted ones), endocrine disruption and genotoxicity. These areas are of particular interest to the cosmetics industry which is facing since 2009 an animal testing ban for cosmetic ingredients and products and since 2013, a complete ban on the marketing, inside of the EU, of cosmetic ingredients and products tested on animals and which, therefore, has invested in the development of non-animal methods in these areas.
With regard to endocrine disrupters, the OECD-endorsed methods are grouped in a conceptual framework (CF) for the testing and assessment of endocrine disrupters which includes five different levels. Level two of this CF includes in vitro assays which provide data on selected endocrine mechanism(s) / pathways, such as hormone receptor binding and transactivation assays. Some of these methods had been developed within ReProTect project under the 6th EU Framework Programme (as well as internationally) for screening purposes and identification of target mechanisms of endocrine active compounds. The submitted test methods were either binding assays or transcriptional assays measuring either androgenic or estrogenic activity which could fit into the OECD conceptual framework. Successful validation and peer review of several estrogen-receptor transactivation assays led to the inclusion of these test methods into an OECD Performance-Based Test Guideline (OECD PBTG 455). An Androgen-Receptor Transactivation assay (ARTA) is currently undergoing a EURL ECVAM coordinated validation study in three of the EU-NETVAL laboratories. If successfully validated, this method, together with other validated ARTAs, will be included in an OECD Performance-Based Test Guideline on ARTAs. Other assays have recently been validated for the detection of chemicals with estrogen binding affinity, leading to the development of a Performance-Based Test Guideline (PBTG 493). Another recently validated test measuring the effects on steroidogenesis was adopted as OECD TG 456.
A number of in vitro assays evaluating different aspects of perturbation of the estrogen-signalling pathway has recently been combined in a computational model by the US Environmental Protection Agency . The model has a good concordance with an in vivo rodent model also evaluating interference with estrogen signalling (Browne et al., 2015). This provides an interesting example of a possible replacement of a current in vivo mechanistic screening assay. Other activities are ongoing to identify knowledge gaps and validation needs on less known endocrine pathways such as the thyroid or the retinoid pathways.
For complex endpoints such as repeated-dose toxicity, carcinogenicity and reproductive toxicology, the lack of suitable and mechanistically based methods and their optimal integration in regulatory testing frameworks remains a challenge. The few methods which are developed and submitted in these areas usually model only one specific mechanism of toxicity that may lead to an adverse effect. In these cases where there is a need to identify complementary endpoints that would have to be assessed with other in vitro/in silico methods and where conceptual frameworks or Integrated Approaches to Testing and Assessment (IATA) are not yet available, regulators are more reluctant to use the results of these methods to inform regulatory decision making. These non-standardised assays then usually remain at the level of screening tools within industry to aid in the prioritisation of substances and are not used for regulatory risk assessment purposes.
Research projects therefore continue to be funded by the European Commission in these complex areas. For example, the Seurat-1 project co-funded by the European Commission and Cosmetics Europe under the 7th EU Framework Programme focused on the safety assessment of chemicals for replacing animal testing for repeated dose toxicity. This initiative attempted to address the safety assessment of chemicals for regulatory use through a series of case studies. It also provided a toolbox developed by the different projects resulting in a large variety of alternative methods, techniques and compiled information, which is available through various databases and websites such as ToxBank, DB-ALM, COSMOS Space, COSMOS KNIME WebPortal and COSMOS Database. Another recent project funded by the European Commission under Horizon2020, under the name of EU-ToxRisk, is building further on the activities started by the SEURAT-1 initiative. EU-ToxRisk continues to evaluate methodologies for repeated dose toxicity, but also for developmental and reproductive toxicity. The project is built up around different case studies to better capture possibilities and shortcomings in safety assessment applications.
Developmental neurotoxicity (DNT) represents another complex endpoint. In October 2016, an OECD/EFSA Workshop on DNT was organised in an effort to develop a consensus on which testing battery of alternative DNT methods could be already applied and used in a fit-for-purpose manner for different regulatory needs, i.e., chemical screening for prioritisation, or hazard identification for specific chemical risk assessment. In recent years, several in vitro assays which assess the impact of chemicals on cellular processes critical to normal brain development have been developed. In particular, assays suitable to measure neural proliferation, differentiation, migration, neurite outgrowth, synaptogenesis, and neural activity have been used to derive mechanistic information for limited numbers of chemicals, and a few of them have been used to screen large numbers of chemicals (Bal-Price et al., 2015). In the longer term, it is expected that in vitro test guidelines will be developed, with a view to achieving a harmonised approach to their regulatory use across countries.
All these research and development activities in the field of alternatives predominantly focus on the integration of a variety of testing and non-testing methods such as in vitro technologies, bioinformatics and computational toxicology into Integrated Approaches to Testing and Assessment (IATA). Ideally and if available, such IATA are based on Adverse Outcome Pathways (AOP), a mechanistic knowledge framework that describes a logical sequence of causally linked events at different levels of biological organisation, which follows exposure to a chemical and leads to an adverse health effect in humans or environment. The AOP concept was developed to better understand, explain and organise the steps that link perturbation of a biological system to an adverse (apical) outcome. This in turn should help to guide the development of relevant methods and their most optimal combination to appropriately mimic the entire range of key biological events from exposure to a xenobiotic to the adverse outcomes of concern for humans or environment.
Activities at the OECD level to develop an IATA framework for the identification of non-genotoxic carcinogens go into that direction. In fact, it has been estimated that 10-20% of recognised human carcinogens classified as Class 1 by the International Agency for Research on Cancer (IARC) act by non-genotoxic mechanisms (Hernandez et al. 2009). However, for virtually all OECD regulatory jurisdictions, including REACH, there are no specific requests to obtain information on non-genotoxic mechanisms of carcinogenicity specifically. Moreover, as mentioned above, there are no in vitro methods available yet. It thus appears likely that many non-genotoxic carcinogens may remain unidentified and the risks they may pose to human health will not be managed.
Of particular interest is also the proposal to change the actual carcinogenicity testing approach for pharmaceuticals (ICH, 2016), in order to satisfy Directive 2010/63/EU. The proposal is based on the concept that "a weight of evidence evaluation can, in certain cases provide sufficient information to conclude that a given pharmaceutical presents a negligible risk or, conversely, a likely risk of human carcinogenicity without conducting a two-year rat carcinogenicity study". A prospective evaluation study to confirm the above hypothesis has been undertaken (ICH, 2016). Preliminary analyses are ongoing (e.g. within EPAA) to investigate if this approach could be translated to other sectors, where only 90-day repeated dose toxicity studies are available (Woutersen et al., 2016). A positive outcome from this exercise could change the classical way of approaching carcinogenicity testing and might yield a significant reduction in the conduct of two-year cancer studies and a consequent reduction in number of animals used.
Environmental effects
In environmental toxicology, the assessment of aquatic toxicity and bioaccumulation are important components of the environmental hazard and risk assessment of all types of chemicals and are therefore information requirements in several pieces of EU and international legislation. EURL ECVAM published its strategy to replace, reduce and refine the use of fish in aquatic toxicity and bioaccumulation testing in 2013. If successfully implemented by all key actors, the strategy will deliver alternative approaches that address standard information requirements in many sectors while ensuring that animal testing is only conducted as a last resort. One important near-term impact could be the reduction of animal testing necessary for the implementation of REACH and the 2018 registration deadline. EURL ECVAM focused its in-house activities on promoting the use of available alternative methods for fish acute toxicity testing, on exploring the usefulness of scientific approaches (e.g. acute-to-chronic relationships) to facilitate the waiving of chronic fish tests, and on supporting activities at OECD level.
Recent achievements are linked to acute fish toxicity testing. The OECD Guidance Document (GD) 126 "Short guidance on the use of the threshold approach for acute fish toxicity testing" is available since 2010 (OECD, 2010) and describes a tiered testing strategy which has the potential to significantly reduce the number of fish used for acute aquatic toxicity testing. The threshold approach has been incorporated into various testing strategies and guidance documents, e.g. the REACH guidance on information requirements and chemical safety assessment (ECHA, 2016) and the OECD Fish Toxicity Testing Framework (OECD, 2012). It is further mentioned as a preferred method for deriving data on acute fish toxicity in the biocidal products regulation (EU, 2012) and in the Commission regulations on data requirements for plant protection products (EU, 2013a & b).
The validated zebrafish embryo acute toxicity test method (ZFET; Busquet et al., 2014) was included in OECD TG236 in 2013. EURL ECVAM recommends the ZFET for generating information on acute fish toxicity where appropriate (EURL ECVAM, 2014). Its use will result in an overall reduction of the numbers of juvenile and adult fish for aquatic toxicity testing. In 2016, ECHA published its "Analysis of the relevance and adequateness of using Fish Embryo Acute Toxicity test (FET) Test Guideline (OECD 236) to fulfil the information requirements and addressing concerns under REACH". ECHA concluded that, at present, the FET can be used in a weight-of-evidence approach and published a summary of ECHA's view.
With regard to chronic fish toxicity testing, EURL ECVAM has recommended options for avoiding chronic fish testing on the basis of existing data and extrapolation approaches (Kienzler et al., 2016). In particular, it was concluded that interspecies extrapolations and acute-to-chronic relationships can be used to scientifically support the waiving of chronic fish tests, according to the specific mode of action.
Moreover, EURL ECVAM is co-leading two OECD projects, i.e the reduction of the number of control fish (co-lead with ICAPO) and drafting of test guidelines to derive fish in vitro hepatic clearance (co-lead with USA; for detailed information see EURL ECVAM Status Report 2016).
Quality control of pharmaceuticals
With respect to the quality control of pharmaceuticals, EURL ECVAM's focus is mainly on vaccines since, traditionally, animals have played an important role in quality control of vaccines and many animals are still used in Europe for this purpose. Over the last decades, several Three Rs methods to classical animal tests have been developed by control authorities, academia and vaccine manufacturers, validated within the framework of the Biological Standardisation Programme (BSP) of the European Directorate for the Quality of Medicines & HealthCare (EDQM; Council of Europe) and incorporated into European Pharmacopoeia monographs.
In 2015, EURL ECVAM released a report on Replacement, Reduction and Refinement of Animal Testing in the Quality Control of Human Vaccines
. The focus of the report is on methods for lot release testing (e.g. safety, pyrogenicity, potency) and projects related to the implementation of the consistency approach to established vaccines such as diphtheria, tetanus, pertussis and rabies vaccines. The report shows that progress has been achieved and new approaches to quality control such as the consistency approach have the potential to further reduce animal use.
Within the EPAA, EURL ECVAM organised in collaboration with vaccine manufacturers a workshop to discuss the consistency approach for the quality control of vaccines (De Mattia et al., 2011). The EPAA Vaccines Consistency Approach project (2010 – 2016) initiated a number of activities aiming at developing and validating non-animal methods with the support of stakeholders from academia, regulators, Official Medicines Control Laboratories (OMCLs), EDQM, European Commission and vaccine manufacturers (De Mattia et al., 2015) summarised the work carried out within the four priority vaccines/vaccine groups (diphtheria/tetanus/acellular pertussis vaccines; human rabies vaccines; veterinary rabies vaccines; clostridial vaccines). Two activities resulted in EDQM BSP studies and cover the validation of in vitro methods for in process control of a clostridial vaccine and potency testing of rabies vaccines for human use. The EPAA Vaccine Consistency Approach project also promoted the inclusion of the topic into the Innovative Medicines Initiative 2 (IMI 2) call published in 2014. IMI 2 is a Joint Undertaking of the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA).
In March 2016, under the new Innovative Medecine Initiative (IMI)2 , the project "Vaccine batch to vaccine batch comparison by consistency testing" (VAC2VAC) was officially launched and brings together 20 public and private partners. The project will provide data to support the consistency approach for quality control of established vaccines for human and veterinary use. The consistency approach moves away from the current focus on final product control often relying on animal testing. In the light of this, VAC2VAC partners will develop, optimise and evaluate non-animal methods, e.g. physicochemical and immunochemical methods, cell-based and other assays for routine batch quality, safety and efficacy testing of vaccines, in collaboration and consultation with regulatory agencies. JRC/EURL ECVAM is participating in the project as leader of the work package related to validation, and will also support project activities related to international dissemination, harmonisation and regulatory acceptance of consistency approaches.
As in other areas, international harmonisation is an important aspect also for the quality control of vaccines. Since 2008 and on behalf of EMA, EURL ECVAM is working with VICH experts on the development of guidelines on harmonisation of criteria to waive the target animal batch safety testing for inactivated and live vaccines for veterinary use. VICH GL50 for inactivated veterinary vaccines was adopted in 2013 and is in force since 1st March 2014 (VICH, 2013). The comparable VICH GL55 for live veterinary vaccines and the revised VICH GL50 underwent public consultation in 2016 and are currently being finalised for adoption by the VICH Steering Committee. A third guideline is under development aiming at the harmonisation of criteria to waive the general batch safety test in laboratory animals (e.g. abnormal toxicity test). Both, the abnormal toxicity test and target animal batch safety testing have been deleted from European Pharmacopoeia monographs several years ago (Schwanig et al., 1997; EDQM, 2012). However, since they may still be required outside of Europe, European manufacturers may need to carry out these tests when exporting to third countries.
EURL ECVAM is a member of the EPAA project "Biologicals" aiming at progressing harmonisation of requirements for batch testing of vaccines and other biological products at a global level. Due to evident differences in the current regional requirements, manufacturers may need to carry out animal tests which are no longer required in Europe, if they want to market their products outside of Europe. EPAA convened an international workshop (15-16 September 2015) with representatives from regulatory bodies and manufacturers to discuss steps towards deletion of general safety tests and to identify means towards implementation of in vitro methods for potency testing of human and veterinary vaccines. The workshop report "Modern science for better quality control of medicinal products: Towards global harmonisation of 3Rs in biologicals" is available on the EPAA website. The major recommendation – agreed by all participants – is the deletion of general safety tests, e.g. abnormal toxicity test, target animal batch safety, from regulatory requirements at a global level. Nowadays, these tests lack scientific relevance and their omission does not compromise the safety of vaccines, or any other pharmaceutical, since more adequate quality control measures are in place. The project team is following up the recommendations in collaboration with workshop participants and relevant stakeholders.
Dissemination
In order to disseminate information on alternative approaches and enhance overall progress in their use, several database services are available at EURL ECVAM (Zuang et al., 2016). One service that is well-known is the EURL ECVAM’s DataBase service on ALternative Methods to animal experimentation (DB-ALM) that provides ready-to-use and evaluated information about the application and development status of advanced alternative methods in a standardised manner. Information at various level of detail is provided and defined according to pre-determined criteria for data content by experts in the field. Current focus is given to in vitro methods and non-experimental approaches used for safety assessments of chemicals and/or formulations, but also includes methods for testing drugs or biologicals or for research purposes. The DB-ALM is a widely used public service with steady increasing interest and usage. To date, it can refer to more than 5000 registrations from over 82 countries covering users from academia, industry, regulatory communities and the animal welfare movement.
Since 2015, the DB-ALM Method Summary data sector provides a harmonised framework for adequately describing alternative methods in an OECD accepted format.
EURL ECVAM also revised its Tracking System for Alternative Test Methods towards Regulatory acceptance (TSAR). TSAR serves to track progress of an alternative method, in a transparent manner, from proposal for validation through to its final adoption and inclusion into the regulatory framework (EU, OECD and other related standards). TSAR includes summary descriptions of individual methods and all available records associated with different steps of the validation and acceptance process. TSAR disseminates information on test methods not only under consideration by EURL ECVAM but by all member organisations of ICATM. In this way an overall view of the methods under evaluation by all international validation centres is provided from one access point.
Of high relevance to Directive 2010/63/EU is the EURL ECVAM Search Guide (first published in 2012 with a re-edition in 2013). It has specifically been developed to inform and support untrained database users in finding high quality information on relevant alternative methods and strategies from the large amount of available information resources in an easy, yet systematic, and efficient way during project preparations in biomedical sciences. It is used as a resource for higher education in academic institutions in life sciences and by scientists and national authorities during the preparation and the evaluation of scientific projects that might involve animal use. It has now entered the Asian market where it was translated and re-published as a handbook and e-book in Korean. A Portuguese version is under development together with Brazilian Authorities within the framework of ICATM.
EURL ECVAM is further investigating Three Rs knowledge sharing opportunities with the aim to explore how sharing of knowhow and access to resources could be enhanced to accelerate overall progress in the Three Rs. This is explored in every domain where animals are used for a scientific purpose, be it for basic biological research, toxicological testing, or for training and education purposes.
Conclusions
Over the last decade, scientists and regulators have increasingly committed to the use of non-animal methods in the area of regulatory toxicity testing. Scientific and technological progress, negative public opinion towards severe animal testing procedures, as well as first successes in including non-animal methods into EU legislation paved the way for this more collective endeavor.
The search for alternatives to animal procedures dates back to the early 90s in Europe and was mainly driven by ethical concerns towards procedures on animals. In the US, an important milestone for advancing progress in this area occurred in 2007 with the publication by the National Research Council of a vision and a strategy for toxicity testing in the 21st century (NRC, 2007) and subsequent massive testing programmes like ToxCast and Tox21, mainly driven by economic interests.
Importantly, it has in general been recognised that new testing approaches often based on human cells and modeling mechanisms of human relevance could be more predictive and informative for human toxicity than the traditional tests based on animals. Notably also, the testing could be faster, cheaper and more efficient, in particular when assays are amenable to high-throughput and high-content screening.
However, for achieving a complete shift from the traditional testing on animals to modern toxicity testing using a combination of in silico and in vitro methods only, there is still some way to go. During this transition period it is therefore important that Directive 2010/63/EU is fully implemented. Thanks to that directive, rules on animal use are harmonised across the EU and meet high standards which should increase the welfare of animals in scientific research and testing.
It should also be borne in mind that the safety testing of chemicals accounts for less than 10% of animals used in the EU and that most animals are used for research purposes. Provisions in the directive to establish animal welfare bodies within the National Committees; systematic project evaluation by a competent authority of any proposed use of live animals; specific requirements on education, training and competence of personnel; and a more detailed and comprehensive reporting on animals used for scientific purposes are all extremely important.
It is difficult to judge if the directive had a direct impact on the number of alternative methods being developed, validated and adopted. The development of new methods (and subsequent validation/evaluation and uptake) mainly occurs when funding and market opportunities arise. This may happen, for example, when a sectorial legislation changes (e.g. banning of animal testing for cosmetic ingredients). However, the directive raised the awareness of scientists, in particular those working in research, regulators and legislators on the existence of alternative methods and approaches and the need to consider them. Therefore, continuing to increase awareness of the existence of Three Rs knowledge sources and free and easy access to them is crucial, starting with education across all three levels (i.e. high school, university and professional levels) extending to how, and in what form, this knowledge is communicated and disseminated to have an important positive impact.
In conclusion, Directive 2010/63/EU is extremely valuable, however many other changes of scientific, economic, social, legal and political nature need to happen in parallel in order to make this "paradigm shift" finally a reality.
References
Bal-Price A, Crofton KM, Leist M, Allen S, Arand M, Buetler T, Delrue N, FitzGerald RE, Hartung T, Heinonen T, Hogberg H, Bennekou SH, Lichtensteiger W, Oggier D, Paparella M, Axelstad M, Piersma A, Rached E, Schilter B, Schmuck G, Stoppini L, Tongiorgi E, Tiramani M, Monnet-Tschudi F, Wilks MF, Ylikomi T, Fritsche E. International STakeholder NETwork (ISTNET): creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes. Arch Toxicol. 2015 Feb;89(2):269-87.
Browne P, Judson R.S, Casey W, Kleinstreuer N.C and Thomas R.S. Screening Chemicals for Estrogen Receptor Bioactivity Using a Computational Model. Environ. Sci. Technol. 2015, 49, 8804−8814.
Busquet F, Strecker R, Rawlings JM, Belanger SE, Braunbeck T, Carr GJ, Cenijn P, Fochtman P, Gourmelon A, Hübler N, Kleensang A, Knöbel M, Kussatz C, Legler J, Lillicrap A, Martínez-Jerónimo F, Polleichtner C, Rzodeczko H, Salinas E, Schneider KE, Scholz S, van den Brandhof EJ, van der Ven LT, Walter-Rohde S, Weigt S, Witters H, Halder M (2014). OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regulatory Toxicology and Pharmacology 69: 496-511.
Ceridono M, Tellner P, Bauer D, et al. (2012). The 3T3 neutral red uptake phototoxicity test: practical experience and implications for phototoxicity testing-the report of an ECVAM-EFPIA workshop. Regul Toxicol Pharmacol. 63(3):480-8.
De Mattia F, Chapsal JM, Descamps J, Halder M, Jarrett N, Kross I, Mortiaux F, Ponsar C, Redhead K, McKelvie J and Hendriksen C (2011). The consistency approach for quality control of vaccines - a strategy to improve quality control and implement 3Rs. Biologicals 39(1): 59-65.
De Mattia F, Hendriksen C, Buchheit KH, Chapsal JM, Halder M, Lambrigts D, Redhead K, Rommel E, Scharton-Kersten T, Sesardic T, Viviani L, Ragan I (2015). The Vaccines Consistency Approach Project: an EPAA initiative. Pharmeuropa Bio & Scientific Notes May 2015, 30-54.
ECHA (2016) Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.7b:
Endpoint specific guidance. Version 3. February 2016. Available at:
https://echa.europa.eu/documents/10162/13632/information_requirements_r7b_en.pdf
EDQM (2012) Press release of 17 April 2012 "Vaccines for veterinary use adopted by the European Pharmacopoeia Commission at the 142nd Session: International harmonisation with VICH guidelines 41 and 42, deletion of the TABST and 3Rs. Available at: http://www.edqm.eu/medias/fichiers/vaccines_for_veterinary_use_adopted.pdf
EFSA (2011) Scientific opinion of the scientific committee on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 9 (9): 2379 [69 pp.] doi: 10.2903/j.efsa.2011.2379
EMA (2016) Guideline on the principles of regulatory acceptance of 3Rs (replacement, reduction, refinement) testing approaches. 15 December 2016 EMA/CHMP/CVMP/JEG-3Rs/450091/2012
EU (2012) Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. Official Journal of the European Union L167: 1-116.
EU (2013a) Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. Official Journal of the European Union L93: 1-84.
EU (2016a) COMMISSION REGULATION (EU) 2016/863 of 31 May 2016 amending Annexes VII and VIII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards skin corrosion/irritation, serious eye damage/eye irritation and acute toxicity. Official Journal of the European Union, L144, 27-31.
EU (2016b) Commission Regulation (EU) 2016/1688 of 20 September 2016 amending Annex VII to
Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration,
Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards skin sensitisation. Official Journal of the European Union L 255, p.14-16
EURL ECVAM (2014). EURL ECVAM recommendation on the Zebrafish Embryo Acute Toxicity Test Method (ZFET) for Acute Aquatic Toxicity Testing. Available at:
https://eurl-ecvam.jrc.ec.europa.eu/eurl-ecvam-recommendations/zfet-recommendation
Schwanig M, Nagel M, Duchow K, and Krämer B. (1997). Elimination of abnormal toxicity test for sera and certain vaccines in the European Pharmacopoeia. Vaccine 15, 1047–1048.
VICH (2013). VICH GL50 Harmonisation of criteria to waive target animal batch safety testing for inactivated vaccines for veterinary use. Available at:
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/03/WC500140354.pdf
Woutersen RA, et al., 2016 Regulatory Toxicology and Pharmacology 81: 242-249.
Zuang V et al. (2013) EURL ECVAM progress report on the development, validation and regulatory acceptance of alternative methods (2010-2013) prepared in the framework of directive 76/768/EEC and regulation (EC) No 1223/2009 on cosmetic products. Available from:
http://bookshop.europa.eu/en/eurl-ecvam-progress-report-on-the-development-validation-and-regulatory-acceptance-of-alternative-methods-2010-2013--pbLBNA25981/
Zuang V et al. (2014) EURL ECVAM status report on the development, validation and regulatory acceptance of alternative methods and approaches (2013-April 2014). JRC report EUR 26702 EN. Available from:
http://publications.jrc.ec.europa.eu/repository/
Zuang V et al. (2015) EURL ECVAM Status Report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches (2015). JRC report EUR 27474 EN. Available from:
http://publications.jrc.ec.europa.eu/repository/
Zuang V et al. (2016) EURL ECVAM Status Report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches (2016); JRC report EUR 28156 EN. Available from:
http://publications.jrc.ec.europa.eu/repository/

Figure 1: The role of EURL ECVAM in the evolution of regulatory methods/approaches and interactions with stakeholders
 EUROPEAN COMMISSION
EUROPEAN COMMISSION























































