EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document 02009R0669-20140101
Commission Regulation (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non-animal origin and amending Decision 2006/504/EC (Text with EEA relevance)
Consolidated text: Commission Regulation (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non-animal origin and amending Decision 2006/504/EC (Text with EEA relevance)
Commission Regulation (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non-animal origin and amending Decision 2006/504/EC (Text with EEA relevance)
2009R0669 — EN — 01.01.2014 — 015.001
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
COMMISSION REGULATION (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non-animal origin and amending Decision 2006/504/EC (OJ L 194, 25.7.2009, p.11) |
Amended by:
COMMISSION REGULATION (EC) No 669/2009
of 24 July 2009
implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non-animal origin and amending Decision 2006/504/EC
(Text with EEA relevance)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules ( 1 ), and in particular Article 15(5) and Article 63(1) thereof,
Having regard to Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety ( 2 ), and in particular Article 53(1),
Whereas:|
(1) |
Regulation (EC) No 882/2004 establishes a harmonised framework of general rules for the organisation of official controls at Community level, including official controls on the introduction of food and feed from third countries. In addition, it provides for a list to be drawn up of feed and food of non-animal origin that is on the basis of a known or emerging risk to be subject to an increased level of official controls at the point of entry into the territories referred to in Annex I thereto (the list). Such an increased level of control should allow, on the one hand, the known or emerging risk to be countered more effectively, and, on the other hand, the collection of accurate monitoring data on the occurrence and prevalence of unfavourable results from laboratory analysis. |
|
(2) |
In order to draw up the list, certain criteria, which would allow the identification of a known or emerging risk linked to a specific feed or food of non-animal origin, should be taken into account. |
|
(3) |
Pending the adoption of a standardised methodology and criteria for the setting up of the list, data resulting from notifications received through the rapid alert system for food and feed (RASFF), as established by Regulation (EC) No 178/2002, reports by the Food and Veterinary Office, reports received from third countries, exchanges of information between the Commission, Member States, and the European Food Safety Authority and scientific assessments, should be considered for the purposes of drawing up and updating the list. |
|
(4) |
Regulation (EC) No 882/2004 provides that Member States are, for the organisation of the increased level of controls, to designate particular points of entry which have access to the appropriate control facilities for the different types of feed and food. Accordingly, it is appropriate to set out in the present Regulation minimum requirements for designated points of entry in order to ensure a degree of uniformity in the effectiveness of the controls. |
|
(5) |
Regulation (EC) No 882/2004 provides that Member States are, for the organisation of the increased level of controls, to require feed and food business operators, responsible for consignments, to give prior notification of the arrival and nature of such consignments. Accordingly, a model form of common entry document (CED) should be laid down for imports of feed and food of non-animal origin covered by this Regulation, in order to ensure a uniform approach throughout the Community. The CED should be made available to the customs authorities when consignments are declared for the release for free circulation. |
|
(6) |
In addition, in order to ensure a certain level of uniformity at Community level with regard to the increased level of official controls, it is appropriate to lay down in this Regulation that those controls should cover documentary, identity and physical checks. |
|
(7) |
Adequate financial resources should be made available for organising the increased levels of official controls. Therefore, the Member States should collect the fees necessary to cover the costs occasioned by those controls. The calculation of those fees should be in accordance with the criteria laid down in Annex VI to Regulation (EC) No 882/2004. |
|
(8) |
Commission Decision 2005/402/EC of 23 May 2005 on emergency measures regarding chilli, chilli products, curcuma and palm oil ( 3 ) provides that all consignments of such products are to be accompanied by an analytical report demonstrating that the product does not contain any of the following substances: Sudan I (CAS number 842-07-9), Sudan II (CAS number 3118-97-6), Sudan III (CAS number 85-86-9) or Sudan IV (85-83-6). Since the adoption of those measures, the frequency of the notifications to the RASFF has decreased, which indicates a significant improvement in the situation as regards the presence of Sudan dyes in relevant products. It is therefore appropriate to discontinue the requirement to provide the analytical report for each consignment of imported products laid down in Decision 2005/402/EC and to establish instead a uniform, increased level of controls on those consignments at the point of entry into the Community. Decision 2005/402/EC should therefore be repealed. |
|
(9) |
Commission Decision 2006/504/EC of 12 July 2006 on special conditions governing certain foodstuffs imported from certain third countries due to contamination risks of these products by aflatoxins ( 4 ), provides for an increased frequency of controls (50 % of all consignments) to be carried for the presence of aflatoxins in peanuts originating from Brazil. Since the adoption of those measures, the frequency of the notifications to the RASFF in relation to aflatoxins in peanuts from Brazil has decreased. It is therefore appropriate to discontinue the measures provided for in Decision 2006/504 as regards such commodities, which should instead be subject to a uniform, increased level of controls at the point of entry into the Community. Decision 2006/504/EC should be amended accordingly. |
|
(10) |
The application of the minimum requirements for designated points of entry may present practical difficulties for the Member States. Therefore, this Regulation should provide for a transitional period during which those requirements may be progressively implemented. Accordingly, the competent authorities in the Member States should be allowed, during that transitional period, to carry out the required identity and physical checks at control points other than the designated point of entry. In those cases, such control points should comply with the minimum requirements for designated points of entry set out in this Regulation. |
|
(11) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS REGULATION:
Article 1
Subject matter
This Regulation lays down rules concerning the increased level of official controls to be carried out pursuant to Article 15(5) of Regulation (EC) No 882/2004 at the points of entry into the territories referred to Annex I thereto, on imports of the feed and food of non-animal origin listed in Annex I to this Regulation.
Article 2
Updates to Annex I
In order to set up and regularly amend the list in Annex I, at least the following sources of information shall be taken into account:
(a) data resulting from notifications received through the RASFF;
(b) reports and information resulting from the activities of the Food and Veterinary Office;
(c) reports and information received from third countries;
(d) information exchanged between the Commission and Member States, and the European Food Safety Authority;
(e) scientific assessments, where appropriate.
The list in Annex I shall be reviewed on a regular basis, and at least quarterly.
Article 3
Definitions
For the purposes of this Regulation, the following definitions shall apply:
(a) ‘common entry document (CED)’ means the document to be completed by the feed and food business operator or its representative as provided for in Article 6, a model of which is set out in Annex II, and by the competent authority confirming completion of official controls;
(b) ‘designated point of entry (DPE)’ means the point of entry provided for in the first indent of Article 17(1) of Regulation (EC) No 882/2004, into one of the territories referred to in Annex I thereto; in cases of consignments arriving by sea, which are unloaded for the purposes of being loaded on another vessel for onwards transportation to a port in another Member State, the designated point of entry shall be the latter port;
(c) ‘consignment’ means a quantity of any of the feed or food of non-animal origin listed in Annex I to this Regulation of the same class or description, covered by the same document(s), conveyed by the same means of transport and coming from the same third country or part of such country.
Article 4
Minimum requirements for designated points of entry
Without prejudice to Article 19, the designated points of entry shall have at least the following available:
(a) a sufficient number of suitably qualified and experienced staff to perform the prescribed checks on consignments;
(b) appropriate facilities for the competent authority to undertake the necessary checks;
(c) detailed instructions regarding sampling for analysis and the sending of such samples for analysis to a laboratory designated pursuant to Article 12(1) of Regulation (EC) No 882/2004 (the designated laboratory);
(d) facilities to store consignments (and containerised consignment) in appropriate conditions during the period of detention, where appropriate, awaiting the results of the analysis referred to in point (c), and a sufficient number of storage rooms, including cold stores, in cases where a controlled temperature is required due to the nature of the consignment;
(e) unloading equipment and appropriate equipment for carrying out sampling for analysis;
(f) the possibility to perform the unloading and the sampling for analysis in a sheltered place, where appropriate;
(g) a designated laboratory which can perform the analysis referred to in point (c), situated at a place to which the samples can be transported within a short period of time.
Article 5
List of designated points of entry
The Member States shall maintain and make publicly available on the Internet for each of the products listed in Annex I an up-to-date list of the designated points of entry. The Member States shall communicate the Internet addresses of these lists to the Commission.
The Commission shall display the national links to those lists on the Commission’s website, for information purposes.
Article 6
Prior notification of consignments
Feed and food business operators or their representatives shall give adequate prior notification of the estimated date and time of physical arrival of the consignment at the designated point of entry and of the nature of the consignment.
For that purpose, they shall complete Part I of the common entry document and transmit that document to the competent authority at the designated point of entry, at least one working day prior to the physical arrival of the consignment.
Article 7
Language of common entry documents
Common entry documents shall be drawn up in the official language, or in one of the official languages, of the Member State where the designated point of entry is located.
However, a Member State may consent to common entry documents being drawn up in another official language of the Community.
Article 8
Increased level of official controls at designated points of entry
1. The competent authority at the designated point of entry shall carry out without undue delay:
(a) documentary checks on all consignments within 2 working days from the time of arrival at the DPE, unless exceptional and unavoidable circumstances arise;
(b) identity and physical checks, including laboratory analysis, at the frequencies set out in Annex I, and in such a way that it is not possible for feed and food business operators or their representatives to predict whether any particular consignment will be subjected to such checks; the results of physical checks must be available as soon as technically possible.
2. After completion of the checks provided for in paragraph 1, the competent authority shall:
(a) complete the relevant part of Part II of the common entry document; and the responsible official of the competent authority shall stamp and sign the original of that document;
(b) make and retain a copy of the signed and stamped common entry document.
The original of the common entry document shall accompany the consignment on its onward transport until it reaches its destination as indicated in the CED.
The competent authority at the DPE may authorise onward transportation of the consignment pending the results of the physical checks. Where authorisation is given, the competent authority at the DPE shall notify the competent authority at the point of destination and appropriate arrangements shall be put in place to ensure that the consignment remains under the continuous control of the competent authorities and cannot be tampered with in any manner pending the results of the physical checks.
In cases where the consignment is transported pending the availability of results from the physical checks, a certified copy of the original CED shall be issued for that purpose.
Article 9
Special circumstances
1. On request of the Member State concerned, the Commission may authorise the competent authorities of certain designated points of entry operating under specific geographical constraints to carry out physical checks at the premises of a feed and food business operator, provided that the following conditions are met:
(a) the efficiency of controls carried out at the DPE is not adversely affected;
(b) the premises fulfil the requirements indicated in Article 4, as relevant, and are approved for that purpose by the Member State;
(c) appropriate arrangements are in place to guarantee that the consignment remains under the continuous control of the competent authorities of the DPE as from the moment of its arrival at the DPE and cannot be tampered with in any manner throughout all checks.
2. By derogation to Article 8(1), under exceptional circumstances, the decision to list a new product in Annex I may provide that identity and physical checks on consignments of that product can be carried out by the competent authority of the place of destination as indicated in the CED, if appropriate at the premises of the feed and food business operator if the conditions laid down in paragraph 1 (b) and (c) are satisfied, provided that the following conditions are met:
(a) the highly perishable nature of the product or the specific characteristics of the packaging are such that the performance of sampling operations at the DPE would inevitably result in a serious risk to food safety or in the product being damaged to an unacceptable extent;
(b) appropriate cooperation arrangements are put in place by the competent authorities at the DPE and the competent authorities performing the physical checks to ensure that:
(i) the consignment cannot be tampered with in any manner throughout all checks;
(ii) the reporting requirements laid down in Article 15 are fully met.
Article 10
Release for free circulation
The release for free circulation of consignments shall be subject to the presentation by the feed and food business operator or their representative to the custom authorities of a common entry document or its electronic equivalent duly completed by the competent authority once all controls required in accordance with Article 8(1) have been carried out and favourable results from physical checks, where such checks are required, are known.
Article 11
Obligations of feed and food business operators
In cases where the special characteristics of the consignment so warrant, feed and food business operator or their representative shall make available to the competent authority:
(a) sufficient human resources and logistics to unload the consignment, in order that the official controls may take place;
(b) the appropriate equipment for sampling for analysis as regards special transport and/or specific packaging forms, insofar as such sampling cannot be representatively performed with standard sampling equipment.
Article 12
Splitting of consignments
Consignments shall not be split until the increased level of official controls has been completed, and the common entry document has been completed by the competent authority as provided for in Article 8.
In the case of subsequent splitting of the consignment, an authenticated copy of the common entry document shall accompany each part of the consignment until it is released for free circulation.
Article 13
Non-compliance
If the official controls establish non-compliance, the responsible official of the competent authority shall complete Part III of the common entry document and action shall be taken pursuant to Articles 19, 20 and 21 of Regulation (EC) No 882/2004.
Article 14
Fees
1. Member States shall ensure the collection of fees occasioned by the increased level of official controls provided for in this Regulation in accordance with Article 27(4) of Regulation (EC) No 882/2004, and the criteria laid down in Annex VI to Regulation (EC) No 882/2004.
2. Feed and food business operators responsible for the consignment or their representatives shall pay the fees referred to in paragraph 1.
Article 15
Reporting to the Commission
1. Member States shall submit to the Commission a report on consignments, for the purposes of a continuous assessment of the feed and food of non-animal origin listed in Annex I.
That report shall be submitted quarterly by the end of the month following each quarter.
2. The report shall include the following information:
(a) details of each consignment, including:
(i) the size in terms of net weight of the consignment;
(ii) the country of origin of each consignment;
(b) the number of consignments subjected to sampling for analysis;
(c) the results of the checks as provided for in Article 8(1);
3. The Commission shall compile the reports received pursuant to paragraph 2 and make them available to the Member States.
Article 16
Amendment to Decision 2006/504/EC
Decision 2006/504/EC is amended as follows:
1. in Article 1, point (a) (iii), (iv) and (v) are deleted,
2. in Article 5, paragraph 2 (a) is replaced by the following:
‘(a) each consignment of foodstuffs from Brazil’,
3. in Article 7, paragraph 3 is deleted.
Article 17
Repeal of Decision 2005/402/EC
Commission Decision 2005/402/EC is repealed.
Article 18
Applicability
This Regulation shall enter into force on the 20th day following its publication in the Official Journal of the European Union.
It shall apply from 25 January 2010.
Article 19
Transitional measures
1. For a period of five years from the date of entry into force of this Regulation, where a designated point of entry is not equipped with the facilities required to carry out identity and physical checks as provided for in Article 8(1)(b), those checks may be carried out at another control point in the same Member State, authorised for that purpose by the competent authority, before goods are declared for release for free circulation, provided that such control point complies with the minimum requirements laid down in Article 4.
2. Member States shall make publicly available, by electronic publication on their website, a list of the control points authorised in accordance with paragraph 1.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
ANNEX I
Feed and food of non-animal origin subject to an increased level of official controls at the designated point of entry
|
Feed and food (intended use) |
CN code (1) |
TARIC sub-division |
Country of origin |
Hazard |
Frequency of physical and identity checks (%) |
|
Dried grapes (vine fruit) |
0806 20 |
Afghanistan (AF) |
Ochratoxin A |
50 |
|
|
(Food) |
|||||
|
— Groundnuts (peanuts), in shell |
— 1202 41 00 |
Brazil (BR) |
Aflatoxins |
10 |
|
|
— Groundnuts (peanuts), shelled |
— 1202 42 00 |
||||
|
— Peanut butter |
— 2008 11 10 |
||||
|
— Groundnuts (peanuts), otherwise prepared or preserved |
— 2008 11 91; 2008 11 96; 2008 11 98 |
||||
|
(Feed and food) |
|||||
|
Strawberries (frozen) |
0811 10 |
China (CN) |
Norovirus and hepatitis A |
5 |
|
|
(Food) |
|||||
|
Brassica oleracea (other edible Brassica, ‘Chinese Broccoli’) (2) |
ex070490 90 |
40 |
China (CN) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (3) |
20 |
|
(Food — fresh or chilled) |
|||||
|
Dried Noodles |
ex190211 00; ex190219 10; ex190219 90; ex190220 10; ex190220 30; ex190220 91; ex190220 99; ex190230 10; ex190230 10 |
10 10 10 10 10 10 10 10 91 |
China (CN) |
Aluminium |
10 |
|
(Food) |
|||||
|
Pomelos |
ex080540 00 |
31; 39 |
China (CN) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (4) |
20 |
|
(Food — fresh) |
|||||
|
Tea, whether or not flavoured |
0902 |
China (CN) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (5) |
10 |
|
|
(Food) |
|||||
|
— Aubergines |
— 0709 30 00; ex071080 95 |
72 |
Dominican Republic (DO) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (6) |
10 |
|
— Bitter melon (Momordica charantia) |
— ex070999 90; ex071080 95 |
70 70 |
|||
|
(Food — fresh, chilled or frozen vegetables) |
|||||
|
— Yardlong beans (Vigna unguiculata spp. sesquipedalis) |
— ex070820 00; ex071022 00 |
10 10 |
Dominican Republic (DO) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (6) |
20 |
|
— Peppers (sweet and other than sweet) (Capsicum spp.) |
— 0709 60 10; ex070960 99 |
20 |
|||
|
(Food — fresh, chilled or frozen vegetables) |
— 0710 80 51; ex071080 59 |
20 |
|||
|
— Oranges (fresh or dried) |
— 0805 10 20; 0805 10 80 |
Egypt (EG) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (7) |
10 |
|
|
— Strawberries |
— 0810 10 00 |
||||
|
(Food fresh fruits) |
|||||
|
Peppers (sweet and other than sweet) (Capsicum spp.) |
0709 60 10; ex070960 99; |
20 |
Egypt (EG) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (8) |
10 |
|
(Food — fresh, chilled or frozen) |
0710 80 51; ex071080 59 |
20 |
|||
|
— Capsicum annuum, whole |
— 0904 21 10 |
India (IN) |
Aflatoxins |
10 |
|
|
— Capsicum annuum, crushed or ground |
— ex090422 00 |
10 |
|||
|
— Dried fruit of the genus Capsicum, whole, other than sweet peppers (Capsicum annuum) |
— 0904 21 90 |
||||
|
— Curry (chilli products) |
— 0910 91 05 |
||||
|
— Nutmeg (Myristica fragrans) |
— 0908 11 00; 0908 12 00 |
||||
|
(Food — dried spices) |
|||||
|
— Nutmeg (Myristica fragrans) |
— 0908 11 00; 0908 12 00 |
Indonesia (ID) |
Aflatoxins |
20 |
|
|
(Food — dried spices) |
|||||
|
— Peas with pods (unshelled) |
— ex070810 00 |
40 |
Kenya (KE) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (9) |
10 |
|
— Beans with pods (unshelled) |
— ex070820 00 |
40 |
|||
|
(Food — fresh and chilled) |
|||||
|
Mint |
ex121190 86 |
30 |
Morocco (MA) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (10) |
10 |
|
(Food — fresh herb) |
|||||
|
Dried beans |
0713 39 00 |
Nigeria (NG) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (11) |
50 |
|
|
(Food) |
|||||
|
Watermelon (Egusi, Citrullus lanatus) seeds and derived products |
ex120770 00; ex110630 90; ex200899 99 |
10 30 50 |
Sierra Leone (SL) |
Aflatoxins |
50 |
|
(Food) |
|||||
|
Peppers (other than sweet) (Capsicum spp.) |
ex070960 99 |
20 |
Thailand (TH) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (12) |
10 |
|
(Food — fresh) |
|||||
|
— Coriander leaves |
— ex070999 90 |
72 |
Thailand (TH) |
Salmonella (13) |
10 |
|
— Basil (holy, sweet) |
— ex121190 86 |
20 |
|||
|
— Mint |
— ex121190 86 |
30 |
|||
|
(Food — fresh herbs) |
|||||
|
— Coriander leaves |
— ex070999 90 |
72 |
Thailand (TH) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (14) |
10 |
|
— Basil (holy, sweet) |
— ex121190 86 |
20 |
|||
|
(Food — fresh herbs) |
|||||
|
— Yardlong beans (Vigna unguiculata spp. sesquipedalis) |
— ex070820 00; ex071022 00 |
10 10 |
Thailand (TH) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (14) |
20 |
|
— Aubergines |
— 0709 30 00; ex071080 95 |
72 |
|||
|
(Food — fresh, chilled or frozen vegetables) |
|||||
|
— Sweet Peppers (Capsicum annuum) |
— 0709 60 10; 0710 80 51 |
Turkey (TR) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (15) |
10 |
|
|
(Food — fresh, chilled or frozen vegetables) |
|||||
|
Dried grapes (vine fruit) |
0806 20 |
Uzbekistan (UZ) |
Ochratoxin A |
50 |
|
|
(Food) |
|||||
|
— Coriander leaves |
— ex070999 90 |
72 |
Vietnam (VN) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (16) |
20 |
|
— Basil (holy, sweet) |
— ex121190 86 |
20 |
|||
|
— Mint |
— ex121190 86 |
30 |
|||
|
— Parsley |
— ex070999 90 |
40 |
|||
|
(Food — fresh herbs) |
|||||
|
— Okra |
— ex070999 90 |
20 |
Vietnam (VN) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (16) |
20 |
|
— Peppers (other than sweet) (Capsicum spp.) |
— ex070960 99 |
20 |
|||
|
(Food — fresh) |
|||||
|
(1) Where only certain products under any CN code are required to be examined and no specific subdivision under that code exists in the goods nomenclature, the CN code is marked ‘ex’. (2) Species of Brassica oleracea L. convar. Botrytis (L) Alef var. Italica Plenck, cultivar alboglabra. Also known as ‘Kai Lan’, ‘Gai Lan’, ‘Gailan’, ‘Kailan’, ‘Chinese bare Jielan’. (3) In particular residues of: Chlorfenapyr, Fipronil (sum fipronil + sulfone metabolite (MB46136) expressed as fipronil), Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim), Acetamiprid, Dimethomorph, Propiconazole. (4) In particular residues of: Triazophos, Triadimefon and Triadimenol (sum of triadimefon and triadimenol), Parathion-methyl (sum of Parathion-methyl and paraoxon-methyl expressed as Parathion-methyl), Phenthoate, Methidathion. (5) In particular residues of: Buprofezin; Imidacloprid; Fenvalerate and Esfenvalerate (sum of RS & SR isomers); Profenofos; Trifluralin; Triazophos; Triadimefon and Triadimenol (sum of triadimefon and triadimenol), Cypermethrin (cypermethrin including other mixtures of constituent isomers (sum of isomers)). (6) In particular residues of: Amitraz (amitraz including the metabolites containing the 2,4 -dimethylaniline moiety expressed as amitraz), Acephate, Aldicarb (sum of aldicarb, its sulfoxide and its sulfone, expressed as aldicarb), Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim), Chlorfenapyr, Chlorpyrifos, Dithiocarbamates (dithiocarbamates expressed as CS2, including maneb, mancozeb, metiram, propineb, thiram and ziram), Diafenthiuron, Diazinon, Dichlorvos, Dicofol (sum of p, p' and o,p' isomers), Dimethoate (sum of dimethoate and omethoate expressed as dimethoate), Endosulfan (sum of alpha- and beta-isomers and endosulfan-sulphate expresses as endosulfan), Fenamidone, Imidacloprid, Malathion (sum of malathion and malaoxon expressed as malathion), Methamidophos, Methiocarb (sum of methiocarb and methiocarb sulfoxide and sulfone, expressed as methiocarb), Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl), Monocrotophos, Oxamyl, Profenofos, Propiconazole, Thiabendazole, Thiacloprid. (7) In particular residues of: Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim), Cyfluthrin (cyfluthrin including other mixtures of constituent isomers (sum of isomers)) Cyprodinil, Diazinon, Dimethoate (sum of dimethoate and omethoate expressed as dimethoate), Ethion, Fenitrothion, Fenpropathrin, Fludioxonil, Hexaflumuron, Lambda-cyhalothrin, Methiocarb (sum of methiocarb and methiocarb sulfoxide and sulfone, expressed as methiocarb), Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl), Oxamyl, Phenthoate, Thiophanate-methyl. (8) In particular residues of: Carbofuran (sum of carbofuran and 3-hydroxy-carbofuran expressed as carbofuran), Chlorpyrifos, Cypermethrin (cypermethrin including other mixtures of constituent isomers (sum of isomers)), Cyproconazole, Dicofol (sum of p, p' and o,p' isomers), Difenoconazole, Dinotefuran, Ethion, Flusilazole, Folpet, Prochloraz (sum of prochloraz and its metabolites containing the 2,4,6-Trichlorophenol moiety expressed as prochloraz), Profenofos, Propiconazole, Thiophanate-methyl, Triforine. (9) In particular residues of: Dimethoate (sum of dimethoate and omethoate expressed as dimethoate), Chlorpyrifos, Acephate, Methamidophos, Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl), Diafenthiuron, Indoxacarb as sum of the isomers S and R. (10) In particular residues of: Chlorpyrifos, Cypermethrin (cypermethrin including other mixtures of constituent isomers (sum of isomers)), Dimethoate (sum of dimethoate and omethoate expressed as dimethoate), Endosulfan (sum of alpha- and beta-isomers and endosulfan-sulphate expresses as endosulfan), Hexaconazole, Parathion-methyl (sum of Parathion-methyl and paraoxon-methyl expressed as Parathion-methyl), Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl), Flutriafol, Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim), Flubendiamide, Myclobutanyl, Malathion (sum of malathion and malaoxon expressed as malathion). (11) In particular residues of Dichlorvos. (12) In particular residues of: Carbofuran (sum of carbofuran and 3-hydroxy-carbofuran expressed as carbofuran), Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl), Dimethoate (sum of dimethoate and omethoate expressed as dimethoate), Triazophos, Malathion (sum of malathion and malaoxon expressed as malathion), Profenofos, Prothiofos, Ethion, Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim), Triforine, Procymidone, Formetanate: Sum of formetanate and its salts expressed as formetanate(hydrochloride). (13) Reference method EN/ISO 6579 or a method validated against it as referred to in Article 5 of Commission Regulation (EC) No 2073/2005 (OJ L 338, 22.12.2005, p. 1). (14) In particular residues of: Acephate, Carbaryl, Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim), Carbofuran (sum of carbofuran and 3-hydroxy-carbofuran expressed as carbofuran), Chlorpyrifos, Chlorpyrifos-methyl, Dimethoate (sum of dimethoate and omethoate expressed as dimethoate), Ethion, Malathion (sum of malathion and malaoxon expressed as malathion), Metalaxyl and metalaxyl-M (metalaxyl including other mixtures of constituent isomers including metalaxyl-M (sum of isomers)), Methamidophos, Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl), Monocrotophos, Profenofos, Prothiofos, Quinalphos, Triadimefon and Triadimenol (sum of triadimefon and triadimenol), Triazophos, Dicrotophos, EPN, Triforine. (15) In particular residues of: Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl), Oxamyl, Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim), Clofentezine, Diafenthiuron, Dimethoate (sum of dimethoate and omethoate expressed as dimethoate), Formetanate: Sum of formetanate and its salts expressed as formetanate(hydrochloride), Malathion (sum of malathion and malaoxon expressed as malathion), Procymidone, Tetradifon, Thiophanate-methyl. (16) In particular residues of: Carbofuran (sum of carbofuran and 3-hydroxy-carbofuran expressed as carbofuran), Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim), Chlorpyrifos, Profenofos, Permethrin (sum of isomers), Hexaconazole, Difenoconazole, Propiconazole, Fipronil (sum fipronil + sulfone metabolite (MB46136) expressed as fipronil), Propargite, Flusilazole, Phenthoate, Cypermethrin (cypermethrin including other mixtures of constituent isomers (sum of isomers)), Methomyl and Thiodicarb (sum of methomyl and thiodicarb expressed as methomyl), Quinalphos, Pencycuron, Methidathion, Dimethoate (sum of dimethoate and omethoate expressed as dimethoate), Fenbuconazole. |
|||||
ANNEX II
COMMON ENTRY DOCUMENT (CED)
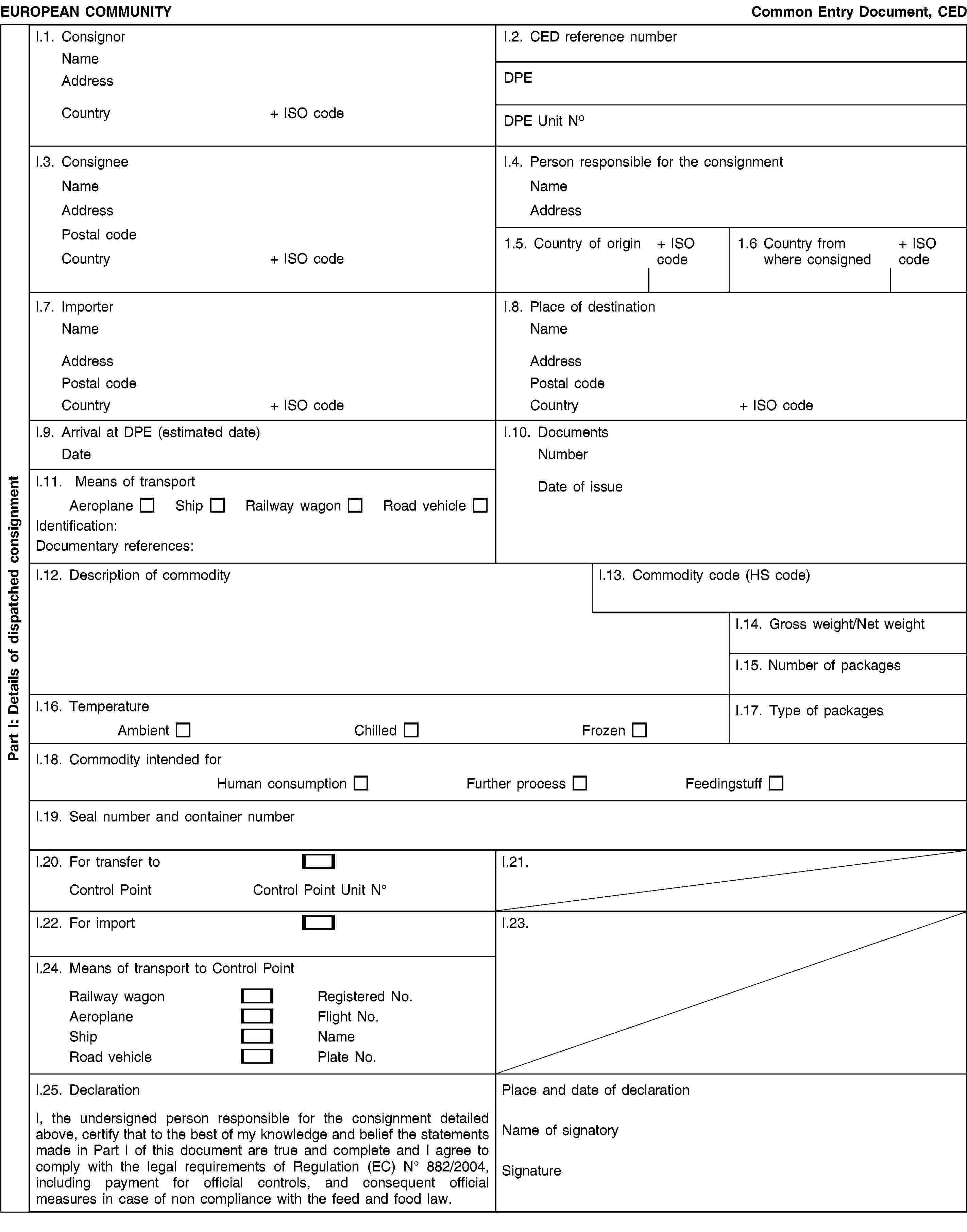
Notes for guidance for the CED
|
General |
: |
Complete the common entry document in capital letters. Notes are shown against the relevant box number. |
|
Part I |
This Part is to be completed by the feed and food business operator or their representative, unless otherwise indicated. |
|
Box I.1. |
Consignor: name and full address of the natural or legal person (feed and food business operator) dispatching the consignment. Information concerning telephone and fax numbers or an e-mail address is recommended. |
|
Box I.2. |
This box must be filled in by the competent authority of the designated point of entry (DPE). |
|
Box I.3. |
Consignee: name and full address of the natural or legal person (feed and food business operator) to whom the consignment is destined. Information on telephone and fax numbers or an e-mail address is recommended. |
|
Box I.4. |
The person responsible for the consignment: the person (feed and food business operator or their representative or the person making the declaration on their behalf) who is in charge of the consignment when it is presented at the DPE and who makes the necessary declarations to the competent authority at the DPE on behalf of the importer. Insert the name and full address. Information on telephone and fax numbers or an e-mail address is recommended. |
|
Box I.5. |
Country of origin: this refers to the third country where the commodity is originating from, grown, harvested or produced. |
|
Box I.6. |
Country from where consigned: this refers to the third country where the consignment was placed aboard the means of final transport for the journey to the Union. |
|
Box I.7. |
Importer: name and full address. Information on telephone and fax numbers or an e-mail address is recommended. |
|
Box I.8. |
Place of destination: delivery address in the Union. Information on telephone and fax numbers or an e-mail address is recommended. |
|
Box I.9. |
Arrival at DPE: insert the estimated date on which the consignment is expected to arrive at the DPE. |
|
Box I.10. |
Documents: insert the date of issue and the number of official documents accompanying the consignment, as appropriate. |
|
Box I.11. |
Give full details of the means of arrival transport: for aircrafts the flight number, for vessels the ship name, for road vehicles the registration number plate with trailer number if appropriate, for railway vehicles the train identity and wagon number. Documentary references: number of airway bill, bill of lading or commercial number for railway or road vehicle. |
|
Box I.12. |
Description of the commodity: provide a detailed description of the commodity (including for feed the type of feed). |
|
Box I.13. |
Commodity or HS code of the Harmonized System of the World Customs Organization. |
|
Box I.14. |
Gross weight: overall weight in kg. This is defined as the aggregate mass of the products and of the immediate containers and all their packaging, but excluding transport containers and other transport equipment. Net weight: weight of actual product in kg, excluding packaging. This is defined as the mass of the products themselves without immediate containers or any packaging. |
|
Box I.15. |
Number of packages. |
|
Box I.16. |
Temperature: tick the appropriate mode of transport/storage temperature. |
|
Box I.17. |
Type of packages: identify the type of packaging of products. |
|
Box I.18. |
Commodity intended for: tick the appropriate box depending on whether the commodity is destined for human consumption without prior sorting or other physical treatment (in this case tick ‘human consumption’) or is intended for human consumption after such treatment (tick ‘further process’ in this case), or is intended for use as ‘feedingstuff’ (in this case tick ‘feedingstuffs’). |
|
Box I.19. |
Give all seal and container identification numbers where relevant. |
|
Box I.20. |
Transfer to a control point: During the transitional period provided for in Article 19(1), the DPE shall tick this box to allow onward transportation to another control point. |
|
Box I.21. |
Not applicable. |
|
Box I.22. |
For import: this box is to be ticked where the consignment is intended for importation into the Union (Article 8). |
|
Box I.23. |
Not applicable. |
|
Box I.24. |
Tick the appropriate means of transport. |
|
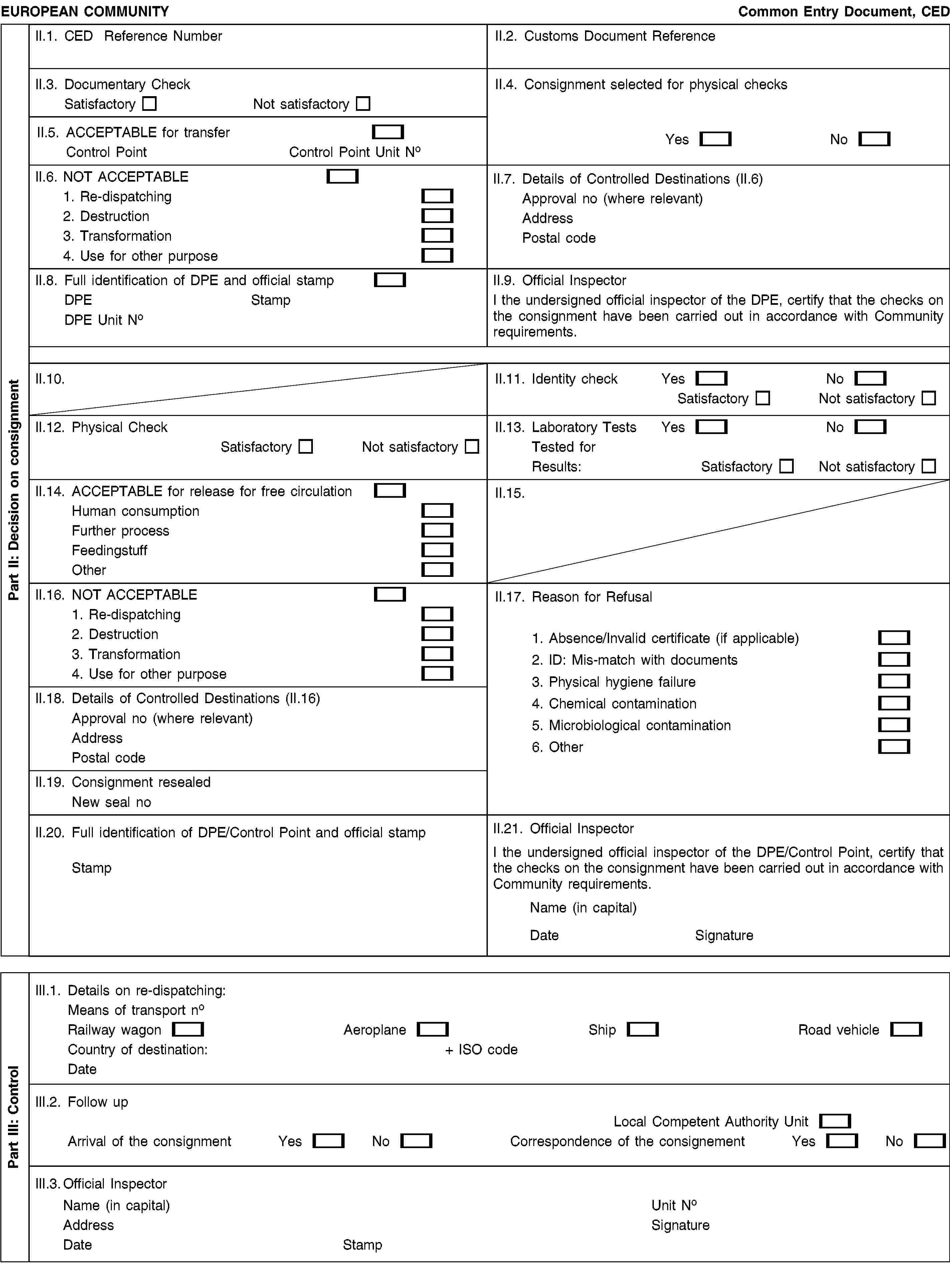
Part II |
This Part is to be completed by the competent authority. |
|
Box II.1. |
Use the same reference number as in Box I.2. |
|
Box II.2. |
For use by customs services, if necessary. |
|
Box II.3. |
Documentary check: to be completed for all consignments. |
|
Box II.4. |
The competent authority of the DPE shall indicate whether the consignment is selected for physical checks, which during the transitional period provided for in Article 19(1) may be carried out at a different control point. |
|
Box II.5. |
The competent authority of the DPE shall indicate, during the transitional period provided for in Article 19(1), following a satisfactory documentary check, to which control point the consignment may be transported in order for identity and physical checks to be carried out. |
|
Box II.6. |
Indicate clearly the action to be taken in the case of rejection of the consignment due to the unsatisfactory outcome of the documentary checks. The address of the establishment of destination in case of ‘Re-dispatching’, ‘Destruction’, ‘Transformation’ and ‘Use for other purpose’ must be entered in Box II.7. |
|
Box II.7. |
Give as appropriate approval number and address (or ship name and port) for all destinations where further control of the consignment is required, for example for Box II.6, ‘Re-dispatching’, ‘Destruction’, ‘Transformation’ or ‘Use for other purpose’. |
|
Box II.8. |
Put the official stamp of the competent authority of the DPE here. |
|
Box II.9. |
Signature of the responsible official of the competent authority of the DPE. |
|
Box II.10. |
Not applicable. |
|
Box II.11. |
The competent authority of the DPE or, during the transitional period provided for in Article 19(1), the competent authority of the control point, shall indicate the results of the identity checks here. |
|
Box II.12. |
The competent authority of the DPE or, during the transitional period provided for in Article 19(1), the competent authority of the control point, shall indicate the results of the physical checks here. |
|
Box II.13. |
The competent authority of the DPE or, during the transitional period provided for in Article 19(1), the competent authority of the control point, shall indicate the results of the laboratory test here. Complete this box with the category of substance or pathogen for which a laboratory test has been carried out. |
|
Box II.14. |
This box is to be used for all consignments to be released for free circulation within the Union. |
|
Box II.15. |
Not applicable. |
|
Box II.16. |
Indicate clearly the action to be taken in the case of rejection of the consignment due to the unsatisfactory outcome of the identity or physical checks. The address of the establishment of destination in case of ‘Re-dispatching’, ‘Destruction’, ‘Transformation’ and ‘Use for other purpose’ must be entered in Box II.18. |
|
Box II.17. |
Reasons for refusal: use, as appropriate, to add relevant information. Tick the appropriate box. |
|
Box II.18. |
Give, as appropriate, the approval number and address (or ship name and port) for all destinations where further control of the consignment is required, for example, for Box II.16, ‘Re-dispatching’, ‘Destruction’, ‘Transformation’ or ‘Use for other purpose’. |
|
Box II.19. |
Use this box when the original seal recorded on a consignment is destroyed on opening the container. A consolidated list of all seals that have been used for this purpose must be kept. |
|
Box II.20. |
Put the official stamp of the competent authority of the DPE here or, during the transitional period provided for in Article 19(1), of the competent authority of the control point. |
|
Box II.21. |
Signature of the responsible official of the competent authority of the DPE or, during the transitional period provided for in Article 19(1), of the competent authority of the control point. |
|
Part III |
This Part is to be completed by the competent authority. |
|
Box III.1. |
Details on re-dispatching: the competent authority of the DPE or, during the transitional period provided for in Article 19(1), the competent authority of the control point, shall indicate the means of transport used, its identification details, the country of destination and the date of re-dispatching, as soon as they are known. |
|
Box III.2. |
Follow-up: indicate the Local Competent Authority Unit responsible, as appropriate, for the supervision in case of ‘Destruction’, ‘Transformation’ or ‘Use for other purpose’ of the consignment. That authority shall report the result of the arrival of the consignment and the correspondence of the consignment in this box. |
|
Box III.3. |
Signature of the responsible official for the competent authority of the DPE or, during the transitional period provided for in Article 19(1), the responsible official for the control point, in case of ‘Re-dispatching’. Signature of the responsible official for the local competent authority in case of ‘Destruction’, ‘Transformation’ or ‘Use for other purpose’. |
( 1 ) OJ L 165, 30.4.2004, p. 1.
( 2 ) OJ L 31, 1.2.2002, p. 1.
( 3 ) OJ L 135, 28.5.2005, p. 34.
( 4 ) OJ L 199, 21.7.2006, p. 21.


