EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document C:2015:421:FULL
Official Journal of the European Union, C 421, 17 December 2015
Official Journal of the European Union, C 421, 17 December 2015
Official Journal of the European Union, C 421, 17 December 2015
Display all documents published in this Official Journal
|
ISSN 1977-091X |
||
|
Official Journal of the European Union |
C 421 |
|

|
||
|
English edition |
Information and Notices |
Volume 58 |
|
Notice No |
Contents |
page |
|
|
II Information |
|
|
|
INFORMATION FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES |
|
|
|
European Commission |
|
|
2015/C 421/01 |
Non-opposition to a notified concentration (Case M.7678 — Equinix/Telecity) ( 1 ) |
|
|
2015/C 421/02 |
Non-opposition to a notified concentration (Case M.7848 — ATP/AXA/Club Quarters/Cleavon) ( 1 ) |
|
|
IV Notices |
|
|
|
NOTICES FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES |
|
|
|
Council |
|
|
2015/C 421/03 |
||
|
2015/C 421/04 |
||
|
|
European Commission |
|
|
2015/C 421/05 |
||
|
|
Court of Auditors |
|
|
2015/C 421/06 |
||
|
|
NOTICES CONCERNING THE EUROPEAN ECONOMIC AREA |
|
|
|
European Commission |
|
|
2015/C 421/07 |
Public holidays in 2016: EEA EFTA States and EEA institutions |
|
|
V Announcements |
|
|
|
PROCEDURES RELATING TO THE IMPLEMENTATION OF THE COMMON COMMERCIAL POLICY |
|
|
|
European Commission |
|
|
2015/C 421/08 |
||
|
|
PROCEDURES RELATING TO THE IMPLEMENTATION OF COMPETITION POLICY |
|
|
|
European Commission |
|
|
2015/C 421/09 |
Prior notification of a concentration (Case M.7823 — Acciona/Nordex) — Candidate case for simplified procedure ( 1 ) |
|
|
2015/C 421/10 |
Prior notification of a concentration (Case M.7880 — Mitsubishi Heavy Industries/UniCarriers Holdings) — Candidate case for simplified procedure ( 1 ) |
|
|
2015/C 421/11 |
Prior notification of a concentration (Case M.7887 — Goldman Sachs/Astorg Asset Management/HRA Pharma) — Candidate case for simplified procedure ( 1 ) |
|
|
|
|
|
(1) Text with EEA relevance |
|
EN |
|
II Information
INFORMATION FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES
European Commission
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/1 |
Non-opposition to a notified concentration
(Case M.7678 — Equinix/Telecity)
(Text with EEA relevance)
(2015/C 421/01)
On 13 November 2015, the Commission decided not to oppose the above notified concentration and to declare it compatible with the internal market. This decision is based on Article 6(1)(b) in conjunction with Article 6(2) of Council Regulation (EC) No 139/2004 (1). The full text of the decision is available only in English and will be made public after it is cleared of any business secrets it may contain. It will be available:
|
— |
in the merger section of the Competition website of the Commission (http://ec.europa.eu/competition/mergers/cases/). This website provides various facilities to help locate individual merger decisions, including company, case number, date and sectoral indexes, |
|
— |
in electronic form on the EUR-Lex website (http://eur-lex.europa.eu/homepage.html?locale=en) under document number 32015M7678. EUR-Lex is the online access to European law. |
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/1 |
Non-opposition to a notified concentration
(Case M.7848 — ATP/AXA/Club Quarters/Cleavon)
(Text with EEA relevance)
(2015/C 421/02)
On 10 December 2015, the Commission decided not to oppose the above notified concentration and to declare it compatible with the internal market. This decision is based on Article 6(1)(b) of Council Regulation (EC) No 139/2004 (1). The full text of the decision is available only in English and will be made public after it is cleared of any business secrets it may contain. It will be available:
|
— |
in the merger section of the Competition website of the Commission (http://ec.europa.eu/competition/mergers/cases/). This website provides various facilities to help locate individual merger decisions, including company, case number, date and sectoral indexes, |
|
— |
in electronic form on the EUR-Lex website (http://eur-lex.europa.eu/homepage.html?locale=en) under document number 32015M7848. EUR-Lex is the online access to European law. |
IV Notices
NOTICES FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES
Council
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/2 |
Council conclusions on personalised medicine for patients
(2015/C 421/03)
THE COUNCIL OF THE EUROPEAN UNION,
|
1. |
RECALLS that under Article 168 of the Treaty on the Functioning of the European Union, a high level of human health protection shall be ensured in the definition and implementation of all Union policies and activities, and that Union action, which is to complement national policies, shall be directed towards improving public health. The Union shall encourage cooperation between the Member States in the field of public health and, if necessary, lend support to their action. Union action shall fully respect the responsibilities of the Member States for the organisation and delivery of health services and medical care, including allocation of the resources assigned to them; |
|
2. |
RECALLS the Council conclusions on common values and principles in European Union health systems adopted on 2 July 2006 (1), which define a set of operating principles shared across the European Union, especially regarding patient involvement and quality and safety of care, and which emphasise in particular that all European Union health systems aim to be patient-centred; |
|
3. |
RECALLS the Council conclusions on innovation in the medical device sector adopted on 6 June 2011 (2), which recognise that innovative medical devices could improve health and quality of life for patients and could contribute to addressing the sustainability of healthcare systems, and that innovation should be increasingly patient-centred; |
|
4. |
RECALLS the Council recommendation of 8 June 2009 on an action in the field of rare diseases (2009/C 151/02) and the incentives offered by Regulation (EC) No 141/2000 of the European Parliament and of the Council (3) on orphan medicinal products which are also used to encourage the development and authorisation of medicinal products for small populations; |
|
5. |
RECALLS the Council conclusions on the reflection process on modern, responsive and sustainable health systems adopted on 10 December 2013 (4), the Council conclusions on the economic crisis and healthcare adopted on 20 June 2014 (5), as well as the Council conclusions on innovation for the benefit of patients adopted on 1 December 2014 (6), which, while stressing the need to fully respect areas of Member States competence, advocate the need for cooperation on strategies to effectively manage expenditure on pharmaceuticals and medical devices, while ensuring equitable access to effective medicines within sustainable national healthcare systems; the Council conclusions on innovation for the benefit of patients have been followed-up by work in the Working Party on Public Health at Senior Level, including possible topics to serve as a basis for future discussions (7); |
|
6. |
TAKES NOTE of the European Commission Staff Working Document on the use of ‘-omics’ technologies in the development of personalised medicine (8), which highlights the potential and issues in the development of personalised medicine and concludes that the development of personalised medicine offers through the use of ‘-omics’ technologies new opportunities for the treatment of patients in the European Union. It proposes that through this approach, healthcare providers may be able to offer better-targeted treatment, avoid medical errors and reduce adverse reactions to medicinal products. It also identifies several challenges to the implementation and uptake of personalised medicine in health systems; |
|
7. |
TAKES NOTE of the World Health Organisation (WHO) 2013 Priority Medicines Report (9), that discusses the role and the current limitations of personalised medicine, called ‘stratified medicine’ in the context of the report, and recommends investments to further strengthen research in and knowledge of stratified medicine and pharmacogenomics; |
|
8. |
NOTES that there is no commonly agreed definition of the term ‘personalised medicine’. However, it is widely understood that personalised medicine refers to a medical model using characterisation of individuals’ phenotypes and genotypes (e.g. molecular profiling, medical imaging, lifestyle data) for tailoring the right therapeutic strategy for the right person at the right time, and/or to determine the predisposition to disease and/or to deliver timely and targeted prevention. Personalised medicine relates to the broader concept of patient-centred care, which takes into account that, in general, healthcare systems need to better respond to patient needs; |
|
9. |
NOTES that, as DNA sequencing technologies, and other advanced ‘-omics’ technologies for the identification of multiple biomarkers are developing rapidly, there is the expectation that these developments could make it possible to use detailed risk profiling as an additional tool for targeted interventions, aiming at and potentially improving health outcomes and over time allowing for a more cost-efficient use of healthcare; |
|
10. |
NOTES that, with the development of personalised medicine, individuals and health systems face new challenges, including balancing its risks and benefits while also considering its ethical, financial, social and legal implications, particularly regarding pricing and reimbursement, data protection and public interest in processing personal data; |
|
11. |
NOTES that the development and implementation of personalised medicine goes hand-in-hand with the development of relevant diagnostics; |
|
12. |
NOTES WITH CONCERN that not all patients have access to innovative methods of better-targeted prevention, diagnosis and treatments, and that a significant challenge for Member States consists in promoting appropriate uptake in healthcare systems, in order to ensure integration into clinical practice in line with the principles of solidarity and universal and equal access to high quality of care, while fully respecting Member States’ competences, and ensuring the sustainability of their national health systems; |
|
13. |
NOTES that personalised medicine is becoming a reality in research, particularly following the support of the seventh framework programme for research, technological development and demonstration activities, which dedicated over EUR 1 billion to underpin personalised medicine for the period 2007-2013 (10). Funding research for personalised medicine will continue through the framework programme for research and innovation, Horizon 2020 (11), including through actions carried out under the Innovative Medicines Initiative (IMI) (12); |
|
14. |
WELCOMES the high-level conference of 8 July 2015‘Making access to personalised medicine a reality for patients’, which addressed obstacles to the integration of personalised medicine into European Union healthcare systems, identified best practices and their added value, and outlined the potential benefits of personalised medicine for public health and its impact on policy-making in the European Union. Involving public health decision-makers, regulators, payers and patients, the conference also underlined the need to define a patient-centred approach to personalised medicine at European Union level, as well as a comprehensive approach integrating the different phases along the life cycle of personalised medicine products in such a way as to facilitate its integration into clinical practice. |
INVITES THE MEMBER STATES TO:
|
15. |
SUPPORT access, as appropriate, according to national provisions, to clinically effective and financially sustainable personalised medicine by developing patient-centred policies including, as appropriate, patient empowerment and the integration of patient perspectives in the development of regulation processes, in cooperation with patient organisations and other relevant stakeholders; |
|
16. |
USE genomics information with a view to integrating advances in human genomics into public health research, policy and programmes, in compliance with existing national provisions concerning personal data and genomics; |
|
17. |
DEVELOP OR STRENGTHEN, if necessary, public health communication strategies, based on available, objective, balanced and non-promotional data to increase public awareness as regards both the benefits and risks of personalised medicine, as well as the citizens’ role and rights, thus supporting appropriate access to innovative diagnostic methods and better-targeted treatment; |
|
18. |
PUT in place information and awareness strategies for patients, based on available, objective, balanced and non-promotional data, in order to improve health literacy and access to reliable, relevant and understandable information on existing treatment options, including expected benefits and risks, thus enabling patients to actively cooperate with healthcare professionals in choosing the most appropriate treatment strategies; |
|
19. |
PROVIDE education, training and continuing professional development for health professionals in order to equip them with the necessary knowledge, skills and competences to make the most of the benefits that personalised medicine brings to patients and healthcare systems; |
|
20. |
FOSTER cooperation in the collection, sharing, management and appropriate standardisation of data necessary for effective research into, and development and application of personalised medicine, in compliance with data protection legislation; |
|
21. |
PROMOTE cross-disciplinary interaction, notably between specialists in genetics, in using statistical methodologies, bio- and health informatics and epidemiology and among health professionals, in order to ensure better understanding of the available data, more efficient integration and interpretation of information from multiple sources and appropriate decision-making on treatment options; |
|
22. |
DEVELOP OR ADJUST, where necessary, procedures aiming to evaluate the impact of personalised medicine, in particular health technology assessment (HTA) procedures, to the specific nature of personalised medicine, taking into account, inter alia, added value from the patients perspective as well as enhanced cooperation and exchange of best practices, while fully respecting Member States’ competences; |
|
23. |
RECOGNISE the potential of clinical and population-based biobanks for accelerating the discovery and development of new medicinal products; support the standardisation and networking of biobanks to combine and share resources, in compliance with data protection legislation; |
|
24. |
CONSIDER exchange of information and best practices within the existing fora, which could support both appropriate access for patients to personalised medicines, as well as the sustainability of health systems; |
|
25. |
CONSIDER developing long-term, patient-centred, strategic approaches on how to meet, with a public health perspective, the challenges associated with access to personalised medicine, while ensuring the sustainability of national health systems and fully respecting Member States’ competences; |
|
26. |
EXCHANGE best practices in the field of personalised medicine and facilitate its appropriate use in health care practice. |
INVITES THE MEMBER STATES AND THE COMMISSION TO:
|
27. |
CONTINUE voluntary joint work, including the development of guidance and the definition of criteria, to support HTA on personalised medicine in accordance with the HTA strategy (13), while fully respecting Member States competences; |
|
28. |
FOSTER enhanced cooperation between Member States within the HTA Network established in accordance with the Directive on the application of patients’ rights in cross-border healthcare and HTA bodies under the future Joint Action; |
|
29. |
PROMOTE the interoperability of electronic health records to facilitate their use for public health and research, through the eHealth Network established in accordance with the Directive on the application of patients’ rights in cross-border healthcare, taking advantage of the support from the Connecting Europe Facility (14); |
|
30. |
DEVELOP common principles on data collection based on standards and a sound legal framework and enabling the processing of patient data and the availability of comparable data at European Union level, allowing secondary use and analysis of data on a larger scale in compliance with data protection legislation, while fully respecting Member States’ competences; |
|
31. |
ENCOURAGE early dialogue and provision of parallel scientific advice between innovators, regulators and HTA bodies, taking into account, as appropriate, input from patients, healthcare professionals and payers, to support evidence generation and regulatory authorisation, while fully respecting Member States competences; |
|
32. |
ENCOURAGE dialogue with Member States’ authorities and stakeholders to facilitate step-by-step implementation of the public health genomics approach both at European Union and national level on the basis of past European Union initiatives, such as the European Best Practice Guidelines for Quality Assurance, Provision and Use of Genome-based Information and Technologies — Public Health Genomics European Network (15), and facilitate ongoing European Union initiatives such as the position paper on Public Health Genomics in Cancer, to be developed under the Joint Action on Comprehensive Cancer Control with the support of the Commission expert groups on cancer control and on rare diseases; |
|
33. |
TAKE personalised medicine into account in the broader context of the future framework for sustainable European Union collaboration on patient safety and quality of care, requested in the Council conclusions on patient safety and quality of care of 1 December 2014; |
|
34. |
CONTINUE the work of the Expert Group on Safe and Timely Access to Medicines for Patients (STAMP), which analyses issues related to the implementation of European Union pharmaceutical legislation with the aim of identifying ways to maximise effective use of existing European Union regulatory tools and further improve safe and timely access of medicines for patients, including innovative medicinal products; continue, within the STAMP expert group, to monitor progress on the adaptive pathway pilot project undertaken by the European Medicines Agency and its potential to allow early authorisation of a medicine for use in a well-defined patient population with a high level of medical need. |
INVITES THE COMMISSION TO:
|
35. |
EXAMINE, based on a study under the third health programme (2014-2020), how to realise the potential of ‘Big Data’, which is used in personalised medicine, in contributing to innovative, efficient and sustainable health systems, respecting the right to protection of personal data. This study should also consider ethical, legal and social aspects; |
|
36. |
FACILITATE cooperation and PROMOTE exchange of best practices on education training and continuing professional development of health professionals in the field of personalised medicine; |
|
37. |
PROMOTE the possibilities offered by the European Reference Networks within the framework of the Directive on patients’ rights in cross-border healthcare, to help facilitate the implementation of translational cross-sectorial research, including, where appropriate, into personalised medicine for patients suffering from rare or low-prevalence diseases or complex diseases; |
|
38. |
CONTINUE to promote the important contributions to personalised medicine from research carried out under the framework programme for research and innovation, Horizon 2020, including through actions carried out under the Innovative Medicines Initiative (IMI), in order to speed up the development of more effective preventive and diagnostic tools as well as better and safer medicines for patients. |
(1) http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2006:146:0001:0003:EN:PDF
(4) OJ C 376, 21.12.2013, p. 3.
(5) OJ C 217, 10.7.2014, p. 2.
(6) OJ C 438, 6.12.2014, p. 12.
(7) 9869/15 (Innovation for the benefit of patients: Follow-up to the Council’s conclusions)
11039/1/15 REV1 (Outcome of proceedings of the Working Party on Public Health at Senior Level on 15 July 2015).
(8) European Commission Staff Working Document, October 2013.
(9) http://www.who.int/medicines/areas/priority_medicines/MasterDocJune28_FINAL_Web.pdf
(10) http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:i23022
For example, the project PerMed (www.permed2020.eu).
(11) http://ec.europa.eu/research/participants/data/ref/h2020/legal_basis/fp/h2020-eu-establact_en.pdf
(12) http://www.imi.europa.eu/
(13) http://ec.europa.eu/health/technology_assessment/docs/2014_strategy_eucooperation_hta_en.pdf
(14) http://ec.europa.eu/digital-agenda/en/connecting-europe-facility
(15) http://www.phgen.eu/typo3/fileadmin/downloads/QA_Report.pdf
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/6 |
Council conclusions on ‘Lessons learned for Public Health from the Ebola outbreak in West Africa — Health Security in the European Union’
(2015/C 421/04)
THE COUNCIL OF THE EUROPEAN UNION,
|
1. |
RECALLS that under Article 168 of the Treaty on the Functioning of the European Union, a high level of human health protection shall be ensured in the definition and implementation of all Union policies and activities; that Union action, which shall complement national policies, shall be directed towards improving public health, preventing physical and mental illness and diseases, and obviating sources of danger to physical and mental health. Such action shall cover the fight against the major health scourges, by promoting research into their causes, their transmission and their prevention, as well as health information and education, and monitoring, early warning of and combating serious cross-border threats to health. Member States shall, in liaison with the Commission, coordinate among themselves their policies and programmes in those areas; |
|
2. |
NOTES with concern that the Ebola Virus Disease (EVD) epidemic in West Africa has proved to be the largest epidemic of the disease on record, with more than 28 000 reported confirmed, probable and suspected cases and over 11 000 reported deaths (1), including about 500 of healthcare workers, since March 2014 and that, since its outbreak in December 2013, the epidemic has evolved into a public health, humanitarian and socioeconomic crisis with an unprecedented impact on families and communities in affected countries; |
|
3. |
RECALLS the International Health Regulations (2005) (2) (IHR) adopted by the 58th World Health Assembly on 23 May 2005 which reinforced coordination among States Parties to the IHR as regards preparedness for and the response to a public health emergency of international concern; |
|
4. |
NOTES the response to the outbreak of EVD by the Member States, the European Commission, the Health Security Committee (HSC), the European Centre for Disease Prevention and Control (ECDC) and the World Health Organisation (WHO); |
|
5. |
WELCOMES the extensive response to the outbreak of EVD by the affected countries and the remarkable work by civil society and non-governmental organisations; |
|
6. |
RECALLS that improving citizens' health security was a core aim of the second EU Health Programme (2008-2013) (3) and NOTES the overarching objective to ‘protect Union citizens from serious cross-border health threats’ as enshrined in the third EU Health Programme (2014-2020) (4); |
|
7. |
RECALLS that Decision No 1082/2013/EU of the European Parliament and of the Council (5) lays down rules on epidemiological surveillance, monitoring, early warning of, and combating serious cross-border threats to health, including preparedness and response planning related to those activities, with a view to coordinate and in order to complement national policies and ACKNOWLEDGES that the Decision enabled the Union to address the public health aspects of the Ebola outbreak while also reinforcing the interoperability of its preparedness and response capacities and that it provides a solid framework to tackle future public health crises similar to the Ebola outbreak; |
|
8. |
WELCOMES that medical evacuation of Ebola patients to Europe was implemented through the collaboration between the WHO, the Commission services, Member States and the HSC; |
|
9. |
UNDERLINES the importance of coordination of preparedness research at the European and global level and of the efforts made by the respective networks; |
|
10. |
UNDERLINES the important role of the HSC, established by Decision No 1082/2013/EU, in supporting the exchange of information between the Member States and the Commission, as well as in facilitating the coordination of the preparedness and response planning to the outbreak and of risk and crisis communication; |
|
11. |
WELCOMES that the EU and its Member States have invested EUR 2 billion in addressing the Ebola crisis (6) and to ensure better preparation to tackle possible future outbreaks; |
|
12. |
RECALLS that under ‘Horizon 2020 — the Framework Programme for Research and Innovation (2014-2020)’ (7) the EU has provided EUR 140 million on research on communicable diseases, such as Ebola; |
|
13. |
RECALLS the Council Conclusions of 30 April 2009 on ‘Influenza A/H1N1 infection’ (8) as well as the Council Conclusions of 12 October 2009 on ‘Pandemic (H1N1) 2009 — a strategic approach’ (9) and the Council Conclusions of 13 September 2010 on ‘Lessons learned from the A/H1N1 pandemic — Health security in the European Union’ (10), in which the Member States are invited to continue and to extend cooperation on preparation, monitoring, early warning and coordinated responses for all matters relating to public health emergencies; |
|
14. |
SUPPORTS the ongoing efforts in reforming WHO's preparedness and response capacity as recommended in Resolution EBSS3.R1 on ‘Ebola: ending the current outbreak, strengthening global preparedness and ensuring the WHO's capacity to prepare for and respond to future large scale outbreaks and emergencies with public health consequences’ adopted on 25 January 2015 (11) and as a follow-up to the Final Report of the Ebola Interim Assessment Panel published on 7 July 2015 (12); |
|
15. |
WELCOMES the resolution of the European Parliament of 18 September 2014 on EU response to the Ebola outbreak (13) as well as its own-initiative report of 27 October 2015 on ‘the Ebola crisis: the long-term lessons and how to strengthen health systems in developing countries to prevent future crises’ (14); |
|
16. |
RECALLS the Ebola High Level Coordination meeting held in Brussels on 16 October 2014, co-organised by the Commission and the Italian Presidency of the Council of the European Union, where EU and EEA Ministers of Health reaffirmed joint efforts to reinforce preparedness and response activities to fight Ebola; |
|
17. |
RECALLS the high level conference ‘Ebola: From Emergency to Recovery’ held in Brussels on 3 March 2015 (15) under the organisation of the European Union, which aimed to sustain the international mobilisation and to plan the next steps in the fight both against the current outbreak and the Ebola virus in general; |
|
18. |
TAKES NOTE of the discussions on lessons learned from the Ebola epidemic that have taken place in various international fora since its outbreak and notably the G7 Health Ministers' Commitment ‘Lessons Learned from Ebola’ adopted on 8 and 9 October 2015 (16) underlining the need for better global public health crisis management and calling for greater cooperation in view of developing and maintaining core capacities for IHR implementation; |
|
19. |
WELCOMES the Conference ‘Lessons learned for Public Health from the Ebola outbreak in West Africa’ co-organised by the Commission and the Luxembourg Presidency of the Council of the European Union on 12 and 14 October 2015 in Luxembourg (17), which stressed the need for improved cross-sectoral cooperation as well as strengthened health security in the European Union in order to enhance and maintain the response and preparedness capacities of Member States in case of future outbreaks; |
|
20. |
RECOGNISES that while preparedness and response planning as well as its implementation remain primarily a matter of national competence to be decided on by Member States, it is necessary to work together with a view to coordinate, where appropriate, national measures at EU level, in coherence with public health crises management at international level, notably within WHO and in line with Decision No 1082/2013/EU on serious cross-border health threats; |
INVITES MEMBER STATES TO:
|
21. |
MAINTAIN appropriate capacities, during and in between emergencies, in order to strengthen national preparedness and response activities, the international coordination and the implementation of lessons learned from previous incidents; |
INVITES THE MEMBER STATES AND THE COMMISSION TO:
|
22. |
IDENTIFY, ASSESS and TAKE FORWARD, as appropriate and while fully respecting Member States' competences, discussions of the following issues at EU level, notably within the HSC on the basis of the relevant provisions of Decision No 1082/2013/EU and while taking into account relevant work at international level:
|
INVITES THE COMMISSION TO:
|
23. |
IDENTIFY opportunities to improve coordination mechanisms for future incidents that extend across different policy areas. |
(1) http://apps.who.int/ebola/ebola-situation-reports
(2) http://apps.who.int/iris/bitstream/10665/43883/1/9789241580410_eng.pdf
(3) Decision No 1350/2007/EC of the European Parliament and of the Council of 23 October 2007 establishing a second programme of Community action in the field of health (2008-13) (OJ L 301, 20.11.2007, p. 3).
(4) Regulation (EU) No 282/2014 of the European Parliament and of the Council of 11 March 2014 on the establishment of a third Programme for the Union's action in the field of health (2014-2020) (OJ L 86, 21.3.2014, p. 1).
(5) Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross-border threats to health and repealing Decision No 2119/98/EC (OJ L 293, 5.11.2013, p. 1).
(6) http://europa.eu/rapid/press-release_MEMO-15-5339_en.htm
(7) Regulation (EU) No 1291/2013 of the European Parliament and of the Council of 11 December 2013 establishing Horizon 2020 — the Framework Programme for Research and Innovation (2014-2020) and repealing Decision No 1982/2006/EC (OJ L 347, 20.12.2013, p. 104).
(8) 9392/09.
(9) 13635/09.
(10) 12665/10.
(11) http://apps.who.int/gb/ebwha/pdf_files/EBSS3/EBSS3_R1-en.pdf?ua=1&ua=1
(12) http://who.int/csr/resources/publications/ebola/report-by-panel.pdf?ua=1
(13) 2014/2842(RSP), http://www.europarl.europa.eu/sides/getDoc.do?type=TA&language=EN&reference=P8-TA-2014-0026
(14) 2014/2204(INI), http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+REPORT+A8-2015-0281+0+DOC+XML+V0//EN&language=en
(15) http://europa.eu/rapid/press-release_IP-15-4521_en.htm
(16) http://www.bmg.bund.de/fileadmin/dateien/Downloads/G/G7-Ges.Minister_2015/G7_Health_Ministers_Declaration_AMR_and_EBOLA.pdf
(17) Conference report, http://ec.europa.eu/health/preparedness_response/events/ev_20151012_en.htm#c
(18) OJ L 347, 20.12.2013, p. 924.
European Commission
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/9 |
Euro exchange rates (1)
16 December 2015
(2015/C 421/05)
1 euro =
|
|
Currency |
Exchange rate |
|
USD |
US dollar |
1,0933 |
|
JPY |
Japanese yen |
133,18 |
|
DKK |
Danish krone |
7,4617 |
|
GBP |
Pound sterling |
0,72830 |
|
SEK |
Swedish krona |
9,2991 |
|
CHF |
Swiss franc |
1,0824 |
|
ISK |
Iceland króna |
|
|
NOK |
Norwegian krone |
9,5555 |
|
BGN |
Bulgarian lev |
1,9558 |
|
CZK |
Czech koruna |
27,030 |
|
HUF |
Hungarian forint |
316,41 |
|
PLN |
Polish zloty |
4,3186 |
|
RON |
Romanian leu |
4,5035 |
|
TRY |
Turkish lira |
3,2416 |
|
AUD |
Australian dollar |
1,5186 |
|
CAD |
Canadian dollar |
1,5050 |
|
HKD |
Hong Kong dollar |
8,4732 |
|
NZD |
New Zealand dollar |
1,6196 |
|
SGD |
Singapore dollar |
1,5407 |
|
KRW |
South Korean won |
1 285,09 |
|
ZAR |
South African rand |
16,4708 |
|
CNY |
Chinese yuan renminbi |
7,0726 |
|
HRK |
Croatian kuna |
7,6425 |
|
IDR |
Indonesian rupiah |
15 293,02 |
|
MYR |
Malaysian ringgit |
4,7045 |
|
PHP |
Philippine peso |
51,754 |
|
RUB |
Russian rouble |
76,8689 |
|
THB |
Thai baht |
39,431 |
|
BRL |
Brazilian real |
4,3042 |
|
MXN |
Mexican peso |
18,7228 |
|
INR |
Indian rupee |
72,9350 |
(1) Source: reference exchange rate published by the ECB.
Court of Auditors
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/10 |
Special Report No 16/2015
‘Improving the security of energy supply by developing the internal energy market: more efforts needed’
(2015/C 421/06)
The European Court of Auditors hereby informs you that Special Report No 16/2015 ‘Improving the security of energy supply by developing the internal energy market: more efforts needed’ has just been published.
The report can be accessed for consultation or downloading on the European Court of Auditors’ website: http://eca.europa.eu
A hard copy version of the report may be obtained free of charge on request to the Court of Auditors:
|
European Court of Auditors |
|
Publications (PUB) |
|
12, rue Alcide De Gasperi |
|
1615 Luxembourg |
|
LUXEMBOURG |
|
Tel. +352 4398-1 |
|
E-mail: eca-info@eca.europa.eu |
or by filling in an electronic order form on EU-Bookshop.
NOTICES CONCERNING THE EUROPEAN ECONOMIC AREA
European Commission
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/11 |
Public holidays in 2016: EEA EFTA States and EEA institutions
(2015/C 421/07)
|
|
Iceland |
Liechtenstein |
Norway |
EFTA Surveillance Authority |
EFTA Court |
|
1 January |
X |
X |
X |
X |
X |
|
6 January |
|
X |
|
|
|
|
2 February |
|
X |
|
|
|
|
8 February |
|
|
|
|
X |
|
9 February |
|
X |
|
|
|
|
24 March |
X |
|
X |
X |
X |
|
25 March |
X |
X |
X |
X |
X |
|
28 March |
X |
X |
X |
X |
X |
|
21 April |
X |
|
|
|
|
|
5 May |
X |
X |
X |
X |
X |
|
6 May |
|
|
|
X |
X |
|
16 May |
X |
X |
X |
X |
X |
|
17 May |
|
|
X |
|
|
|
26 May |
|
X |
|
|
|
|
17 June |
X |
|
|
|
|
|
23 June |
|
|
|
|
X |
|
24 June |
|
|
|
|
X |
|
1 August |
X |
|
|
|
|
|
15 August |
|
X |
|
X |
X |
|
29 August |
|
|
|
|
X |
|
8 September |
|
X |
|
|
|
|
1 November |
|
X |
|
X |
X |
|
2 November |
|
|
|
|
X |
|
8 December |
|
X |
|
|
|
|
23 December |
|
|
|
X |
|
|
26 December |
X |
X |
X |
X |
X |
|
27 December |
|
|
|
X |
X |
|
28 December |
|
|
|
X |
X |
|
29 December |
|
|
|
X |
X |
|
30 December |
|
|
|
X |
X |
Public holidays falling on Saturdays and Sundays are not listed.
V Announcements
PROCEDURES RELATING TO THE IMPLEMENTATION OF THE COMMON COMMERCIAL POLICY
European Commission
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/13 |
Notice of initiation of an anti-dumping proceeding concerning imports of certain manganese oxides originating in Brazil, Georgia, India and Mexico
(2015/C 421/08)
The European Commission (‘the Commission’) has received a complaint pursuant to Article 5 of Council Regulation (EC) No 1225/2009 of 30 November 2009 on protection against dumped imports from countries not members of the European Community (1) (‘the basic Regulation’), alleging that imports of certain manganese oxides, originating in Brazil, Georgia, India and Mexico, are being dumped and are thereby causing material injury to the Union industry.
1. Complaint
The complaint was lodged on 20 November 2015 by Erachem Comilog SPRL (‘the complainant’), the sole producer of certain manganese oxides in the Union, thus representing 100 % of the total Union production of certain manganese oxides.
2. Product under investigation
The product subject to this investigation is manganese oxides (chemical formula: MnO) with a purity in net weight of 50 per cent and more, but less than 77 per cent of manganese (‘the product under investigation’).
3. Allegation of dumping
The product allegedly being dumped is the product under investigation, originating in Brazil, Georgia, India and Mexico (‘the countries concerned’), currently falling within CN codes ex 2820 90 90 and ex 2602 00 00. These CN codes are given for information only.
In the absence of reliable data on domestic prices for Brazil and India, the allegation of dumping is based on a comparison of a constructed normal value (manufacturing costs, selling, general and administrative costs — SG&A — and profit) with the export price (at ex-works level) of the product under investigation when sold for export to the Union.
The allegation of dumping from Mexico is based on a comparison of the domestic price with the export price (at ex-works level) of the product under investigation when sold for export to the Union.
The allegation of dumping from Georgia is based on a comparison of the price in the United States of America and on constructed normal value (manufacturing costs, selling, general and administrative costs — SG&A — and profit) in Georgia. The allegation of dumping is based on a comparison of the normal value thus established with the export price (at ex-works level) of the product under investigation when sold for export to the Union.
On this basis the dumping margins calculated are significant for all the countries concerned.
4. Allegation of injury and causation
The complainant has provided evidence that imports of the product under investigation from the countries concerned have increased overall in absolute terms and have increased in terms of market share.
The prima facie evidence provided by the complainant shows that the volume and the prices of the imported product under investigation have had, among other consequences, a negative impact on the quantities sold, the level of prices charged and the market share held by the Union industry, resulting in substantial adverse effects on the overall performance and the financial situation of the Union industry.
5. Procedure
Having determined, after informing the Member States, that the complaint has been lodged by or on behalf of the Union industry and that there is sufficient evidence to justify the initiation of a proceeding, the Commission hereby initiates an investigation pursuant to Article 5 of the basic Regulation.
The investigation will determine whether the product under investigation originating in the countries concerned is being dumped and whether the dumped imports have caused injury to the Union industry. If the conclusions are affirmative, the investigation will examine whether the imposition of measures would not be against the Union interest.
5.1. Investigation period and period considered
The investigation of dumping and injury will cover the period from 1 October 2014 to 30 September 2015 (‘the investigation period’). The examination of trends relevant for the assessment of injury will cover the period from 1 January 2012 to the end of the investigation period (‘the period considered’).
5.2. Procedure for the determination of dumping
Exporting producers (2) of the product under investigation from the countries concerned are invited to participate in the Commission investigation.
5.2.1. Investigating exporting producers
5.2.1.1.
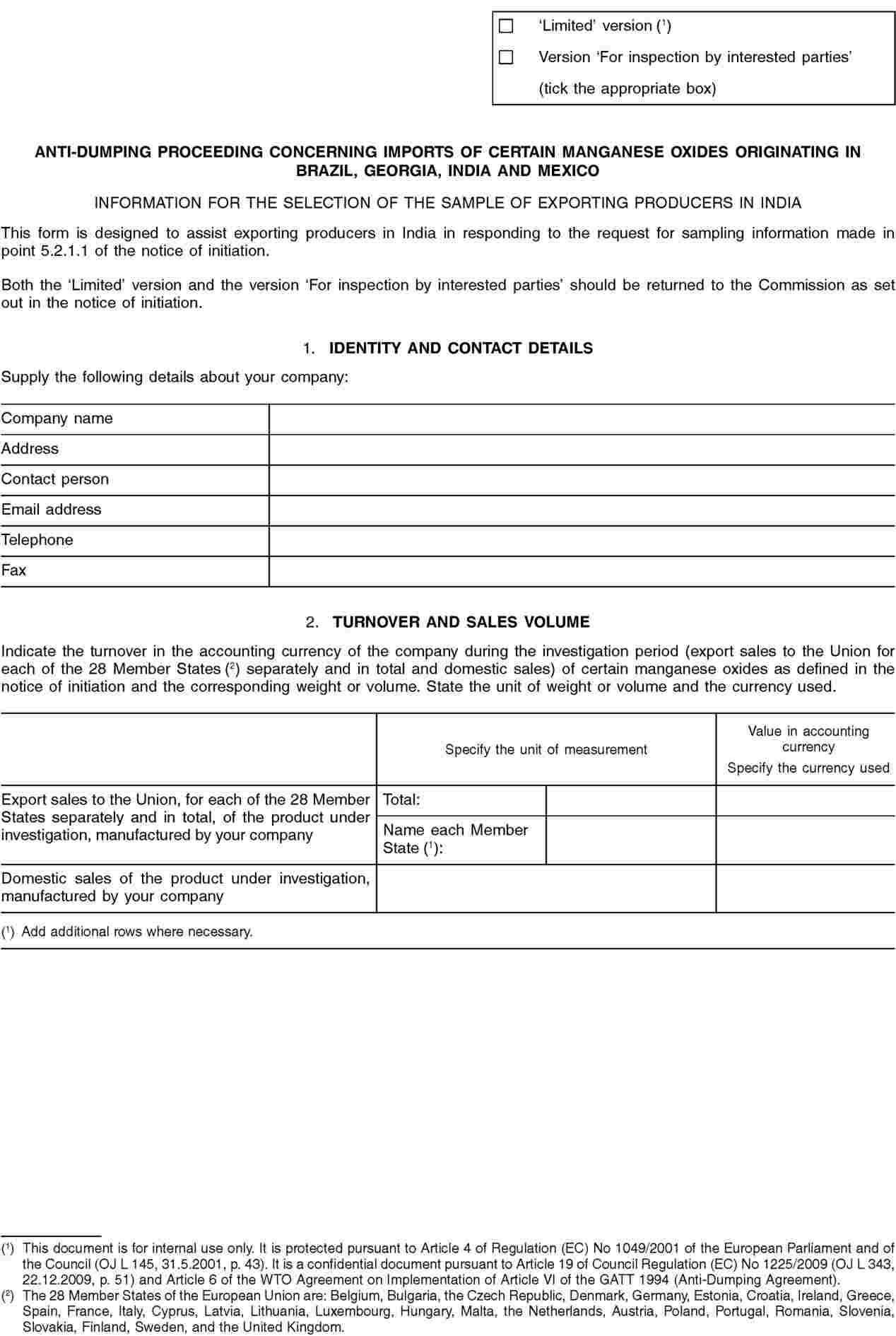
(a) Sampling
In view of the potentially large number of exporting producers in India involved in this proceeding and in order to complete the investigation within the statutory time limits, the Commission may limit the exporting producers to be investigated to a reasonable number by selecting a sample (this process is also referred to as ‘sampling’). The sampling will be carried out in accordance with Article 17 of the basic Regulation.
In order to enable the Commission to decide whether sampling is necessary, and if so, to select a sample, all exporting producers, or representatives acting on their behalf, are hereby requested to make themselves known to the Commission. These parties have to do so within 15 days of the date of publication of this Notice in the Official Journal of the European Union, unless otherwise specified, by providing the Commission with information on their company(ies) requested in Annex I to this Notice.
In order to obtain information it deems necessary for the selection of the sample of exporting producers, the Commission will also contact the authorities of India and may contact any known associations of exporting producers.
All interested parties wishing to submit any other relevant information regarding the selection of the sample, excluding the information requested above, must do so within 21 days of the publication of this Notice in the Official Journal of the European Union, unless otherwise specified.
If a sample is necessary, the exporting producers may be selected based on the largest representative volume of exports to the Union which can reasonably be investigated within the time available. All known exporting producers, the authorities of India and associations of exporting producers will be notified by the Commission, via the authorities of India if appropriate, of the companies selected to be in the sample.
In order to obtain information it deems necessary for its investigation with regard to exporting producers, the Commission will send questionnaires to the exporting producers selected to be in the sample, to any known association of exporting producers, and to the authorities of India.
All exporting producers selected to be in the sample will have to submit a completed questionnaire within 37 days from the date of notification of the sample selection, unless otherwise specified.
Without prejudice to the possible application of Article 18 of the basic Regulation, companies that have agreed to their possible inclusion in the sample but are not selected to be in the sample will be considered to be cooperating (‘non-sampled cooperating exporting producers’). Without prejudice to section (b) below, the anti-dumping duty that may be applied to imports from non-sampled cooperating exporting producers will not exceed the weighted average margin of dumping established for the exporting producers in the sample (3).
(b) Individual dumping margin for companies not included in the sample
Non-sampled cooperating exporting producers may request, pursuant to Article 17(3) of the basic Regulation, that the Commission establish their individual dumping margins (‘individual dumping margin’). The exporting producers wishing to claim an individual dumping margin must request a questionnaire and return it duly completed within 37 days of the date of notification of the sample selection, unless otherwise specified. The Commission will examine whether they can be granted an individual duty in accordance with Article 9(5) of the basic Regulation.
However, exporting producers claiming an individual dumping margin should be aware that the Commission may nonetheless decide not to determine their individual dumping margin if, for instance, the number of exporting producers is so large that such determination would be unduly burdensome and would prevent the timely completion of the investigation.
5.2.1.2.
All exporting producers and associations of exporting producers in Brazil, Georgia and Mexico are invited to contact the Commission, preferably by email, immediately but no later than 15 days after the publication of this Notice in the Official Journal of the European Union, unless otherwise specified, in order to make themselves known and request a questionnaire. In order to obtain information it deems necessary for its investigation with regard to exporting producers, the Commission will send questionnaires to known exporting producers in Brazil, Georgia and Mexico, to any known association of exporting producers, and to the authorities of those countries.
The exporting producers and, where applicable, the associations of exporting producers must submit the completed questionnaire within 37 days of the date of publication of this Notice in the Official Journal of the European Union, unless otherwise specified.
5.2.2. Additional procedure with regard to exporting producers in Georgia
5.2.2.1.
Subject to the provisions of section 5.2.2.2 below, in accordance with Article 2(7)(a) of the basic Regulation and without prejudice to the relevant provisions of the Association Agreement signed between the EU and Georgia on 27 June 2014 which is provisionally applied since 1 September 2014 (hereinafter referred to as the DCFTA), normal value will be determined on the basis of the price or constructed value in a market economy third country. For this purpose the Commission will select an appropriate market economy third country. The complainant has proposed the United States of America as analogue country, but Brazil, India and Mexico may also be considered as analogue country on the basis of Article 2(7)(a). According to the information available to the Commission, there are no other suppliers of the Union from market economy third countries. With the aim of finally selecting the market economy third country the Commission will examine whether there is production and sales of the product under investigation in other market economy third countries. Interested parties are hereby invited to comment on the choice of the analogue country within 10 days of the date of publication of this Notice in the Official Journal of the European Union.
5.2.2.2.
In accordance with Article 2(7)(b) of the basic Regulation and without prejudice to the relevant provisions of the DCFTA, individual exporting producers in the country concerned, which consider that market economy conditions prevail for them in respect of the manufacture and sale of the product under investigation, may submit a properly substantiated market economy treatment claim to this effect (‘MET claim’). MET will be granted if the assessment of the MET claim shows that the criteria laid down in Article 2(7)(c) of the basic Regulation (4) are fulfilled. The dumping margin of the exporting producers granted MET will be calculated, to the extent possible and without prejudice to the use of facts available pursuant to Article 18 of the basic Regulation, by using their own normal value and export prices in accordance with Article 2(7)(b) of the basic Regulation.
The Commission will send MET claim forms to all known exporting producers in Georgia, to any known association of exporting producers, and to the authorities of Georgia. Any exporting producers wishing to apply for MET must request the MET claim form from the Commission no later than 10 days after the publication of this Notice in the Official Journal of the European Union. All exporting producers claiming MET must submit the completed MET claim form within 21 days of the date of publication of this Notice in the Official Journal of the European Union, unless otherwise specified.
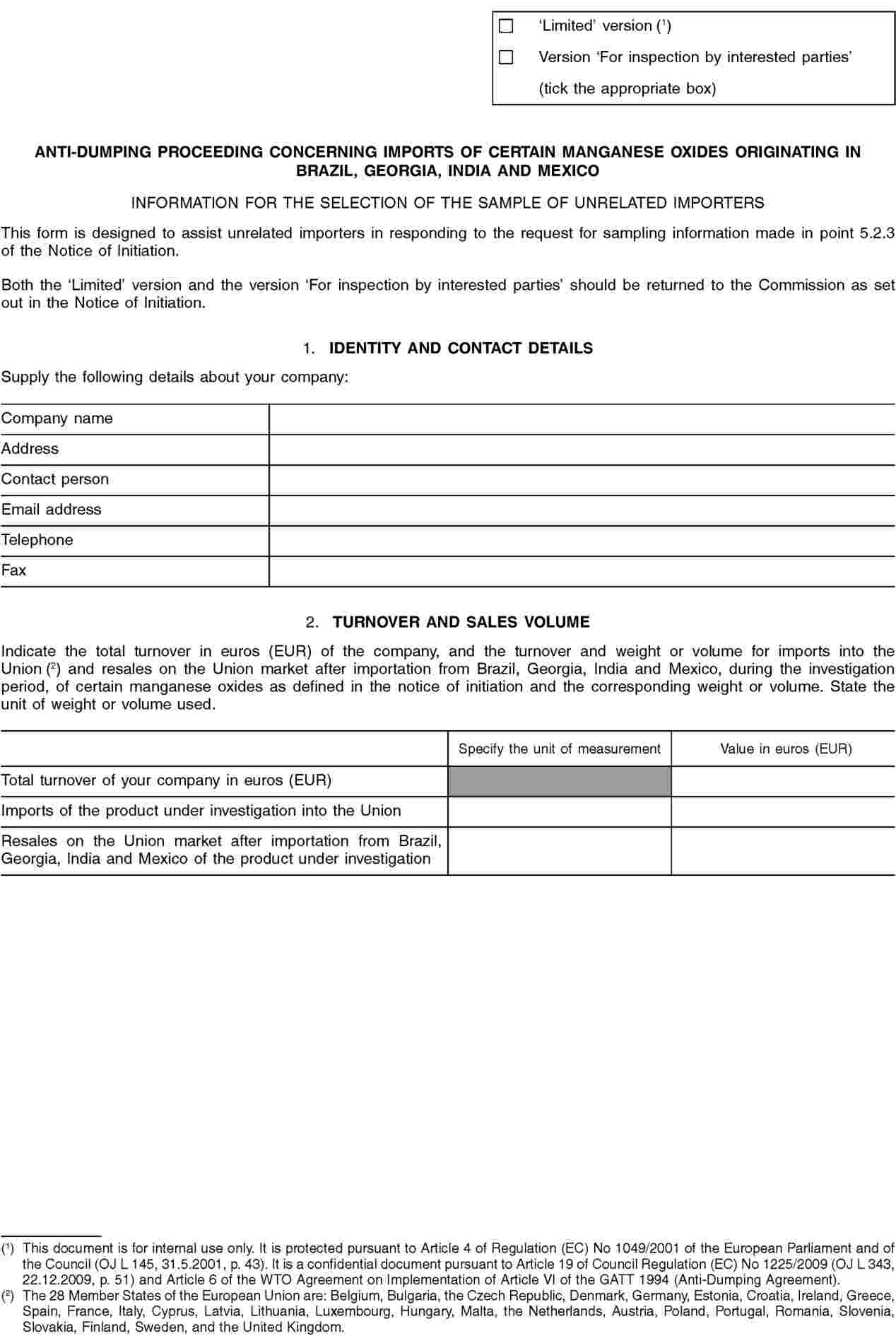
5.2.3. Investigating unrelated importers (5) (6)
Unrelated importers of the product under investigation from the countries concerned to the Union are invited to participate in this investigation.
In view of the potentially large number of unrelated importers involved in this proceeding and in order to complete the investigation within the statutory time limits, the Commission may limit to a reasonable number the unrelated importers that will be investigated by selecting a sample (this process is also referred to as ‘sampling’). The sampling will be carried out in accordance with Article 17 of the basic Regulation.
In order to enable the Commission to decide whether sampling is necessary and, if so, to select a sample, all unrelated importers, or representatives acting on their behalf, are hereby requested to make themselves known to the Commission. These parties must do so within 15 days of the date of publication of this Notice in the Official Journal of the European Union, unless otherwise specified, by providing the Commission with the information on their company(ies) requested in Annex II to this Notice.
In order to obtain information it deems necessary for the selection of the sample of unrelated importers, the Commission may also contact any known associations of importers.
All interested parties wishing to submit any other relevant information regarding the selection of the sample, excluding the information requested above, must do so within 21 days of the publication of this Notice in the Official Journal of the European Union, unless otherwise specified.
If a sample is necessary, the importers may be selected based on the largest representative volume of sales of the product under investigation in the Union which can reasonably be investigated within the time available. All known unrelated importers and associations of importers will be notified by the Commission of the companies selected to be in the sample.
In order to obtain information it deems necessary for its investigation, the Commission will send questionnaires to the sampled unrelated importers and to any known association of importers. These parties must submit a completed questionnaire within 37 days from the date of the notification of the sample selection, unless otherwise specified.
5.3. Procedure for the determination of injury and investigating Union producers
A determination of injury is based on positive evidence and involves an objective examination of the volume of the dumped imports, their effect on prices on the Union market and the consequent impact of those imports on the Union industry. In order to establish whether the Union industry is injured, Union producers of the product under investigation are invited to participate in the Commission investigation.
In order to obtain information it deems necessary for its investigation with regard to Union producers the Commission will send questionnaires to the known Union producer, namely to Erachem Comilog SPRL, and to any known association of Union producers where available.
The aforementioned Union producer and the associations of Union producers must submit the completed questionnaire within 37 days of the date of publication of this Notice in the Official Journal of the European Union, unless otherwise specified.
Any Union producer and association of Union producers not listed above is invited to contact the Commission, preferably by email, immediately but no later than 15 days after the publication of this Notice in the Official Journal of the European Union, unless otherwise specified, in order to make itself known and request a questionnaire.
5.4. Procedure for the assessment of Union interest
Should the existence of dumping and injury caused thereby be established, a decision will be reached, pursuant to Article 21 of the basic Regulation, as to whether the adoption of anti-dumping measures would not be against the Union interest. The Commission will in particular take into account the competition aspects of the present investigation, and interested parties are invited to make relevant comments on that issue.
Union producers, importers and their representative associations, users and their representative associations, and representative consumer organisations are invited to make themselves known within 15 days of the date of publication of this Notice in the Official Journal of the European Union, unless otherwise specified. In order to participate in the investigation, the representative consumer organisations have to demonstrate, within the same deadline, that there is an objective link between their activities and the product under investigation.
Parties that make themselves known within the above deadline may provide the Commission with information on the Union interest within 37 days of the date of publication of this Notice in the Official Journal of the European Union, unless otherwise specified. This information may be provided either in a free format or by completing a questionnaire prepared by the Commission. In any case, information submitted pursuant to Article 21 will only be taken into account if supported by factual evidence at the time of submission.
5.5. Other written submissions
Subject to the provisions of this Notice, all interested parties are hereby invited to make their views known, submit information and provide supporting evidence. Unless otherwise specified, this information and supporting evidence must reach the Commission within 37 days of the date of publication of this Notice in the Official Journal of the European Union.
5.6. Possibility to be heard by the Commission investigation services
All interested parties may request to be heard by the Commission investigation services. Any request to be heard should be made in writing and should specify the reasons for the request. For hearings on issues pertaining to the initial stage of the investigation the request must be submitted within 15 days of the date of publication of this Notice in the Official Journal of the European Union. Thereafter, a request to be heard must be submitted within the specific deadlines set by the Commission in its communication with the parties.
5.7. Instructions for making written submissions and sending completed questionnaires and correspondence
Information submitted to the Commission for the purpose of trade defence investigations shall be free from copyrights. Interested parties, before submitting to the Commission information and/or data which is subject to third party copyrights, must request specific permission to the copyright holder explicitly allowing a) the Commission to use the information and data for the purpose of this trade defence proceeding and b) to provide the information and/or data to interested parties to this investigation in a form that allows them to exercise their rights of defence.
All written submissions, including the information requested in this Notice, completed questionnaires and correspondence provided by interested parties for which confidential treatment is requested shall be labelled ‘Limited’ (7).
Interested parties providing ‘Limited’ information are required to furnish non-confidential summaries of it pursuant to Article 19(2) of the basic Regulation, which will be labelled ‘For inspection by interested parties’. These summaries should be sufficiently detailed to permit a reasonable understanding of the substance of the information submitted in confidence. If an interested party providing confidential information does not furnish a non-confidential summary of it in the requested format and quality, such information may be disregarded.
Interested parties are invited to make all submissions and requests by email including scanned powers of attorney and certification sheets, with the exception of voluminous replies which shall be submitted on a CD-ROM or DVD by hand or by registered mail. By using email, interested parties express their agreement with the rules applicable to electronic submissions contained in the document ‘CORRESPONDENCE WITH THE EUROPEAN COMMISSION IN TRADE DEFENCE CASES’ published on the website of the Directorate-General for Trade: http://trade.ec.europa.eu/doclib/docs/2011/june/tradoc_148003.pdf The interested parties must indicate their name, address, telephone and a valid email address and they should ensure that the provided email address is a functioning official business email which is checked on a daily basis. Once contact details are provided, the Commission will communicate with interested parties by email only, unless they explicitly request to receive all documents from the Commission by another means of communication or unless the nature of the document to be sent requires the use of a registered mail. For further rules and information concerning correspondence with the Commission including principles that apply to submissions by email, interested parties should consult the communication instructions with interested parties referred to above.
Commission address for correspondence:
|
European Commission |
|||
|
Directorate-General for Trade |
|||
|
Directorate H |
|||
|
Office: CHAR 04/039 |
|||
|
1040 Bruxelles/Brussel |
|||
|
BELGIQUE/BELGIË |
|||
|
6. Non-cooperation
In cases where any interested party refuses access to or does not provide the necessary information within the time limits, or significantly impedes the investigation, provisional or final findings, affirmative or negative, may be made on the basis of facts available, in accordance with Article 18 of the basic Regulation.
Where it is found that any interested party has supplied false or misleading information, the information may be disregarded and use may be made of facts available.
If an interested party does not cooperate or cooperates only partially and findings are therefore based on facts available in accordance with Article 18 of the basic Regulation, the result may be less favourable to that party than if it had cooperated.
Failure to give a computerised response shall not be deemed to constitute non-cooperation, provided that the interested party shows that presenting the response as requested would result in an unreasonable extra burden or unreasonable additional cost. The interested party should immediately contact the Commission.
7. Hearing Officer
Interested parties may request the intervention of the Hearing Officer in trade proceedings. The Hearing Officer acts as an interface between the interested parties and the Commission investigation services. The Hearing Officer reviews requests for access to the file, disputes regarding the confidentiality of documents, requests for extension of time limits and requests by third parties to be heard. The Hearing Officer may organise a hearing with an individual interested party and mediate to ensure that the interested parties' rights of defence are being fully exercised.
A request for a hearing with the Hearing Officer should be made in writing and should specify the reasons for the request. For hearings on issues pertaining to the initial stage of the investigation the request must be submitted within 15 days of the date of publication of this Notice in the Official Journal of the European Union. Thereafter, a request to be heard must be submitted within specific deadlines set by the Commission in its communication with the parties.
The Hearing Officer will also provide opportunities for a hearing involving parties to take place which would allow different views to be presented and rebuttal arguments offered on issues pertaining, among other things, to dumping, injury, causal link and Union interest. Such a hearing would, as a rule, take place at the latest at the end of the fourth week following the disclosure of provisional findings.
For further information and contact details interested parties may consult the Hearing Officer's web pages on DG Trade's website: http://ec.europa.eu/trade/trade-policy-and-you/contacts/hearing-officer/
8. Schedule of the investigation
The investigation will be concluded, pursuant to Article 6(9) of the basic Regulation within 15 months of the date of the publication of this Notice in the Official Journal of the European Union. In accordance with Article 7(1) of the basic Regulation, provisional measures may be imposed no later than nine months from the publication of this Notice in the Official Journal of the European Union.
9. Processing of personal data
Any personal data collected in this investigation will be treated in accordance with Regulation (EC) No 45/2001 of the European Parliament and of the Council of 18 December 2000 on the protection of individuals with regard to the processing of personal data by the Community institutions and bodies and on the free movement of such data (8).
(1) OJ L 343, 22.12.2009, p. 51.
(2) An exporting producer is any company in the countries concerned which produces and exports the product under investigation to the Union market, either directly or via a third party, including any of its related companies involved in the production, domestic sales or exports of the product under investigation.
(3) Pursuant to Article 9(6) of the basic Regulation, any zero and de minimis margins, and margins established in accordance with the circumstances described in Article 18 of the basic Regulation will be disregarded.
(4) The exporting producers have to demonstrate in particular that: (i) business decisions and costs are made in response to market conditions and without significant State interference; (ii) firms have one clear set of basic accounting records which are independently audited in line with international accounting standards and are applied for all purposes; (iii) there are no significant distortions carried over from the former non-market economy system; (iv) bankruptcy and property laws guarantee legal certainty and stability and (v) exchange rate conversions are carried out at market rates.
(5) Only importers not related to exporting producers can be sampled. Importers that are related to exporting producers have to fill in Annex I to the questionnaire for these exporting producers. In accordance with Article 143 of Commission Regulation (EEC) No 2454/93 concerning the implementation of the Community Customs Code, persons shall be deemed to be related only if: (a) they are officers or directors of one another's businesses; (b) they are legally recognised partners in business; (c) they are employer and employee; (d) any person directly or indirectly owns, controls or holds 5 % or more of the outstanding voting stock or shares of both of them; (e) one of them directly or indirectly controls the other; (f) both of them are directly or indirectly controlled by a third person; (g) together they directly or indirectly control a third person; or (h) they are members of the same family. Persons shall be deemed to be members of the same family only if they stand in any of the following relationships to one another: (i) husband and wife, (ii) parent and child, (iii) brother and sister (whether by whole or half blood), (iv) grandparent and grandchild, (v) uncle or aunt and nephew or niece, (vi) parent-in-law and son-in-law or daughter-in-law, (vii) brother-in-law and sister-in-law (OJ L 253, 11.10.1993, p. 1). In this context ‘person’ means any natural or legal person.
(6) The data provided by unrelated importers may also be used in relation to aspects of this investigation other than the determination of dumping.
(7) A ‘Limited’ document is a document which is considered confidential pursuant to Article 19 of Council Regulation (EC) No 1225/2009 (OJ L 343, 22.12.2009 p. 51) and Article 6 of the WTO Agreement on Implementation of Article VI of the GATT 1994 (Anti-Dumping Agreement). It is also a document protected pursuant to Article 4 of Regulation (EC) No 1049/2001 of the European Parliament and of the Council (OJ L 145, 31.5.2001, p. 43).
ANNEX I

ANNEX II

PROCEDURES RELATING TO THE IMPLEMENTATION OF COMPETITION POLICY
European Commission
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/24 |
Prior notification of a concentration
(Case M.7823 — Acciona/Nordex)
Candidate case for simplified procedure
(Text with EEA relevance)
(2015/C 421/09)
|
1. |
On 10 December 2015, the Commission received a notification of a proposed concentration pursuant to Article 4 of Council Regulation (EC) No 139/2004 (1) by which Acciona of Spain will acquire within the meaning of Article 3(1)(b) of the Merger Regulation control of part of the undertaking Nordex SE (‘Nordex’) of Germany by way of purchase of shares. |
|
2. |
The business activities of the undertakings concerned are:
|
|
3. |
On preliminary examination, the Commission finds that the notified transaction could fall within the scope of the Merger Regulation. However, the final decision on this point is reserved. Pursuant to the Commission Notice on a simplified procedure for treatment of certain concentrations under Council Regulation (EC) No 139/2004 (2) it should be noted that this case is a candidate for treatment under the procedure set out in this Notice. |
|
4. |
The Commission invites interested third parties to submit their possible observations on the proposed operation to the Commission. Observations must reach the Commission not later than 10 days following the date of this publication. Observations can be sent to the Commission by fax (+32 22964301), by email to COMP-MERGER-REGISTRY@ec.europa.eu or by post, under reference M.7823 — Acciona/Nordex, to the following address:
|
(1) OJ L 24, 29.1.2004, p. 1 (the ‘Merger Regulation’).
(2) OJ C 366, 14.12.2013, p. 5.
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/25 |
Prior notification of a concentration
(Case M.7880 — Mitsubishi Heavy Industries/UniCarriers Holdings)
Candidate case for simplified procedure
(Text with EEA relevance)
(2015/C 421/10)
|
1. |
On 9 December 2015, the Commission received a notification of a proposed concentration pursuant to Article 4 of Council Regulation (EC) No 139/2004 (1) by which the undertaking Mitsubishi Heavy Industries, Ltd (Japan), acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of the undertaking UniCarriers Holdings Corporation (Japan) by way of purchase of shares. |
|
2. |
The business activities of the undertakings concerned are: — for Mitsubishi Heavy Industries, Ltd: various industrial activities, including the production and sale of forklifts in the EEA; — for UniCarriers Holdings Corporation: development, manufacture and marketing of forklifts, containers carriers, transfer cranes, other material handling machinery and forklift engines. |
|
3. |
On preliminary examination, the Commission finds that the notified transaction could fall within the scope of the Merger Regulation. However, the final decision on this point is reserved. Pursuant to the Commission Notice on a simplified procedure for treatment of certain concentrations under the Council Regulation (EC) No 139/2004 (2) it should be noted that this case is a candidate for treatment under the procedure set out in this Notice. |
|
4. |
The Commission invites interested third parties to submit their possible observations on the proposed operation to the Commission. Observations must reach the Commission not later than 10 days following the date of this publication. Observations can be sent to the Commission by fax (+32 22964301), by e-mail to COMP-MERGER-REGISTRY@ec.europa.eu or by post, under reference number M.7880 — Mitsubishi Heavy Industries/UniCarriers Holdings, to the following address:
|
(1) OJ L 24, 29.1.2004, p. 1 (the ‘Merger Regulation’).
(2) OJ C 366, 14.12.2013, p. 5.
|
17.12.2015 |
EN |
Official Journal of the European Union |
C 421/26 |
Prior notification of a concentration
(Case M.7887 — Goldman Sachs/Astorg Asset Management/HRA Pharma)
Candidate case for simplified procedure
(Text with EEA relevance)
(2015/C 421/11)
|
1. |
On 9 December 2015, the Commission received a notification of a proposed concentration pursuant to Article 4 of Council Regulation (EC) No 139/2004 (1) by which the undertakings Astorg Asset Management Sàrl. acting on behalf of Astorg VI (‘Astorg’, Luxembourg) and The Goldman Sachs Group, Inc. (‘Goldman Sachs’, United States) acquire within the meaning of Article 3(1)(b) of the Merger Regulation control over Laboratoire HRA Pharma SAS and its subsidiaries (‘HRA Pharma’, France), by way of purchase of shares. |
|
2. |
The business activities of the undertakings concerned are:
|
|
3. |
On preliminary examination, the Commission finds that the notified transaction could fall within the scope of the Merger Regulation. However, the final decision on this point is reserved. Pursuant to the Commission Notice on a simplified procedure for treatment of certain concentrations under Council Regulation (EC) No 139/2004 (2) it should be noted that this case is a candidate for treatment under the procedure set out in this Notice. |
|
4. |
The Commission invites interested third parties to submit their possible observations on the proposed operation to the Commission. Observations must reach the Commission not later than 10 days following the date of this publication. Observations can be sent to the Commission by fax (+32 22964301), by email to COMP-MERGER-REGISTRY@ec.europa.eu or by post, under reference M.7887 — Goldman Sachs/Astorg Asset Management/HRA Pharma, to the following address:
|
(1) OJ L 24, 29.1.2004, p. 1 (the ‘Merger Regulation’).
(2) OJ C 366, 14.12.2013, p. 5.

