EUROPEAN COMMISSION
EUROPEAN COMMISSION
Brussels, 25.6.2019
SWD(2019) 199 final
COMMISSION STAFF WORKING DOCUMENT
FITNESS CHECK
of the most relevant chemicals legislation (excluding REACH), as well as related aspects of legislation applied to downstream industries
Accompanying the document
REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
Findings of the Fitness Check of the most relevant chemicals legislation (excluding REACH) and identified challenges, gaps and weaknesses
{COM(2019) 264 final}
Table of contents
1
INTRODUCTION
2
BACKGROUND TO THE INTERVENTION
2.1
Description of the initiative
2.2
An overview of the EU chemicals industry and related sectors
2.3
Baseline
3
STATE OF PLAY
3.1
Implementation of the EU chemicals legislation
3.2
Enforcement of the EU chemicals legislation
4
METHODOLOGY
4.1
Description of methodology: quantification and data collection
4.2
Limitations and robustness of findings
ANSWERS TO THE EVALUATION QUESTIONS
5
EFFECTIVENESS
5.1
Evaluation question: to what extent does the EU legislative framework for the risk management of chemicals meet its objectives?
5.2
Evaluation question: what factors affect (either positively or negatively) the correct functioning of the EU legislative framework for the hazard identification and risk management of chemicals? What are the consequences or effects that were not originally planned for?
6
EFFICIENCY
6.1
Evaluation question: what are the costs and benefits associated with the implementation of the legislative framework for chemicals? What are the key drivers for those costs and benefits? To what extent are the costs proportionate to the benefits?
6.2
Evaluation question: what aspects of the functioning of the framework are the most efficient and what are the least efficient?
7
COHERENCE
7.1
Evaluation question: to what extent are the legal acts consistent in how they attempt to reach the stated objectives and can differences in the hazard identification and risk management of chemicals be justified?
7.2
Evaluation question: what, if any, are the inconsistencies, contradictions, unnecessary duplication, overlap or missing links between different pieces of legislation? Are these leading to unintended results?
8
RELEVANCE
8.1
Evaluation question: to what extent do the objectives of the legislative framework for chemicals meet the current needs?
8.2
Evaluation question: to what extent does the current legislative framework for chemicals take into account health, environmental, social and economic consequences that are relevant to citizens and stakeholders?
8.3
Evaluation question: to what extent are the current procedures transparent and robust enough to enable decisions related to hazard identification, risk assessment and risk management to be relevant and evidence-based?
9
EU VALUE ADDED
10
CONCLUSIONS
A comprehensive and generally well-functioning framework
Burden reduction and simplification
Needs for improvement
11
LIST OF ANNEXES
1INTRODUCTION
Chemicals are everywhere in our modern society. They are an integral part of most human activities and production processes and they are present in most consumer products, be it for food, electronics, toys, clothes or industrial machines. They have contributed to the improvement of human health and life expectancy, and to our societal comfort and wellbeing. They play an important role in the EU industrial competitiveness and creating jobs. On the flipside, however, are the potential and actual human health and environment risks that result from exposures to hazardous chemicals. The overall aim of 50 years of EU policy on chemicals is to promote their safe use with a view to improving their overall sustainability including human health and environment protection, competitiveness, innovation, internal market, growth and jobs. To do so the EU chemicals legislation (what we call today, 'the European Union chemicals acquis') identifies hazardous chemicals and, for those chemicals where the human health and environmental risks require action, establishes measures to manage these risks.
The Commission decided to undertake this Fitness Check of chemicals legislation other than REACH ('the Fitness Check') to see what elements of the European chemicals acquis work well and what needs to be improved, both in terms of meeting the policy objectives and in terms of reducing regulatory burden. Unlike most evaluations, the Fitness Check is not an evaluation of one piece of legislation but covers more than 40 different pieces of legislation (see Annex 4 Table 1). It covers legislation that addresses chemical hazard identification, assessment classification and labelling, risk assessment, and risk management, including worker safety, transport, environmental protection, chemical-specific and product-specific legislation.
This Fitness Check focuses on how the chemical risk assessment and management processes work across the EU chemicals acquis. This means that in some cases, the focus is on the entire piece of legislation as all of its requirements and, hence, associated regulatory costs relates to chemical hazard/risk assessment and risk management. Examples include the CLP Regulation, the Plant Protection Products Regulation, the Residues of Pesticides Regulation, the Biocidal Products Regulation, the Cosmetics Products Regulation, the Detergents Regulation, the Chemical Agents Directive, and the Carcinogens and Mutagens Directive. For many of the other pieces of legislation only certain requirements were relevant for the purposes of this Fitness Check, for example: the Toy Safety Directive, the water and water-related legislation, the Waste Shipments Directive, the Industrial Emissions Directive and the Seveso III Directive (see Annex 8).
To assess this, the Fitness Check has:
·Mapped out links between hazard identification and consequent risk management in downstream legislation on the basis of generic risk considerations (GRC).
·Mapped out the links between specific risk assessments (SRA) and the consequent risk management.
·Examined the overall effectiveness, efficiency, relevance, coherence and EU added value of the two risk management approaches (GRC and SRA), on their own but also compared to one another, as adopted in the chemicals legislation.
Moreover, as announced in the Circular Economy Action Plan, the Commission has assessed the interface between waste, products and chemicals legislations. The Fitness Check takes into consideration the findings presented in the related 'Interface' Communication.
This Fitness Check complements the REACH Evaluation. Together, they cover the core EU legislative framework for the risk management of chemicals. The interface between REACH and other legislation is covered by the REACH review. Some REACH-related aspects are also covered under this Fitness Check in particular where REACH is a central consideration in assessing the coherence of different pieces of chemicals legislation (e.g. the identification, assessment and classification of persistent, bioaccumulative and toxic and very persistent, very bioaccumulative substances (PBTs/vPvBs)).
Figure 1
presents the intervention logic of the chemicals legislation covered by this Fitness Check. It summarises how the EU chemicals acquis is envisaged to lead to positive impacts on health, the environment and the functioning of the internal market as well as to enhanced competitiveness and innovation. It presents the links between the needs, the objectives, and the actions taken by different actors for each of the key steps in the hazard and risk assessment processes. It also sets out the related output of all these actions and general outcomes of the implementation and application of the EU chemicals acquis (e.g. improved knowledge on substances, hazardous substances identified, etc.).
Figure 1 The intervention logic evaluated in this Fitness Check
2BACKGROUND TO THE INTERVENTION
2.1Description of the initiative
2.1.1Objectives of EU chemicals legislation
The primary objectives of EU chemicals acquis are:
1.Ensuring a high level of protection of human health from the adverse effects of hazardous chemicals.
2.Ensuring a high level of protection of the environment from the adverse effects of hazardous chemicals.
3.Supporting and enhancing the efficient functioning of the internal market for chemicals and the competitiveness and innovativness of EU industry and business.
Specific pieces of legislation may have more specific objectives related to chemicals (see Annex 4 Table 1), such as protecting selected vulnerable groups (e.g. children), encouraging substitution to less hazardous alternatives, reducing the number of animals used for testing chemicals, ensuring the free movement of specific products or encouraging improvements in the occupational safety and health of workers. It is also a general, if not always explicitly stated, objective of the EU chemicals legislation to improve the knowledge of chemical hazards and risks. Furthermore, some of the legislation within the scope of this Fitness Check may also include objectives that concern other policy areas, such as ensuring agricultural productivity and sustainability or promoting products that have a high level of environmental performance.
The EU has also played a leading role in the development of, and is committed to, several global objectives related to chemicals. The EU and its Member States, committed to the UN objective of a sound management of chemicals throughout their life cycle in 2002, often referred to as the ‘World Summit of Sustainable Development (WSSD) 2020 goal’. In 2006, governments and stakeholders agreed on the Strategic Approach to International Chemicals Management (SAICM) (UNEP, 2006), a global policy framework to promote safe chemicals management with the explicit aim of implementing the WSSD 2020 Goal on chemicals and waste. In 2015, the EU committed to the United Nations’ 2030 Agenda for Sustainable Development including the Sustainable Development Goals (SDGs) (UN, 2015). Several of the SDGs relate directly or indirectly to chemicals and chemical policy (in particular SDGs 3.9, 6.3, 12.4). It should be noted, however, that apart from some international competitiveness assessment aspects, the Fitness Check scope did not include a detailed assessment of performance against the abovementioned international objectives and commitments. The focus was on the performance of the EU chemicals acquis in delivering against the core policy objectives within the EU context.
2.1.2The Framework of EU Chemicals Legislation
The EU legal framework for chemicals comprises not only chemicals legislation in the strict sense of the word – directly regulating chemical substances and mixtures – but also legislation regulating conditions under which chemicals are manufactured, treated or used (e.g. occupational health and safety or environmental legislation) or regulating products, in which chemicals are used (e.g. toys, medical devices and food contact materials). Furthermore, there are chemicals-related provisions in several pieces of environmental protection legislation such as the Water Framework Directive, the Waste Framework Directive and the Industrial Emission Directive.
The development of EU legislation on chemicals started in 1967 with the adoption of a Directive that harmonised the Member States' rules for the classification, packaging and labelling of chemical substances across the then European Economic Community. Since then a multitude of different pieces of legislation have been adopted (see
Figure 2
; see also Annex 4 Table 2) that, to a greater or lesser degree, address the risk management of hazardous chemicals. In 2001 the European Commission adopted a White Paper setting out the strategy for a future chemicals policy, ultimately leading to the adoption of REACH in 2006, the Classification, Labelling and Packaging Regulation ('the CLP Regulation' which repealed the Dangerous Substances and Dangerous Preparations Directives in 2008), and to the establishment of the European Chemicals Agency in Helsinki (ECHA) in June 2007.
The EU has also committed to a number of legally binding international agreements related to chemicals, which are implemented through EU chemicals-related legislation, for example, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) and the Basel, Minamata, Rotterdam, and Stockholm Conventions as well as the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR) (see Annex 8 Section 8.1.1 for further detail).
Figure 2 Some key pieces of EU chemicals legislation adopted since 1967
The EU chemicals legislation has been a model for policy development in other parts of the world. The knowledge base resulting from the implementation of different pieces of EU legislation is, in many instances, made available to government, industry and stakeholders beyond the EU.
2.1.3Scope of the Fitness Check
This Fitness Check focuses on more than 40 pieces of legislation (see Annex 4 Table 1). Those, together with REACH (which is outside the scope of this exercise) form the core of the EU framework of chemicals and chemicals-related legislation. The primary criteria for determining which pieces of legislation to include within the scope of the Fitness Check was the existence of requirements in the legislation relating to hazard/risk assessment and risk management of chemicals. This meant including horizontal legislation that supports the overall process of chemical hazard and risk assessment such as the Test Methods Regulation (440/2008/EC) and the Good Laboratory Practice Directives (2004/9/EC and 2004/10/EC).
A meaningful way to categorise these pieces of legislation, given the risk management focus of this Fitness Check, is as follows (see also Annex 8 section 8.1.2):
1)Legislation covering chemical hazard identification and classification: Chemical Agents Directive (98/24/EC), Carcinogens and Mutagens at Work Directive (2004/37/EC), CLP Regulation (1272/2008/EC), Plant Protection Products Regulation (1107/2009/EC), Asbestos Directive (2009/148/EC) and Biocidal Products Regulation (528/2012/EU).
2)Legislation covering chemical risk assessment and risk management measures:
a)Worker safety and transport legislation: Pregnant Workers Directive (1992/85/EEC), Young People at Work Directive (1994/33/EC), the Chemical Agents Directive (1998/24/EC) and Carcinogens and Mutagens at Work Directive (2004/37/EC).
b)Environmental protection legislation: the Urban Waste Water Directive (91/271/EEC), Water Framework Directive (2000/60/EC) and Industrial emissions (integrated pollution prevention and control) Directive (2010/75/EU).
c)Chemicals control legislation: Contaminants in Food and Feed Regulation (315/93/EEC), Persistent Organic Pollutants Regulation (850/2004/EC), and Directive (2002/32/EC), Residues of Pesticides Regulation (396/2005/EC), Plant Protection Products Regulation (1107/2009/EC), Biocidal Products Regulation (528/2012/EU) and Export and Import of Hazardous Chemicals Regulation (649/2012/EU).
d)Products control legislation: Medical Devices Directives (93/42/EEC; 90/385/EEC; 98/79/EC), Drinking Water Directive (98/83/EC), General Product Safety Directive (2001/95/EC), Detergents Regulation (648/2004/EC), Toy Safety Directive (2009/48/EC), Cosmetic Products Regulation (1223/2009/EC), Food Contact Materials Regulations (10/2011/EC and 450/2009/EC) and Pressure Equipment Directive (2014/68/EU).
3)Supporting and horizontal legislation: Good Laboratory Practice Directives (2004/9/EC and 2004/10/EC), Test Methods Regulation (440/2008/EC), and Protection of Animals Used For Scientific Purposes Directive (2010/63/EU).
This Fitness Check is not an in-depth evaluation of each individual piece of legislation within its scope. Instead, it aims to assess the functioning, performance and coherence of the overall framework with a particular focus on the hazard/risk assessment and risk management of chemicals. In addition and in parallel, the Commission is conducting targeted Better Regulation evaluations of a number of pieces of chemicals legislation within the scope of the Fitness Check, including the Plant Protection Products and the Residues of Pesticides Regulations, the Urban Waste Water Treatment Directive, the Water Framework Directive, the Food Contact Materials legislation and the Detergents Regulation (see Annex 4 Table 4).
At the margins, there is some additional legislation that this Fitness Check could have covered e.g. pharmaceuticals legislation (human and veterinary products) and food additives legislation. It was, however, considered that the risk and hazard assessments performed under these pieces of legislation are used slightly differently compared with those performed under the main body of EU chemicals legislation (e.g. an assessment of the risk trade-offs between the health benefits of the medical product versus potential undesired side-effects). REACH is generally outside the scope of this exercise. It is subject to its own legal review deadlines. While the first evaluation of REACH was finished in 2013, the second evaluation had already started when this Fitness Check was launched and was completed by the time that this Fitness Check entered its finalisation phase. Nevertheless, given the importance of hazard identification and classification criteria under this Fitness Check, Annex XIII to REACH covering persistent, bioaccumulative, toxic (PBT) and very persistent and very bioaccumulative (vPvB) criteria was included in the scope of this exercise. In general, where considered relevant from a comparative perspective, links to these pieces of legislation are covered as part of the coherence analysis.
2.1.4Main steps: from hazard identification to risk management measure
Chemical risk assessment involves the analysis of the inherent hazardous properties of a substance or a mixture and the extent of exposure to that substance or mixture. The human health and environmental risks related to exposure to hazardous chemicals are addressed via the hazard and risk assessment procedures and requirements set out in the different key pieces of the EU chemicals legislation such as the CLP, the Plant Protection Products and Biocidal Products Regulations, etc. The main steps of these procedures involve:
·hazard identification (based on toxicity tests and other relevant information);
·dose (concentration) – response (effect) assessment;
·exposure assessment – exposure scenarios (based on models and measurements of the occurrence of the chemical);
·risk characterisation; and
·risk estimation.
Risk management measures – which can be policy-based and/or technical in nature - are then decided in light of the identified hazards and/or risks. Risk management measures can range from (and involve a mix of) a total ban to any condition to the manufacture, use or placing on the market of chemicals (such as setting emission/concentration/migration limits, obligations to communicate hazards and risks, labelling requirements, obligations to use personal protection equipment, etc.).
2.1.5Risk management approaches
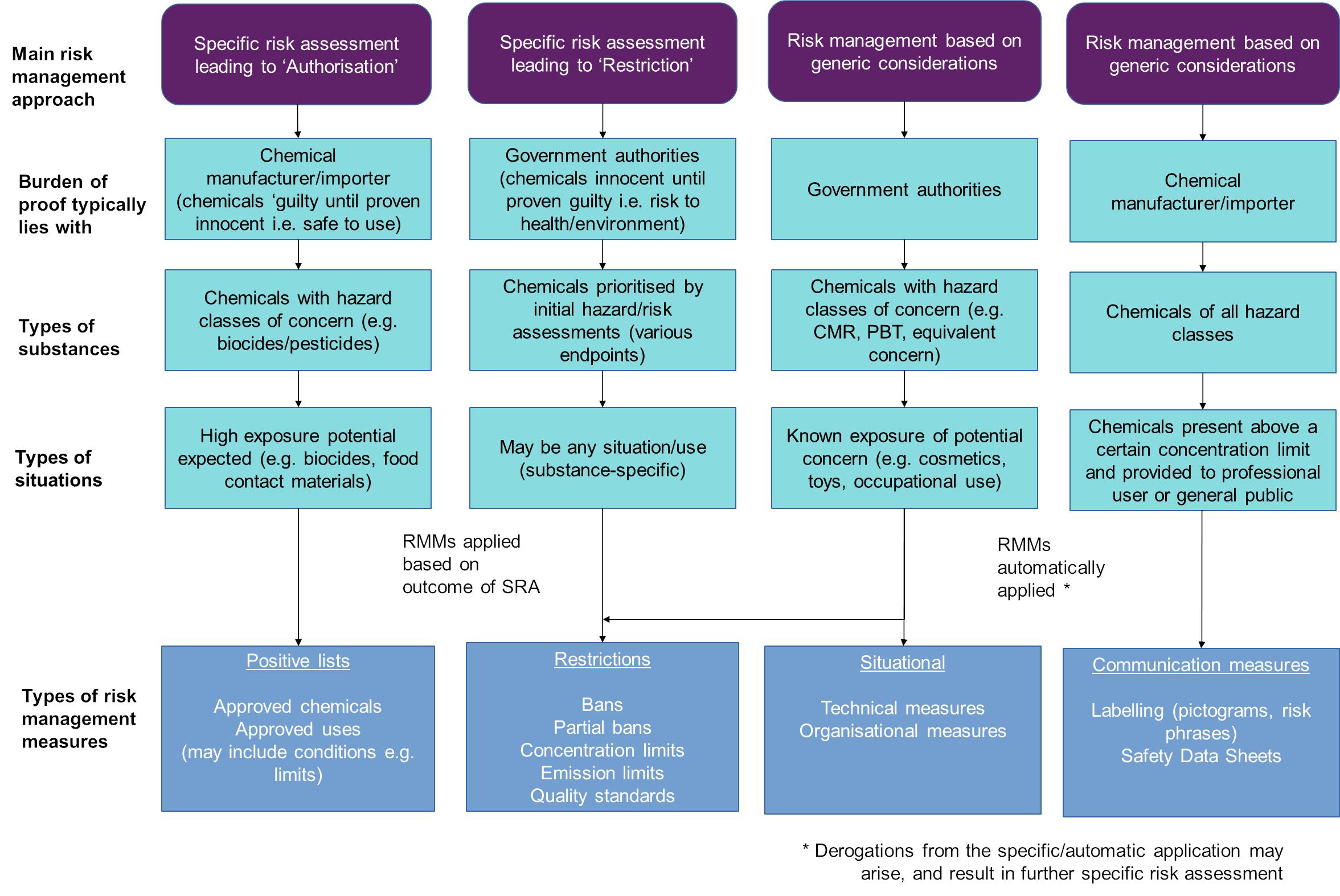
There are two basic approaches to risk management often used in combination, in the EU chemicals acquis: one based on specific risk assessment (SRA) and the other one based on generic risk considerations (GRC) (see Annex 8 Section 8.2.1).
The main difference between these two approaches is the point in time when the exposure assessment is considered and the specificity of the exposure assessment. For risk management based on GRC, the potential exposures and risks are considered generically, prior to the adoption of legislation. The GRC-based approach is built into the legislation in the form of an automatic trigger of pre-determined risk management measures (e.g. packaging requirement, communication requirement, restrictions, bans, etc.) based on the hazardous properties of the chemical, without the need or possibility to assess and take into account specific exposure levels for a specific situation or use. For example, under the Cosmetic Products Regulation any substance classified as carcinogenic, mutagenic or toxic for reproduction (CMR) categories 1A/B and 2, shall be banned from use in cosmetics (subject to strict derogations), given the fact that direct exposure of humans is taking place through the application of a cosmetic product on the external parts of the human body (or teeth or mucous membranes of the oral cavity). Similar approaches have been taken for active ingredients in plant protection products and biocides, for substances in toys, etc.
The decision to link particular hazard properties (e.g. CMR, persistent bioaccumulative and toxic substances (PBTs), endocrine disruptors (EDs)) to automatic risk management measures without the intervening step of a specific risk assessment is done on the basis of generic risk consideration without prejudice to performing also a full risk assessment for the other properties of the substances which are not linked to the related hazard properties. In the legislation evaluated in this Fitness Check, the generic risk consideration approach is typically applied for the following use applications and the following substances:
Use applications:
·when there is a need to obtain and pass on information to enable [further/specific] risk assessment or risk management (e.g. labelling obligations under the CLP, labelling requirements and use instructions under the Plant Protection Products and the Biocidal Products Regulations).
·for use in widely dispersive or open applications which result in a significant exposure of humans or the environment (e.g. plant protection products).
·for use in applications where the exposure is considered to be more difficult to control and monitor (e.g. plant protection products).
·for use in applications resulting in exposure of vulnerable groups (e.g. children).
·for use to prioritise the risk assessment of certain chemicals and under certain conditions (e.g. food contact materials)
Substances:
·for substances with hazard properties that result in severe adverse effects on human health or the environment should exposures occur (e.g. CMRs, PBTs, EDs, chemicals with Single Target Organ Toxicity (STOT) properties); and
·for substances where it is difficult/impossible to identify a safe threshold and, therefore, where most specific risk assessments are likely to identify risks that lead to a need for risk management measures (e.g. PBTs, vPvBs, respiratory sensitisers).
On the other hand, in the case of the specific risk assessment approach, the exposure assessment is performed on a case-by-case basis when each substance is risk assessed under a specific legal framework. The risk management measures are triggered based on the outcomes of the specific risk assessment which considers the use of the substances and in which both the hazards and the potential specific exposure scenarios for humans and the environment to the hazardous substance or mixture in question are assessed at the same time.
The specific risk assessment approach is used more widely for uses which are not necessarily or obviously going to lead to widespread and difficult to control exposures and/or where the hazard properties of a substance are of less concern.
In many instances, individual pieces of chemicals legislation use a combination of both of these approaches. For example, the Cosmetic Products Regulation applies the specific risk management approach to establish lists of authorised substances as well as, where necessary, restrictions on the use of certain substances in certain situations. In addition, for substances identified and classified as a CMRs categories 1A/B and 2, the generic risk management approach is applied (such substances shall be banned and cannot, therefore, be used in cosmetic products subject to strict derogations).
2.1.6Risk assessment and risk management processes and bodies involved
The human health and environmental risks from the exposure to hazardous chemicals are addressed via hazard and risk assessment procedures prescribed in the EU chemicals legislation. The main steps of the chemicals risk assessment and management process (i.e. decision making and implementation and enforcement) usually involve:
The necessary hazard identification, exposure assessment and risk assessment of chemicals are undertaken through a number of separate (but closely aligned) processes involving EU expert committees/bodies associated (see Annex 8 Section 8.2.2). These committees/expert groups are mainly established in association with different pieces or groups of legislation. Examples include:
·the European Chemicals Agency (ECHA): covering the CLP, the Export and import of hazardous chemicals (PIC) Regulation, the Biocidal Products Regulations) and REACH;
·the European Food Safety Authority (EFSA): covering the Plant Protection Products and Residues of Pesticides Regulations as well as the Food Contact Materials and the Contaminants in Food and Feed legislation;
·the Scientific Committee on Consumer Safety (SCCS): covering the Cosmetic Products Regulation, the Toy Safety Directive and the General Product Safety Directive (GPSD)
·the Scientific Committed on Occupational Exposure Limits (SCOEL): previously covering occupational safety and health legislation; and
·the Scientific Committee on Health, Environment and Emerging Risks (SCHEER): covering health, environmental and emerging risks and broad, complex or multidisciplinary issues that require a comprehensive assessment of risks to consumer safety or public health and related issues not covered by other European Union risk assessment bodies.
2.2An overview of the EU chemicals industry and related sectors
The chemicals industry covers five main sectors (petrochemicals, polymers, basic inorganics, specialties and consumer chemicals) broken down into 16 subsectors. Five of these subsectors (paints, varnishes and similar coatings; printing ink and mastics; soap and detergents, and cleaning and polishing preparations; perfumes and toilet preparations; plastics in primary forms; and other organic basic chemicals) account for over 65% of EU chemical companies.
The chemicals industry is also characterised by geographical concentration with 85% of the EU turnover in the chemical industry concentrated in seven countries — Germany (30%), France (14%), the Netherlands (10%), Italy (10%), Spain (7%), the United Kingdom (7%), and Belgium (7%).
As an "enabling industry", the chemical industry is at the heart of the EU manufacturing industry, supplying two-thirds of its production to other industry sectors. Thus, a large range of downstream sectors rely on the use of chemicals in their everyday activities, such as the automotive and aerospace sectors, the paper and pulp sector, as well as the manufacture of everyday goods such as textiles, cosmetics, toys, etc. Other important links exist with agriculture activities and services.
The chemical manufacturing industry is the fifth largest in the EU, accounting for 7% of the EU’s industrial production. With annual EU chemicals sales of EUR 507 billion, the sector comprises over 28 000 companies and it directly employs around 1.2 million people as well as generating additional estimated 3.6 million indirect jobs. SMEs account for around 96% of the number of companies in the sector, approximately one third of the direct employment and one third of the sector's value-added. The EU chemical sector generated a value-added of approximately EUR 115 billion in 2014 representing about 0.8% of EU GDP. In 2016, extra-EU chemicals exports amounted to EUR 146.2 billion and extra-EU imports reached EUR 99 billion (the EU chemicals trade surplus outside the EU being valued at EUR 47.2). In 2017, there was an increase in both exports and imports compared to 2016 (+ 6.5% and + 8.3%).
In terms of chemicals sales, the EU chemicals industry represented in 2016 15.1% of the global market, behind China (39.6%) ahead of the United States (14.2%). EU chemical sales increased by more than 50% in 20 years, while its world market share halved (down from 32.5% in 1996 to 15.1% in 2016) due to strong chemical demand growth in China, and other emerging countries and low growth in Europe and North America, where Europe sells most of its chemicals.
The main competitive advantage of the EU chemicals manufacturing industry is the high level of technological development, skilled workforce and strong research base. The EU chemicals industry is one of the most research and development intensive manufacturing sectors within advanced economies (behind US and China only). As an input provider for other industries, the chemicals industry is also considered to be at the forefront of innovation and a solution provider for many societal and environmental challenges, with chemical technological breakthroughs spilling over its downstream sectors.
The total sold production of chemicals, including pharmaceuticals in the EU in terms of value increased moderately from 2007 to 2016 with an average annual growth of 0.6%. The production of industrial chemicals in the EU-28, increased each year between 2004 and 2007, rising overall by 4.5 % to peak at 371 million tonnes in 2007. The EU chemicals industry was strongly affected by the economic and financial crisis of 2007-2009. In 2009, total sales revenue in the EU chemicals sector lost more than one fifth of its original value compared to 2008. The recovery trend started in 2010 and peaked in 2012 before declining slightly in line with the global economy. It remained relatively stable during the period 2013–2016 but, in production terms, still 40–50 million tonnes below the pre-crisis peak in 2007. In 2017, and especially if compared to the 2012-2016 period, the EU chemical industry resumed strong growth (+7.9%) which continued in first quarter of 2018.
2.3Baseline
This is a first comprehensive and cross-cutting assessment of the EU chemicals legislation over its 50 years of existence and the progress made towards the achievement of its core objectives. There was no pre-existing assessment that could have been used as a baseline.
The wide scope of the Fitness Check and the selective focus on the hazard and risk assessment and management elements, together with the data limitations and the continuous evolution of EU chemicals legislation led to using for the assessment purposes a number of different points of reference.
For the assessment of the effectiveness the following points of reference were used:
·Achieving human health and environmental protection was measured by looking at the achieved exposure reductions since 1970s through implementation of risk management measures such as bans, restrictions, emission limits, concentration limits, etc. In this regard, a range of different timeframes were considered thus reflecting the fact that different pieces of legislation were adopted at different moments in time (see Annex 4 Table 2). The ‘present’ (at the time the studies were undertaken i.e. between 2015-2017) was also a frequently used reference point for assessing the effectiveness of certain processes and aspects of the EU framework of chemical legislation (e.g. communication of chemical hazards and risks to consumers and workers). In terms of on-going exposures and predicted future health and environmental impacts e.g. future cancer fatalities linked to past, present and future exposures, the timeframes used went as far as 2100.
·In terms of meeting the internal market objective, the most practical way to measure change was the level of harmonisation and the growth in intra-EU trade of chemicals. Although there is good data on trade, it is difficult to know what proportion of the growth in intra-EU trade is due to chemicals legislation versus other market forces. Trade was used as a performance indicator but this was not explicitly compared to what might have happened in the absence of EU chemicals legislation given the difficulty of estimating what this baseline might have been. The timeframe considered for this part of the assessment was 2006-2016.
·Eurostat data on the volumes of hazardous chemicals produced and consumed in the EU during the 2004-2016 period was used to provide a rather general point of reference for assessing the progress made in terms of substitution of the most hazardous chemicals. However, lack of clear link between competitiveness and innovativeness and the EU regulatory intervention, as well as lack of specific performance criteria or meaningful points of reference made the assessment difficult.
The coherence of the EU chemicals legislation was assessed by comparing different pieces of legislation e.g. are the cut-off criteria under the Plant Protection Products Regulation coherent with the cut-off criteria set out in the Biocidal Products Regulation.
For the assessment of costs and benefits, setting a baseline reflecting what would have been the legislation in place in Member State in the absence of the EU legislation since 1970s was not possible. Therefore, a baseline of no legislation in place at the EU or Member State level was used, even though such an assumption also seems hypothetical. For both cost and benefit assessments, the ‘zero counterfactual’ baseline was used except where the costs of transition from older EU legislation to current EU legislation were assessed (for the CLP Regulation). In this case, the counterfactual used for regulatory costs was the pre-existing legislation. The assessed costs represent therefore total costs and not the additional costs of implementing EU legislation i.e. costs above and beyond the costs of assumed Member State legislation that might have already been in place.
Even though the ‘zero counterfactual’ baseline was used in a consistent way, cost and benefit figures used are difficult to compare because of the timelines not lining up. As explained above the benefit assessment was backwards looking, i.e. what are the specific exposure level reductions that we can observe 'today’ that can be reasonably attributable to the pieces of legislation within the scope of this Fitness Check and/or other pieces of legislation considered as chemicals related. The cost assessment was limited in time (2004-2016 period). It does not look at what were the costs to achieve the specific exposure level reductions. It looks at what were the costs that specific sub-sectors of the chemicals industry had to bear in order to comply with the legislation existing at that time.
3STATE OF PLAY
This section describes the state of play of the EU chemicals legislation and the factors affecting its implementation and enforcement. The following conclusions should be seen as a collection of issues identified under the Fitness Check and related evaluations, without being complete for each of the pieces of legislation within its scope. Moreover, it should be noted that under a number of pieces of the EU chemicals legislation Member States are not required to report information on enforcement or information provided is of poor quality. This was a significant obstacle for the assessment carried out for the purposes of this Fitness Check. The situation is however expected to improve as several of the individual pieces of legislation within the scope of this Fitness Check are currently undergoing their own evaluations (see the list of ongoing evaluation in Annex 4 Table 4). The follow up to the Fitness Check of monitoring and reporting of environmental policy will also contribute to improving the current state of knowledge.
3.1Implementation of the EU chemicals legislation
3.1.1Main actors and roles of each
The implementation of the EU chemicals legislation relies increasingly on European harmonised processes in which Member States alone or in cooperation with others and the Commission play important roles. The Commission has been granted delegated and implementing powers, the latter being executed via comitology. Approximately 20 different committees assist the Commission in the chemicals legislation area.
Member States are responsible for the correct application of the acquis and the timely and correct transposition of Directives. Regulations do not need to be transposed i.e. they are directly applicable and legally binding across the EU. In the area of chemicals legislation, the use of Regulations over Directives has increased over the past 10-20 years. Directives are mainly used in the occupational safety and health (OSH) legislation and environmental policy (water and waste) areas. For the OSH legislation this reflects the willingness to provide on the one hand a level playing field for business operating within the internal market and on the other, to leave room for Member States to adopt more stringent protective measures when transposing EU Directives into national law. For environmental policies, this allows taking into account the diversity of environmental situations in the various regions of the EU.
The implementation of the EU chemicals legislation relies also on the activities of different EU agencies (collection of data, scientific opinions, guidance, helpdesks etc.) such as ECHA or EFSA, and scientific committees providing scientific opinions.
3.1.2State of play
The EU chemicals legislation is relatively mature (2nd or 3rd generation). Some transposition issues related to chemicals legislation (hazard/risk assessment and management aspects) have occurred in the past but the great majority of these have been identified and resolved since then. Moreover, the increasing replacement of Directives by Regulations has contributed to this.
Certain stakeholder groups have expressed concern about the Commission's capacity to make risk management decisions in a timely manner. Particular areas of concern include the review programme for the approval of existing active substances under the Biocides Regulation which is closely interlinked with Member States' capacity to carry out assessments, and up in the chain linked to the capacity of industry to deliver good quality dossiers, and authorisation of the use of recycled plastics in food contact materials.
3.2Enforcement of the EU chemicals legislation
3.2.1Main actors and roles of each
The Member States, EU Agencies and the Commission all play a role in enforcement.
The Member States have the legal powers and obligation to enforce against duty holders. Enforcement activities cover all activities aimed at promoting compliance and achieving general and specific legal objectives e.g. allowing free movement of goods, lowering risks to safety, health and the environment, etc. These activities may include enforcement activities in a broader sense such as providing information, guidance and prevention or in a narrower sense such as data collection and analysis, inspections, warnings, improvement notices, fines, prosecutions, legal actions in case of infringement etc.
The Commission's enforcement role is to check the proper application of the legislation. This includes the resort to formal infringement procedures e.g. in case of non-conformity of national transposition law with EU directives or incorrect application of the EU law. The Commission also provides assistance to national authorities through guidance documents, clarifications on interpretation of legal provisions, etc. Some pieces of legislation delegated certain 'enforcement powers' to ECHA or EFSA, for example, in the case of risk assessment dossier evaluation. Guidance to assist national authorities and industry has improved the clarity and consistency of interpretation of legal requirements. National helpdesks for CLP co-operate through the ECHA Helpnet to support companies in understanding their obligations. Although much still needs to be done, networks such as the FORUM and RAPEX and other legislation specific enforcement networks have significantly contributed to improved coherence of enforcement.
3.2.2State of play
The Member States have the legal powers to enforce against duty holders. At Member State level, resource (both financial and human) capacity and expertise constraints, particularly following the financial crisis, are resulting in a number of enforcement challenges:
·Capacity of national competent authorities to conduct the necessary market surveillance activities in respect to consumer goods. The General Product Safety Directive (GPSD) created a horizontal framework ensuring the safety of consumer products. To this end, it sets out a number of obligations for manufacturers, importers and distributors as well as certain obligations for Member States as regards the organisation of market surveillance. The GPSD also established a network of authorities of the Member States competent for product safety aimed at facilitating operational collaboration on market surveillance and other enforcement activities. The GPSD applies to all consumer products including the harmonised sectors like toys, cosmetics, etc., in so far as the relevant harmonisation legislation has not itself provided for specific rules with regards to specific safety aspects. While the GPSD contains an obligation for Member States to take part in the cooperation mechanism, the performance of the obligations it imposes on Member States to organise and perform market surveillance depends on the resources available. For this reason differences in the various Member States still continue to persist, leading to a different level of protection and enforcement within the EU.
·In the case of plant protection products, even though controls on retailers were reported to be generally satisfactory, the majority of Member States do not conduct controls on plant protection products stated to be for use in other Member States or in non-European Union countries. This weakness in control systems can be easily exploited to place non-compliant products on the market.
·Capacity to undertake routine inspections and other compliance and enforcement activities, including monitoring and reporting.
·For example, the need to invest additional resources on enforcement activities is recognised in order to ensure that no biocidal product is illegally placed on the market at national level and that these products are properly labelled.
·Regarding the Toy Safety Directive, Member States considered that the low consistency of national approaches to enforcement (both in terms of the number and the type of control procedures) creates a trade barrier. Limited testing capacity of some Member States was also deplored.
·Another example is non-harmonised food contact materials (FCMs) – i.e. specific food contact materials such as inks, adhesives, or paper for which at EU level no harmonised rules exist. Member States highlighted the lack of resources needed for controls (personnel for the inspections, analytical equipment, facilities, etc.). They also reported that local inspection is not adequate for checking compliance with a supply chain spread throughout the world.
·Regarding online chemicals sales, several enforcement surveys show that various non-authorised chemicals and related products are increasingly being offered for sale via the Internet. As chemicals legislation does not distinguish between different types of trade, all provisions regulating chemicals apply in principle also to Internet trade. Currently, however, access to websites and relevant information on transactions, vendors or service providers for monitoring authorities is difficult and therefore hampers their investigations.
Verification of compliance with and enforcement of chemicals legislation is in many cases complex and resource-intensive. Some of the differences in the level of enforcement are due to differences in the resources allocated and made available by Member States. Other factors, leading to non-uniform application of the EU law include the national control set ups (planning and frequency of controls, number of inspectors, training and other professional qualifications, etc.), differences in the interpretation of the EU law, differences in or lack of standards, lack of harmonised requirements and guidelines, etc. The following specific examples illustrate these observations:
·Differences in administrative organisation of Member States create differences regarding the frequency of controls and inspections. These differences are notable regarding in particular the occupational safety and health (OSH) legislation and the CLP Regulation.
·Different interpretation by Member States of the legislation and lack of guidance documents and/or harmonised analytical methods for testing impact the implementation of the EU chemicals legislation. This was indicated in particular for the CLP, the FCMs, the Plant Protection Products Regulations, and the Toy Safety Directive.
Even though the principle of mutual recognition is one of the means of ensuring the free movement of goods within the EU, whether it is effective in doing so, depends on if and how Member States apply it. In cases where there is an absence of mutual recognition, this leads to duplication of efforts between Member States and exacerbates the existing resource limitations. Because mutual recognition is currently underutilised for plant protection products authorisation, risk assessments are sometimes partially or fully repeated by other Member States thus creating additional costs. The main reasons for this are related to lack of information on how the first Member State reached its conclusion, leading to a lack of confidence. The other major reason is the age of the data, noting that some time may have passed between the first and subsequent assessments.
Where technical standards or detailed harmonised requirements are lacking or are incomplete, or where technical standards do exist but there is no EU-wide shared methodology for assessing them, this can undermine the quality and completeness of the exposure assessments that are needed for conducting the required risk assessments. This issue was highlighted by several Member States regarding the 'safety' (i.e. risk) assessments of toys and their constituent substances, as well as assessing health risks associated with their use. In general, the performance of risk assessment is easier to quality control where there is a requirement to not only document it but also communicate its outcome to the public authorities.
4METHODOLOGY
4.1Description of methodology: quantification and data collection
A roadmap was published in 2015 presenting the scope and the key evaluation questions to be addressed by the Fitness Check, as well as a consultation strategy to ensure stakeholders' engagement in the process (see below and the Annex 2). The Fitness Check was accompanied by an interservice steering group covering all Commission services in charge of the legislation under scrutiny plus horizontal services.
Priorities for assessment were established on the basis of the main areas of improvement identified by the key studies supporting the Fitness Check, considering the concerns raised by stakeholders.
4.1.1Studies
Two key studies and two related studies, carried out by external consultants for the Commission, provide an important part of the evidence base for the Fitness Check.
A.Complementarity of the Fitness Check core studies
The 1st Fitness Check study (1st FC Study) was completed in January 2017. It focuses on the CLP Regulation and related legislation governing hazard identification, communication and risk management of chemicals. This includes an assessment of costs and benefits associated with the CLP Regulation. The on going costs of the CLP are estimated as ‘present day’ costs generated at the time of the study (2015-2016) using a ‘zero counterfactual’ as the point of reference i.e. against a situation where there is no legislation in place at Member State or the EU level. The transition costs from the previsious Dangerous Substances Directive (DSD) and Dangerous Preparations Directive (DPD) to the CLP Regulation cover the time period from 2009 (when the CLP first came into force) to the 2015 deadline for meeting the CLP requirements applicable to mixtures. The (partial) assessment of human health and environmental benefits of classification, labelling and packaging of chemicals was examined across a timeframe of 2000-2016. This allowed a comparison between the partial estimation of benefits accrued under the pre-CLP legislative situation (the DSD and the DPD) against partial estimation of benefits accrued following implementation of the CLP Regulation. The benefits assessment was also done using a zero counterfactual baseline.
The 1st FC Study was complemented by a second study (FC+ study) completed in November 2017. Its focus was pieces of legislation that operate independently of the CLP for chemical hazard identification and classification and pieces of legislation where specific risk assessment procedures form the core part of the risk management process (this was not covered by the 1st FC Study). For the great majority of assessment aspects, including the analysis of cost drivers, the time reference of the FC+ Study was the ‘present day’ situation i.e. situation at the time the study was undertaken (2017). The cost driver analysis was done using a zero counterfactual (i.e. no chemicals legislation in place at Member State and the EU level) as the point of reference.
Because in many cases, the EU chemicals legislation is based on the use of both generic and specific risk assessment and more or less direct link to the CLP Regulation (see Annex 4 Table 1), a number of pieces of legislation were covered by both core studies. Examples include the Toy Safety Directive, the Cosmetic Products Regulation, the Plant Protection and the Biocidal Products Regulations, the Industrial Emissions Directive, the Water Framework Directive, the Detergents Regulation, the Food Contact Materials Regulation (
Table 1
). The REACH Annex XIII and the CLP Regulation were however exclusively covered by the 1st FC study.
In line with the Fitness Check methodology, the studies respond to the evaluation criteria and evaluation questions from the roadmap, while also providing a more detailed analysis of relevant themes through study tasks and case studies.
B.Additional Fitness Check supporting studies
The findings of the two core Fitness Check studies were complemented by a cumulative cost assessment of the chemical industry (CCA1) and a study on the cumulative health and environmental benefits of chemicals legislation (CuBA Study). The CCA1 study provides an estimate of total regulatory costs (i.e. it uses a zero counterfactual as a point of reference which assumes no chemicals legislation at Member State level in the absence of EU chemicals legislation) of the most relevant EU legislation with a bearing on the chemical industry (excluding downstream sectors) during the period 2004-2014.
The CuBA study draws together a large body of evidence on the health and environmental improvements achieved since 1970s as a result of hazardous chemical exposure reductions linked to EU chemicals legislation. The CuBA study also assesses the health and environmental impacts and costs associated with on-going exposures to chemical risks. Again, the benefits are estimated using a zero counterfactual as the point of reference.
C.Methodology, time and legal scope, and topics covered
The main methodologies applied in the context of the abovementioned studies can be described as follows:
·Development of an intervention logic underpinning the rationale for chemicals legislation and the CLP Regulation more specifically, including legal mapping to identify relevant legislation and specific provisions within this. This was then supported by a legal analysis to identify the nature of the obligations for different economic operators, how the legislation was implemented in practice, and areas where there appeared to be inconsistencies, overlaps and incoherence.
·A literature review to pull key information from impact assessments, position papers, academic and scientific research, papers and reports prepared by the relevant scientific bodies, regulatory submissions and other 'grey' literature.
·Development of evaluation questions (see Annex 10) and stakeholder consultation activities (see Annex 2).
·Case study research, which involved a more in-depth examination of some of the most pertinent issues identified as part of initial research (e.g. metals classification and the CLP Regulation, parallel hazard assessments, persistent, bioaccumulative and toxic (PBTs) / very persistent and very Bioaccumulative (vPvBs) substances, carcinogenic, mutagenic or toxic for reproduction substances (CMRs), linkages between the CLP and the occupational safety and health (OSH) legislation and several others), either directly linked to the interface between the CLP and other legislation, the functioning of specific legislation, or examining tools or measures needed to support the legislation.
·Comparative analysis of approaches based on specific risk assessments and generic risk considerations.
More generally, the studies have applied the tools set out in the Better Regulation Toolbox in assessing costs and benefits.
Figure 3
illustrates the time period covered by each of the Fitness Check studies.
Figure 3 Time period covered by the Fitness Check studies
Table 1
illustrates the legal scope covered by studies.
|
COVERED BY:
|
LEGISLATION
|
|
1st FC
FC+
CCA1
CuBA
|
Industrial Emissions Directive
Water Framework Directive
Biocidal Products Regulation
Plant Protection Products Regulation
Toy Safety Directive
Cosmetic Products Regulation
Detergents Regulation
Food contact materials Regulations
|
|
1st FC
CCA1
CuBA
|
CLP Regulation
REACH Annex XIII
Inland transport of dangerous goods Directive
Carcinogens and mutagens at work Directive
Chemicals Agents Directive
Young People at Work
Pregnant Workers Directive
Seveso III Directive
Waste Framework Directive
End of Life Vehicles Directive
Fertilisers Regulation
|
|
1st FC
FC+
CuBA
|
Waste shipments Regulation
EU Ecolabel Regulation
Pressure equipment Directive
General Product Safety Directive
|
|
FC+
CCA1
CuBA
|
RoHS 2 Directive
Batteries Directive
Packaging and Packaging Waste Directive
Export and import of hazardous chemicals (PIC) Regulation
POPs Regulation
Explosives Directive
|
|
1st FC
CCA1
|
Signs at work Directive
|
|
FC+
CuBA
|
Asbestos Directive
Urban Waste Water Directive
Marine Strategy Framework Directive
Contaminants in food and feed Regulation and Directive
Drinking Water Directive
Medical Devices Directives
Protection of animals used for scientific purposes Directive
|
|
1st FC
|
Aerosol dispensers Directive
Test methods Regulation
Good Laboratory Practice Directives
|
|
FC+
|
Residues of pesticides Regulation
|
Table 1 Pieces of legislation covered by the Fitness Check Studies
Annex 4 Table 1 and Table 3 provides more detailed information about how the Fitness Check supporting studies cover the topics discussed in the remainder of this document.
The studies provide evidence for the full scope to a large extent. However, either because of methodological challenges and lack of data or peculiarities of this Fitness Check i.e. focusing on the framework-wide issues rather than on legislation specific issues, some aspects were not assessed in-depth. In order to fill such gaps, other available sources of information were used, including other REFIT supporting studies or interim reports, EU Agencies’ and the Commission’s reports, as well as the other recent chemicals related initiatives and actions (see Annex 4 Table 3).
Annex 4 Table 4 provides a list of finished or still ongoing individual evaluations and how these different sources of information were used for the purposes of this Fitness Check (mainly concerning occupational safety legislation, plant protection products legislation, detergents and waste legislation). It should be noted however that where there is no specific reference to these individual evaluations, it is either because they were already used and refered to in the Fitness Check Studies or because the evaluation has just started and therefore evidence is not yet available.
4.1.2Data collection and stakeholder consultation
Given the wide scope of the whole exercise and, in some cases, the lack of data (costs, benefits, enforcement, performance monitoring, etc.) on individual pieces of legislation, this Fitness Check put a particular emphasis on stakeholder and expert input. Therefore, some of the issues identified may require further assessment as part of a dedicated evaluation of a specific piece of legislation, as mentioned above.
The stakeholder consultation strategy developed for the purpose of this Fitness Check comprised an public consultation (from 4 March to 27 May 2016), an SME panel through the Enterprise Europe Network (from 30 May to 18 July 2016), targeted interviews, stakeholder workshops conducted as part of the two main Fitness Check studies as well as the CCA1 and CuBA studies, and two Eurobarometer surveys (see Annex 2 for more details).
In line with the consultation strategy, input from a wide range of stakeholders was collected:
·public authorities, notably competent authorities responsible for the implementation and enforcement activities;
·industry associations covering both the chemicals industry and downstream sectors (manufacturers and importers of chemicals, distributors of substances and mixtures, formulators);
·companies in both the chemicals industry and downstream sectors, focusing in particular on Small and Medium-sized Enterprises (SMEs) (manufacturers and importers of chemicals, distributors of substances and mixtures, formulators);
·civil society organisations – NGOs (e.g. environmental, health, animal welfare);
·consumer associations;
·trade unions
·other interested groups such as academics / research institutes; and
·consumers / workers /citizens.
The online public consultation was conducted in English, German and French. The SME panel and the two Eurobarometer surveys were conducted in all EU languages.
These different consultation activities and tools allowed receiving feedback from all stakeholder groups. A summary of these views is provided in Annex 2.
Information on the Fitness Check is published on the websites of DG GROW and DG ENV.
4.1.3Use of findings from studies and stakeholder views for the purposes of this Fitness Check
The two core Fitness Check studies and the two additional Fitness Check studies provide the main evidence for the assessment presented in the remainder of this document. The evidence that these studies provide was used in a combined and complementary way. Each study corresponds to a different evidence gathering phase which was followed by an assessment phase.
The 1st FC Study corresponds to the first evidence-gathering phase (March 2015-October 2016; see
Figure 4
) which started with the publication of the Roadmap. It was preceded by the launch of the CCA1 Study. The assessment done during this phase was based on desk research and was followed and complemented by an extensive stakeholder consultation process. This first phase of the assessment provided useful and meaningful input and allowed to identify additional needs in order to cover the full scope of this Fitness Check.
Figure 4 First evidence gathering phase done for the purposes of the Fitness Check
The second evidence gathering phase started with the launch of the FC+ Study (see
Figure 5
). Similarly to the 1st FC Study, it also included targeted interviews with stakeholders as well as a stakeholder workshop.
Figure 5 Second phase of the assessment done for the purposes of the Fitness Check
During the assessment phase (starting in December 2017), all the evidence and stakeholder input gathered went through a thorough selection process. The purpose was to select those elements that affect (positively or negatively) the functioning of the framework and to identify those aspects that were only affecting the functioning of a specific piece of legislation. Therefore, not all of the findings gathered found their way in the final report. This assessment phase was also necessary in order to reality check the findings and to ensure that the Fitness Check supporting studies were used and combined to their utmost potential.
Annex 4 (Table 1 and Table 3) provides more detailed information about how the Fitness Check supporting studies cover the topics discussed in the remainder of this document.
4.2Limitations and robustness of findings
Given the wide scope of the exercise and the impacts of chemicals legislation, there were numerous challenges in gathering the data needed to provide a robust evidence base, as well as in providing quantitative estimates of impacts. As far as possible, data was triangulated with evidence collected from multiple sources e.g. literature review, qualitative assessment based on expert input (e.g. Member State Competent Authorities), stakeholder consultation etc. to provide as robust a picture of the evidence as possible. Nevertheless, whilst some legislation and risk assessment processes are well covered by multiple different stakeholder groups and literature/data sources, other pieces of legislation are less well covered.
Where specific obstacles and challenges were encountered, limitations are mentioned and explained in the relevant sections. The evidence and study limitations presented particular challenges with respect to the Fitness Check findings in the following areas:
·Determining and comparing framework-wide costs and benefits and, therefore, assessing the proportionality of the EU chemicals legislation at the framework level.
·Enforcement and implementation of the EU chemicals legislation.
·Determining the actual significance, in practical terms, of some of the coherence issues identified. It was beyond the scope and resources of the Fitness Check to seek primary evidence in order to test the real life significance of coherence issues flagged by one or more stakeholder groups.
Care was taken to accurately report different opinions and findings while also ensuring that the evidence and sources can be traced back and that therefore the reliability and robustness are ensured.
4.2.1The First Fitness Check Study (‘1st FC Study’)
The key limitations of the 1st FC Study can be described as follows:
·The broad scope of the study and the number of pieces of legislation to be considered.
·The lack of available information on the scale of issues identified (both positive and negative) and the subsequent need to rely on information provided by stakeholders.
·The limited response received from civil society stakeholders. However, further desk-based research of published information from NGOs was undertaken to inform the study.
·The lack of available data to assist in determining the effectiveness and efficiency of the legislative framework (particularly in quantitative terms).
·The inability or unwillingness of companies to provide certain data creating difficulties in quantifying the impacts of the CLP Regulation and other legislation.
·The lack of up-to-date information regarding the effect of the CLP Regulation on consumer behaviour.
4.2.2The Second Fitness Check Study (‘FC+ Study’)
The key limitations of the FC+ Study can be described as follows:
·Stakeholders were identified based on their active engagement with specific pieces of legislation. However, involvement in the study was on a voluntary basis. Therefore, those who felt strongly about particular processes or pieces of legislation were more likely to take part. To offset this possible limitation stakeholders included regulators, industry and NGOs, as well as officers of the European Commission and EU agencies responsible for chemicals legislation.
·In a limited number of cases particular stakeholder groups (e.g. industry, regulators, NGOs) dominated the responses for certain aspects of legislation. The study report states where this is the case.
·The stakeholders engaged, while broadly diverse, could still be argued to be a relatively small sub-set compared to the size and scale of the EU chemicals industry. To offset this limitation the work completed under the FC+ Study included a review of the findings of the 1st FC Study to enable a more complete analysis, and evidence was sought wherever possible to back up opinions. Findings from the 1st FC Study (including its public consultation and SME panel) were used to help corroborate findings in the FC+ Study where appropriate.
·The available economic data on costs and efficiency reported in a quantitative fashion was very limited. Literature data, and two stakeholder engagements were used to gather quantitative and qualitative information on the functioning and efficiency aspects of the risk assessment and risk management processes used under the EU legislation. However, it was not possible to provide extensive costed examples related to efficiency.
·The available information on specific pieces of legislation varied, with some legislation and risk assessment processes well covered by multiple different stakeholder groups and literature/data sources. Other pieces of legislation were not as well covered and the analysis relied more on policy guidance documents and review of the legislation to ascertain how the processes function and what potential issues may exist (see Annex 3 Table 6 for a summary of data availability per piece of legislation).
·The FC+ Study also undertook a semi-quantitative assessment of the key cost drivers for six pieces of legislation.
4.2.3The Study on the Cumualative Health and Environmental Benefits of Chemicals Legislation (‘CuBA Study’)
With respect to the CuBA Study, key limitations can be described as follows:
·The study focused on “cumulative” health and environmental benefits delivered through the cumulative effect (accumulation) of various different pieces of legislation, each addressing a risk or group of risks. It did not, however, seek to attribute specific impacts to every individual piece of legislation. The study presents a combination of qualitative, quantitative and monetary estimates of these benefits. Neither the socio-economic benefits of chemicals legislation (in terms of accelerated innovation) nor of chemicals themselves (facilitating efficiencies or technologies for example) were part of the study scope.
·It is important to note that this is the first time a study on this scale and scope has been attempted. The work is based on drawing together existing information, though a number of calculations/interpretations were done to derive some of the quantitative figures in the report. In some cases the estimates provided are associated with significant uncertainties. These are discussed at length, but are provided as a starting point for additional research and discussion. Where benefits relate to productivity and/or healthcare treatment (“direct financial”) costs, these are compared to GDP in national accounts to provide context on their significance; others reflect “personal valuation” (willingness to pay to avoid certain medical ailments or for ecosystem services, for example). These costs are no less real than those that are linked to GDP: society places a high value on having a long, healthy and fulfilled life. Where appropriate, they are expressed in monetary terms.
4.2.4The Cumulative Costs Assessment Study (‘CCA1 Study’)
The cost estimates provided by the CCA1 Study have to be treated with caution due to differences in scope and in the methodology applied. Firstly, the period covered by the CCA1 Study i.e. 2004-2014, only partially corresponds to the one covered by this Fitness Check. Secondly, the estimated costs relate only to certain subsectors of the EU chemicals industry and not to all the downstream sectors that are also considered by this Fitness Check. Furthermore, regulatory costs were estimated and included in the overall CCA1 estimates for several pieces of legislation that are not in the scope of this Fitness Check while, at the same time, several other pieces of legislation although within the scope of this Fitness Check, were not covered. Finally, the sample size and coverage did not allow for statistically accurate analysis and conclusions. Therefore, additional cost elements were gathered where possible and qualitative assessment is presented instead.
ANSWERS TO THE EVALUATION QUESTIONS
The following sections answer the evaluation questions concerning the five central evaluation criteria of effectiveness, efficiency, relevance, coherence and EU added value. A more detailed analysis of effectiveness, efficiency and coherence related issues can be found in annex (Annex 5, Annex 6 and Annex 7 respectively) as well as in the underlying Fitness Check studies.
Many of the factors that affect the effectiveness of EU chemicals legislation are also closely linked to its efficiency, coherence, relevance and implementation. Issues identified in the effectiveness section are, therefore, sometimes referred to in other sections where they are evaluated in more detail.
5EFFECTIVENESS
5.1Evaluation question: to what extent does the EU legislative framework for the risk management of chemicals meet its objectives?
This section analyses the progress made towards achieving the three core objectives that are shared by nearly all pieces of EU chemicals legislation:
1.Ensuring a high level of protection of human health from the adverse effects of hazardous chemicals.
2.Ensuring a high level of protection of the environment from the adverse effects of hazardous chemicals.
3.Supporting the efficient functioning of the internal market for chemicals and enhancing the competitiveness and innovation of EU industry and business.
As the first two objectives are rather different in their nature from the third objective and, therefore, have a different set of performance indicators, they are assessed separately.
5.1.1The objectives of high level of protection of human health and environment
A.What's the issue?
EU chemicals legislation aims to achieve a high level of protection of human health and the environment by minimising exposures to hazardous chemicals and by stimulating substitution of hazardous substances by less hazardous chemicals (or alternative non-chemical solutions). The effectiveness of the EU chemicals acquis in achieving these objectives can be assessed by analysing the trends in:
·the production and use of hazardous substances;
·the human and environmental exposures to hazardous chemicals; and, ultimately
·the impacts in the form of the main health and environmental impact parameters associated with exposures to hazardous chemicals, such as trends in the EU incidence rates of certain human diseases, trends in animal population levels, trends in eco-system health/resilience.
B.What are the findings?
Conclusions
For the specific hazardous substances that have been targeted over the last 3-4 decades, the EU chemicals acquis has been quite effective in reducing and minimising human and environmental exposures. This includes some notable reductions in exposures to problematic substances such as lead, mercury, benzene, asbestos, polychlorinated biphenyls (PCBs), and a range of other chemicals with carcinogenic, mutagenic or toxic for reproduction (CMR) and persistent, bioaccumulative and toxic (PBT) / very persistent and very bioaccumulative (vPvB) hazard characteristics. However, a range of on-going and emerging health and environmental concerns related to the exposure to hazardous chemicals remain and require further attention.
The analysis finds little evidence of a general shift towards production and/or consumption of less hazardous substances although there are some preliminary positive indications of substitution with respect to substances hazardous to the environment. This may, in part, reflect the effectiveness of risk management measures in reducing exposures and risks, therefore reducing the incentive to substitute to less hazardous substances. Essentially, the share of industrial chemicals hazardous to health and the environment in the total chemicals production has remained relatively unchanged over the last decade.
Trends in endpoint human health and environmental impacts (cancers, reproductive diseases, respiratory sensitization, insect and bird populations, etc.) point to a mixed picture but are difficult to use as direct indicators of chemicals policy performance because of the attribution challenge. Most of these trends are linked to multiple causal factors of which exposure to hazardous chemicals might be just one. Moreover, data is generated, including through the regulatory framework, based on substance-by-substance approach. It is therefore difficult to use it to give a picture of the overall level of protection of human health and the environment. The current approach and indicators used in monitoring and assessing human health and environmental impacts could benefit from being more holistic. On a positive note, the reduction in the incidence rates of workplace-related cancers and in lead-related health impacts are good examples of improvements that can be linked to the EU interventions. There are, however, a few trends such as breast cancer, certain reproductive diseases, and decline of insect and bird populations that are a cause for concern. Further research and a strengthened science-policy interface are needed.
1)Production and consumption of hazardous substances
Trends in the production and consumption of hazardous substances, either expressed in absolute terms or relative to overall chemicals production and consumption, are one potential indicator of the substitution of hazardous substances by less hazardous substances. While not shared by all the pieces of legislation within the scope, it remains one of the goals of some of them e.g. the Plant Protection Products Regulation and the Biocidal Products Regulation. Eurostat has been producing since 2014 relevant data sets regarding substitution trends for industrial chemicals (please see also Annex 5 Section 5.1.1 A)).
The findings of the latest analysis for EU-28 published in December 2017 are:
·The trend in the production of chemicals hazardous to health and the environment followed the trend for the overall chemicals production (
Figure 6
), reaching a peak in 2007, after which there was a significant decline in production during the financial and economic crisis in 2008, followed by a strong rebound between 2009 and 2010 and a subsequent more stable phase.
Figure 6 Production and consumption of chemicals, EU-28, 2004-2016. Source: Eurostat (online data codes: env_chmhaz) Note: some chemicals are hazardous to both the environment and human health therefore adding these total together and subtracting the result from the total production or consumption volume to determine the volume of non-hazardous chemicals cannot be done.
·The share of chemicals hazardous to health and the environment was relatively unchanged over the period 2004–2016. The share of chemicals hazardous to the environment fluctuated between 37% and 39%, while the share of chemicals hazardous to health fell from about 66% in 2004 to 62% in 2016.
The analysis shows substitution of hazardous substances by less hazardous substances has not yet occurred to any notable extent. Essentially, the share of industrial chemicals hazardous to health and the environment in the total chemicals production has remained relatively unchanged over last decade. This may, in part, reflect the effectiveness of risk management measures in reducing exposures and risks, therefore reducing the incentive to substitute to less hazardous substances. The analysis also shows what might be the beginning of a positive substitution trend. The largest overall decrease in EU-28 production between 2004 and 2016 was recorded for chemicals with severe chronic environmental hazard and for chemicals with significant acute environmental hazard (as the production volume was reduced by about 18 % for both classes over the period under consideration). This may indicate that the substitution for these groups to less hazardous chemicals has started to happen (while it does not seem to be the case yet for chemicals hazardous to health). One could also note that no legislation-specific information is available which could allow the assessment of the pace of substitution once such a need is identified and eventually compare across the legislation. These statistics do not allow to link changes in the share of chemicals hazardous to health and the environment to the EU intervention. In order to do so, more in-depth analysis would be required.
Respondents to the public consultation were asked to assign a score of between 1 (no contribution) to 5 (large contribution) to the role of the EU legislative framework in reducing the use of hazardous chemicals and/or substitution with safer alternatives. Scores assigned showed considerable variation among the four groups of respondents. Industry and public authority groups considered the EU chemicals framework to have made the largest contribution to a reduction in number or use of hazardous chemicals and/or an increase in substitution to safer alternatives. In contrast, NGOs and other civil society organisations were considerably less positive.
2)Human and environmental exposures to hazardous chemicals
There is clear evidence that, where targeted EU policy and regulatory action has been taken, human and environmental exposures to a number of well-known individual hazardous chemicals have been successfully reduced or in many cases, minimised. As one example, consumer exposure to lead e.g. in petrol, paints, toys, drinking water, etc., has been reduced by an estimated 89% in the EU between 1990 and 2011, following a variety of risk management measures implemented by Member States, at least in part due to EU legislation. This has resulted in a sustained and significant reduction, on average, in measured levels of lead in blood (see
Figure 7
).
Figure 7 Medians (green dots) and 5th to 95th interval of the distribution of lead levels in the blood of German students from 1981 to 2015, along with levels of lead in blood of children from various European cohorts included in the WHO ENHIS database in grey (no known large lead pollution sources) and red (in the vicinity of known lead pollution sources). Dotted line represents the threshold implied by the WHO IQ loss model.
From the environmental perspective, similar outcomes have been achieved in the EU between 1990 and 2011 for a number of heavy metals such as mercury (66% emissions reduction), cadmium (64% emission reduction) and arsenic (78% emissions reduction) (see
Figure 8
). Reductions in the concentration of a number of other hazardous chemicals in the environment such as tributyltin, PCBs, dioxins, dichlorodiphenyltrichloroethane (DDT), have also been achieved following EU policy.
Figure 8 Mercury, Cadmium and Lead emissions (indexed, 1990-2011) alongside selected regulatory action
There are, however, a number of on-going exposure situations that give cause for concern and which point to some shortcomings in meeting the objectives of protecting human health. These reflect both new, emerging issues, as well as existing ones that require further attention in terms of exposure reduction and control. Based on the current evidence, some of the more notable on-going exposure issues in the EU are exposures (see also Annex 5 Section 5.1.1. B)) to:
·carcinogenic substances at the workplace for which occupational exposure limits (OELs) have not yet been set;
·neurotoxic substances;
·chemicals linked to cardiovascular and respiratory (CVR) disease; and
·endocrine disrupting chemicals.
On-going environmental exposure situations also give cause for concern, the most notable being:
·Hazardous chemical exposures affecting the quality of surface and ground waters, including marine waters, with implications for their ecosystems (and indirectly for human health via seafood and drinking water consumption), despite considerable progress made in reducing the discharge of pollutants such as nutrients, pesticides, industrial chemicals, and household chemicals into Europe's waters over recent decades. Concern has grown regarding, for example, the widespread occurrence of persistent harmful substances such as polybrominated diphenyl ethers, which pose a risk even at very low concentrations. New (stricter) environmental quality standards have been set for these substances and for some others such as fluoranthene, and these are due to be met by 2021. The results from some Member States, e.g. Sweden, Luxembourg and the Netherlands, indicates the new standard will be difficult to achieve. Concern is also growing that the toxicity of mixtures of chemicals is not sufficiently addressed by the legislation, which focuses largely on individual substances (or small groups).
·Hazardous chemical exposures affecting terrestrial eco-system health/resilience such as neonicotinoid pesticides representing a risk to wild bees and honeybees.
Respondents to the public consultation from industry and companies as well as those representing public authorities were overall the most positive about the extent to which the EU legislative framework sufficiently addresses emerging areas of concern while civil society representatives and citizens assigned the lowest scores.
3)Human health and environmental impact evidence and indicators
The trends in the main health and environmental impact parameters that are known, or strongly suspected, to be associated with exposures to hazardous chemicals (e.g. trends in the incidence rates of certain cancers, reproductive diseases, sperm count and quality and trends in animal populations and eco-system health/resilience) are important to consider when examining the effectiveness of EU chemicals policy. However, using human health and environmental adverse effects as direct and reliable indicators of chemicals policy performance needs to be treated with caution because of the attribution challenge: many of the observed health and environmental adverse effects may derive from multiple causes (life-style, genetics, habitat destruction/degradation, etc.) and it is difficult to determine to what extent exposure to hazardous chemicals contributes to the observed adverse effects. Complicating things further is the fact that observable adverse effects in human health and the environment often do not materialise immediately after exposure. For example, the latency between exposure to carcinogens and the development of cancer can often be as much as 20 years or more.
The available evidence regarding the trends in the main health and environmental impact parameters points to a mixed picture. Some clear improvements have been achieved, for example, in the reduction of cancers related to workplace exposure to a number of targeted carcinogens which has resulted in the estimated prevention of 1 million new cancer cases in the EU over the last 20 years partly through the implementation of the occupational safety and health (OSH) legislation. However, a number of other trends suggest there is still cause for concern, for example:
·The health burdens resulting from most cancers continue to rise in the EU (except for lung cancer) (see
Figure 9
for trends for breast cancer). For many cancers, the contributing role of chemical exposures is not yet well understood and defined while at the same time suspected to play a role. As a result, it is often unclear which specific chemical exposures should be targeted by legislation, in an attempt to eliminate preventable disease causes.
Figure 9 Age-standardised incidence rate trends for breast cancer in several European countries
·The same is true for neurodevelopment and reproductive health. While both male and female fertility rates are decreasing in Europe and while some neurodevelopmental disorders (e.g. autism) increase, there is no data on how many of these cases are attributable to exposure to hazardous chemicals. However, it is likely that hazardous chemicals play a role in these adverse health outcomes. Substance categories of concern include certain phthalates, dioxins, perfluorinated chemicals, analgesics, etc. These issues are more generally linked to the need to obtain better information about the spectrum of chemicals with relevance to human exposures and diseases. Achieving this include improvments regarding data requirements, toxicological testing and screening methods human biomonitoring as well as better predictive and prioritisation approaches.
In the area of the environment, the observed trends also point to a mixed picture:
·Improvements in water quality in some areas may have contributed to some recovery of aquatic ecosystems and the restriction on the use of tributyltin (TBT) as an antifoulant in marine paints has resulted in the recovery of mollusc populations in many ports and coastal areas in Europe.
·Major declines (as high as 50-75%) in the populations of a number of animal species in the EU have been observed over the past 3-4 decades including pollinators, other flying insects (see
Figure 10
), amphibians, and birds. Europe’s wild bee population is in decline with nearly one in ten species facing the threat of extinction and more than a quarter of bumblebee species being currently at risk of dying out. The populations of over 20% of bird species in the EU are in significant decline , with the largest declines (46% between 1990 and 2014) for common farmland birds. The causes of these declines requires further research but are likely to be multiple including exposure to hazardous chemicals, changes in agricultural practices, habitat degradation, climate change, etc.
Figure 10 Temporal distribution of insect biomass at selected locations in Germany. Daily biomass across 26 locations in multiple years
The current approach and indicators used in monitoring and assessing human health and environmental impacts could benefit from being more holistic. For instance, such more holistic impact assessments could feed into exposure indicators (e.g. passive sampling, representative mixtures, human biomonitoring) as well as impact indicators (e.g. (eco)epidemiology, effect based methods as proposed in the Water Framework Directive).
5.1.2The objective of ensuring the efficient functioning of the internal market and of enhancing competitiveness and innovation
A.What's the issue?
The EU chemicals legislation aims to ensure the efficient functioning of the internal market and to enhance competitiveness and innovation. The effectiveness of the EU chemicals legislation in achieving these objectives can therefore be measured by analysing:
·trends in the development of intra-EU sales of chemicals compared to domestic sales;
·trends in the EU export of chemicals and global market share;
·the role that the legislation plays in boosting the competitiveness of the EU chemicals industry and innovation.
B.What are the findings?
Conclusions
EU chemicals legislation has been instrumental in ensuring the free circulation of chemicals and products within the internal market through the harmonisation of requirements, standards, risk management measures, labelling, and mutual recognition approach that reduce barriers for intra-EU trade. To a large degree, there is a level playing field in Europe, and chemicals legislation has strengthened the internal market and enhanced the competitiveness of EU industry, this being reflected in the growth in intra EU trade. The EU remains the largest chemicals exporting region in the world and, despite the decline of the share (although absolute sales levels have increased) in the global market the EU chemicals industry remains internationally competitive. However, some interpretation, implementation and enforcement issues at Member States level leave room for further improvements.
While the EU chemicals industry is often seen as a frontrunner in terms of innovation, there is no evidence that the EU chemicals legislation, as such, is either a major trigger of, or barrier to, innovation for companies in general.
EU chemicals legislation has been instrumental in ensuring free circulation of substances, mixtures and articles within the internal market through harmonisation of standards and requirements that reduced barriers for intra-EU trade. There has been a continuous increase of the share of the intra-EU trade of chemicals i.e. EU companies selling in the EU single market rather than in their home country market, in the total sold production of chemicals (from 43% in 2006 to 55% in 2016). For example, the CLP Regulation provides the basis for consistently identifying properties of concern, with this information then used in hazard communication to workers, downstream users and consumers of chemicals. Similar trends have been observed in the fields of cosmetics, detergents, fertilisers, etc., where EU product specific legislation has been adopted. The fact that many Directives have become Regulations contributed to harmonisation across the EU and therefore a better functioning internal market. Nevertheless, there are still areas where divergences in interpretation, implementation and enforcement continue to persist potentially leading to fragmentation of the European market and creating burden and barriers for businesses (see Annex 5 Section 5.1.2).
In terms of international competitiveness, the EU chemical industry in 2016 represented 15.1% of the global market, behind China (39.6%) but ahead of the United States (14.2%). Although the European share of global sales has decreased (32.5% in 1996) the EU chemicals industry remains internationally competitive as evidenced by the trade surplus of EUR 47.2 billion (exports EUR 146.2 billion, imports EUR 99 billion). The decrease in the share of global sales is mainly due to relative growth in other parts of the world, such as China and India, served by their own domestic production. Other potential reasons given for this are high energy prices, currency appreciation, high labour costs, regulatory and tax burdens. Yet the EU remains the largest chemicals exporting region in the world. The main competitive advantage of the EU chemical industry is the high level of technological development, skilled workforce and strong research base.
As an input provider for other industries, the chemicals industry is also considered to be at the forefront of innovation and a solution provider for many societal and environmental challenges, with chemical technological breakthroughs spilling over into downstream sectors. As mentioned above, the beginnings of a possible positive trend can be observed concerning substitution to less hazardous or non-hazardous substances for substances hazardous to the environment. In many cases, hazard classification under the CLP alone for example is an incentive for substitution as it triggers a number of legal obligations, including labelling and communication to downstream users as well as consumers. Indeed, increasing consumer awareness of the health risks associated with certain hazard classifications (most notably carcinogens) is a powerful trigger for substitution in the supply chain. In other cases, risk management measures (such as bans and restrictions) triggered by a certain hazard classification provide such incentives. Innovation and substitution are encouraged by many pieces of legislation acting in concert and supported by drivers, such as consumer demands, market circumstances and initiatives such as e.g. the Substitution Support Portal (SUBSPORT) under the European Union’s Life programme. Overall impacts of chemicals legislation on innovation are, however, more complex, as described in the REACH Evaluation. As no specific indicators exist for assessing these and also given that many other factors play a role e.g. intention to develop new applications in order to conquer new markets, it is currently not possible to know whether the EU chemicals legislation has been a major trigger of innovation.
The EU chemicals legislation was considered by citizens, industry and companies and public authorities as mostly effective in ensuring a well-functioning internal market while civil society considered it to be moderately effective. Regarding this particular aspect, SME Panel results showed that the EU chemicals legislation is considered to be sufficiently harmonised across Member States for the proper functioning of the European single market. While citizens, industry and companies and civil society considered the legislation moderately effective in stimulating competitiveness and innovation, public authorities were of an opinion that it is mostly effective in reaching this objective.
5.2Evaluation question: what factors affect (either positively or negatively) the correct functioning of the EU legislative framework for the hazard identification and risk management of chemicals? What are the consequences or effects that were not originally planned for?
An effective framework of chemicals legislation ensures the timely and sound identification of chemical hazards and risks, the appropriate control of human and environmental exposures to hazardous chemicals and, for hazardous chemicals where the exposures cannot be reliably controlled, a progressive shift towards the use of less hazardous chemicals (substitution) including non-chemical solutions.
The basic steps of the risk management procedures and processes applied to chemicals within the EU framework of chemicals legislation (see Section 2.1 and Annex 8 for further detail) are:
·hazard identification (based on toxicity tests and other relevant information);
·dose (concentration) – response (effect) assessment;
·exposure assessment – exposure scenarios for relevant uses of the chemical (based on models and measurements of the occurrence of the chemical);
·risk characterisation; and
·risk estimation.
The correct functioning of each of these risk management steps can be affected by one or more key performance factors, including:
·Whether the necessary scientific knowledge (including recognised and accepted test methodologies for hazard identification) and data/information (e.g. on chemical uses and exposure scenarios) are available, are used appropriately and can be shared between different risk assessment regimes to ensure the coherence of findings and to avoid duplication of effort.
·Whether and how the hazard identification and risk assessment process is triggered.
·Whether the overall 'speed' of the hazard identification and classification and risk assessment processes can handle the quantity of existing and newly designed hazardous chemicals placed on the market. This is not simply a question of efficiency but, fundamentally, of effectiveness. If the framework fails to identify and address the hazards and risks of chemicals in a timely manner, its effectiveness is reduced. This also requires further discussion on how to better prioritise and in which areas and/or for which substances such prioritisation would be necessary.
·Whether the necessary competences and resources are available at EU and Member State level to ensure robust and timely hazard identification/assessment/classification, risk assessment and risk management decision-making.
·Whether the use of generic risk considerations (GRC) and specific risk assessment (SRA) based approaches is appropriate and balanced.
·Whether the desired transition to non-animal test methods is happening and is effective.
These different factors can affect the performance of one or more of the risk management steps outlined above. For example, poor quality or missing data affects the ability to correctly identify and classify hazards, to determine reliable exposure scenarios, and, therefore, to arrive at a robust risk assessment. The assessment of the effectiveness of the framework of EU chemicals legislation has, therefore, been structured and presented according to these factors.
5.2.1Data, knowledge and information
A.What's the issue?
Scientific understanding and the availability of good-quality, reliable data underpins the effective functioning of EU chemicals legislation. It includes, among other things, knowledge and information on chemical properties, data on eco-toxicity of chemicals and on chemical uses and exposures to chemicals (including occurrence in, and release from, articles (consumer products)).
Please refer to Annex 5 Section 5.2.1 for a more detailed description of data, knowledge and information related aspects.
B.What are the findings?
Conclusions
Enormous efforts have been made at the EU and Member State level to ensure that the necessary data to take effective chemical risk management decisions is available, comparable and of good quality. Likewise, the scientific understanding of how hazardous chemicals impact human health and the environment has improved significantly over the last two decades. Much of this effort has been resourced and underpinned by industry assuming the responsibility of ensuring the safe use of chemicals placed on the market. This has been helped by significant investment in EU-level capacity for supporting the risk assessment processes under the various chemicals legislation regimes (ECHA, EFSA, and a number of scientific committees).
While the existing test guidelines cover the majority of known adverse effects on human health and the environment, they can be further improved. Standardised and internationally recognized test guidelines still need to be developed and/or validated. This is the case for certain environmental adverse effects such as the terrestrial compartments and some specific terrestrial species. This is also the case for neurotoxicity, immunotoxicity and some endocrine disruptors related aspects.
The EU has put considerable efforts and resources in promoting the avoidance or reduced use of animal testing. However, there are still barriers to the use and acceptance of alternative (non-animal) test methods for regulatory purposes, partially linked to lack of test guidelines for certain effects or to gaps in the current knowledge.
The current state of knowledge regarding exposure scenarios i.e. knowledge of which chemicals and their combinations, and at what concentrations, humans and the environment are being exposed to, needs further attention.
The scientific understanding of mechanisms and pathways of how hazardous chemicals interact with organisms has improved considerably over the last two decades with, for example, the support of the Commission's research framework programmes.
Much has also been done to improve the quality, reliability and reproducibility of hazard and risk assessment studies and data. Quality standards are prescribed for how hazard and risk analysis is to be conducted, including the testing methodologies. Toxicity studies submitted by chemicals producers or importers need to be performed according to validated test methods and guidelines. In addition, the laboratories that perform chemical hazard and risk assessment studies must comply with the GLP requirements. During the workshops, there was general agreement amongst participants that the GLP requirements help to ensure that the rigorous documentation about how a study was conducted is made available. This is, good to have as it allows comparability and reproducibility but they are not sufficient to ensure high scientific quality.
Validated test methods and guidelines help to ensure comparability and reproducibility of data produced and thus increase the reliability and quality of data. International agreement on test guidelines (under OECD) ensures the mutual acceptance of the data among countries and regions, which lowers the technical barriers to trade and reduces also the number of animals used for testing. The existing test guidelines cover the majority of known adverse effects on human health and the environment. However, standardised test methods and guidelines are lacking for certain environmental adverse effects (soil biota, reptiles, and other terrestrial animal species). There is also a need to further improve the existing test methods and guidelines regarding neurotoxicity, immunotoxicity, epigenetics, endocrine disruption as well as how to capture peculiarities of nanomaterials.
The policy on the protection of animals used for scientific purposes has streamlined resources and efforts towards the development of alternative methods to replace, reduce, and refine animal testing. To date, this focus has been successful for five human health endpoints, for which tests have been validated and recognised internationally. However, there are still barriers to the use and acceptance of alternative test methods, and no methods are available yet to fully evaluate complex systemic endpoints. The identified gaps in existing test guidelines for endpoints relevant for human health and the environment call for development of new test methods and adequate funding is required for both approaches, non-animal and animal. In addition, there is a need to accelerate the regulatory acceptance of alternative test methods (see also Annex 5 Section 5.2.2).
The EU chemicals legislation requires in principle the use of 'all available information'. A number of stakeholders, however, expressed concern that potentially relevant and useful peer-reviewed scientific studies and data were being ignored or overlooked during regulatory hazard and risk assessments because they are not GLP-compliant. This warrants some attention and action because the peer-reviewed studies may use test designs, test species and test endpoints that are more sensitive and relevant than those used in standardised studies and can, therefore, be an important complement to the standardised studies provided that they are reliable and properly documented. Moreover, lack of awareness of authorities regarding the availability of relevant or new information and data for hazard and risk assessment contributes to a situation where it can take several years between the first concerns and evidence being published in the academic journals and the regulatory hazard and risk assessments being triggered. Tools are currently lacking to ensure continuous monitoring of scientific papers and publications and mechanism for the identification of, and reaction to, early warning signals.
As regards exposure data in particular, there continue to be significant gaps in our knowledge of which chemicals and their combinations, and at what concentrations, humans and the environment are being exposed to. These gaps have an impact on determining realistic, acceptable and robust exposure scenarios. The main difficulties can be summarised as follows:
·Exposure assessments typically make use of a combination of models, laboratory data and monitoring to calculate the potential exposure within a given scenario. In order to successfully conduct exposure assessments, the models in use have to be underpinned by data, and likewise real world analysis is needed to validate results. Additional monitoring to validate models is often a step that is overlooked in the EU risk assessment processes and this undermines the quality of the results.
·Exposure scenarios used in setting ‘safe’ exposure limits, are established based on intended, normal, reasonable and/or foreseeable use of a product (e.g. cosmetic, plant protection, biocidal, detergent products) or foreseeable/predictable situation (e.g. occupational or industrial settings). There is evidence that for hazardous chemicals with a broad range of applications in a myriad of different consumer products, industry and public authorities may be unaware of many uses. In addition, there are no requirements on producers of hazardous chemicals for example to make available substance-specific information on actual amounts marketed. As an initial step, the Commission recently began to tackle this issue for veterinary antibiotics where reporting obligations on volumes used have been introduced.
·Yet, even when all uses and amounts are known, determining realistic exposure scenarios can still be problematic where consumer behaviour is difficult to predict. Determining and characterising exposure in an occupational setting by way of comparison is relatively more straightforward, as the exposure scenario is more controlled and predictable.
To address the issue of human health exposure data, the EU Commission has funded the European Human Biomonitoring Initiative (HBM4EU). However, a similar holistic initiative for animals, plants and eco-systems is currently lacking . The screening of 'unknowns' (i.e. sampling and testing designed to detect unsuspected hazardous chemicals) in humans and the environment is also missing.
5.2.2Hazard and risk (re-)assessment
A.What's the issue?
The obligation to perform hazard and risk assessments or re-assessment sits primarily with industry, in line with the principle of reverse burden of proof. Public authorities (national or EU) intervene only in a limited number of cases (in areas of the highest potential risks to human health and/or the environment).
Please refer to Annex 5 Section 5.2.3 for more detailed description of how hazard/risk assessment is triggered.
B.What are the findings?
Conclusions
Where the initiative to trigger the hazard/risk assessment sits with industry and there is a positive incentive to do the assessment (e.g. seeking authorisation to place a product on the market), the quality of the risk assessment dossiers tend to be good. For the pieces of legislation where the underpinning mechanism relies on the presumption of conformity with the existing rules, information is scarce and therefore does not allow to conclude on the quality of conformity assessments carried out. It appears however clearly that the capacity and resources of the EU and/or Member State authorities to check the quality of these self-assessments are paramount but are often constrained.
The obligation to perform risk assessments sits primarily with the industry in line with the principle of reverse burden of proof. Risk assessment can also be initiated by public authorities, both at the EU and MS level e.g. the Commission will trigger risk assessment under the Water Framework Directive and Industrial Emissions Directive.
The effectiveness of the obligation for industry to carry out a risk assessment, i.e. whether risk assessments are done and to what quality, is influenced by the following aspects:
·existence of a commercial interest to gain approval/authorization,
·existence of a prescription for how the risk assessment should be performed and documented, and
·existence of an obligation to communicate the outcome of the assessment to public authorities and/or downstream users.
Systematic checks by EU and/or national public authorities of the risk assessment done by industry are legally required only for certain pieces of legislation where authorisation/approval/permit is needed before the substance/product can be placed on the market (e.g. plant protection products, biocidal products) or activity can be carried out (e.g. industrial activities, including waste management activities). In such cases, the quality of dossiers submitted by industry and the robustness of the overall assessment are generally good.
Under other product related legislation (e.g. the Cosmetic Products Regulation, the RoHS Directive, the Toy Safety Directive, the Detergents Regulation) and the occupational safety and health (OSH) legislation, the underpinning mechanism is based on conformity/safety assessment done by economic operators themselves and the presumption of conformity with the existing rules. In these cases, assessments carried out are not systematically checked by public authorities. Therefore, ensuring that only safe products are placed on the market or that worker safety rules are complied with, relies primarily on economic operators, including importers, who can be held responsible for non compliance. This approach reduces the administrative burden for public authorities. However, ensuring that this obligation is actually complied with still relies on Member States and depends in particular on market surveillance activities and inspections carried out at national level which requires considerable resources. The recent ECHA report has shown that the compliance with the general safety obligation is challenging but more evidence and information, including data from regular market surveillance or other similar or equal mechanisms, need to be gathered to conclude on the level of compliance of self-assessments (and thus on the level of enforcement).
The EU chemicals legislation requires risk assessments to be updated. However, there are some differences in the level of stringency of the legal provisions. In some cases, the legislation will specify the frequency or conditions that will trigger a re-assessment e.g. the Biocidal Products Regulation, the Plant Protection Products Regulation, the Ecolabel Regulation, the Industrial Emissions Directive and the Water Framework Directive. In most cases however re-assessment is required if and when new scientific knowledge and/or evidence emerge. All the factors identified above for the initial assessment are also valid for re-assessments. Re-assessments seem to be more effective when there is an automatic trigger in the legislation such as expiration of the approval of active substances for plant protection products (usually 10-15 years). More evidence needs to be gathered to conclude on the effectiveness of re-assessments in cases where they are to be triggered by new scientific knowledge.
Respondents to the public consultation were asked to indicate their satisfaction with risk assessment and characterisation which received the lowest weighted score from Citizens and NGOs and others but was scored relatively highly by industry associations and public authorities.
5.2.3Hazard classification
A.What's the issue?
The communication of chemical hazard properties to downstream users is an important risk management measure that helps ensure the safe handling of chemicals and mixtures. It needs to be underpinned by reliable, robust hazard classification. Hazard classification is also crucial for other risk management processes within the framework of EU chemicals legislation, such as restrictions or authorisations.
Please refer to Annex 5 Section 5.2.4 for more detailed description of how hazards are classified under the EU chemicals legislation.
B.What are the findings?
Conclusions
As the primary basis for most chemical hazard assessment and classification in the EU, the CLP Regulation is effective and is considered by the majority of stakeholders as an improvement over the earlier Directives that it replaced. Some issues, however, were identified with respect to the pace and focus of harmonised classifications, the classification of mixtures, and inconsistencies in industry self-classifications.
Two processes are available for classification:
·For hazards of highest concern (carcinogenicity, mutagenicity, reproductive toxicity (CMR) and respiratory sensitisers) and for other substances on a case-by-case basis, classification and labelling should be harmonised throughout the EU to ensure an adequate risk management. This is done through harmonised classification and labelling (CLH). Harmonised classifications are listed in Annex VI to the CLP Regulation.
·Under the CLP, a substance must be self-classified by manufacturers, importers or downstream users when it has no harmonised classification in Annex VI to the CLP and it presents hazardous properties. This classification and labelling information for the substances to be placed on the market is then notified by manufacturers and importers to the Classification and Labelling Inventory (CLI) held by ECHA. Mixtures must always be self-classified before being placed on the market, as they are not subject to CLH.
The harmonised classification is an important instrument for achieving the safe use and enhancing the substitution of hazardous chemicals. It is also linked with the approval process for plant protection product and biocidal product active substances.
According to ECHA the number of assessments for harmonised classifications under the CLP Regulation is relatively low compared to the likely number of chemicals which merit a harmonised classification. During the public consultation, citizens and civil society organisation considered the speed of the procedures for CLH slightly satisfactory, industry considered it to be moderately satisfactory and public authorities considered the speed to be mostly satisfactory. The main consequence of this ‘slow’ pace is that not all of the potentially hazardous chemicals which would therefore merit a harmonised classification are dealt with thus potentially prolonging exposure of EU citizens to such hazardous chemicals.
It seems to be due to the following capacity constraints:
·Currently, the main focus is on active substances used in plant protection and biocidal products. This explains the fact that relatively few harmonised classifications are being done for industrial chemicals.
·Much of the current situation is a reflection of the high resource needs (staff/expert capacity) at Member State level for preparing a classification dossier, combined with reductions in resources and budgets allocated for this work in many Member States, in particular following the 2008 financial crisis. There is also considerable variation between Member States in their capacity and willingness to initiate harmonised classification dossiers with just a few Member States carrying the majority of the burden.
·The current speed also reflects the need to ensure that all the relevant opinions, including stakeholder views are taken into account.
·In many cases, the process is slowed down and there is some reticence because of the consequences that the harmonised classification may trigger in downstream legislation e.g. ban of CMRs under the Cosmetic Products Regulation or cut-off criteria under the Plant Protection Products Regulation. In this regard, it should be noted that efforts have already been made in order to speed up the CLH process. A fast track procedure was introduced by ECHA for discussing non‐controversial endpoints. ECHA indicated that in the RAC meeting where this was introduced, 65% of classification proposals for such endpoints went through without discussion.
·Currently, there is no quantified objective or a point of reference to compare with to evaluate the speed of the classification and to know how many substances and by when these need to have a harmonised classification. In addition, the Commission lacks the legal basis for initiating the harmonised classification process or to ask ECHA to develop dossiers while industry can initiate and submit harmonised classification dossiers only for a limited number of substances. Regarding classification of active substances for plant protection and biocidal products and revision of the existing entries, only Member State Competent Authorities can submit proposals, but, according to industry, they are difficult to approach or not always cooperative.
As regards self-classifications by industry under the CLP Regulation, there are often multiple classifications for the same substance submitted to be registered in the CLI because different notifiers fail to arrive at an agreed entry despite the legal obligation to make every effort to do so. Furthermore, there are concerns about the reliability of some of the self-classifications which is exacerbated by the lack of legal basis for ECHA to correct or delete obvious mistakes, to remove entries by companies which have ceased to exist or for substances which are no longer placed on the market (especially below 1t/y) and to get in direct contact with notifiers/registrants, in order to initiate a correction or obligation for manufacturer/importer to check the quality of the information being notified. This affects the value of the CLI as a hazard communication tool. The Commission and ECHA are actively looking into a number of ways to improve the situation.
The lowering of generic concentration limits for some hazard classifications under the CLP compared to the levels prescribed under the previous regime (i.e. the Directive which the CLP Regulation replaced), in particular for skin and eye irritation or corrosion, has resulted in more stringent classifications when classifying mixtures using the ‘calculation method’. Stakeholders representing the detergent sector stated that it leads to over-classifications. Similarly, because SMEs are more likely to depend on the calculation methods to classify mixtures (due to cost considerations), they are also more likely to place more conservative hazard classifications on their products than companies that can do the necessary testing. In principle, the bridging principle classification method could address this issue. Bridging principles are basic principles used to classify un-tested mixtures under the CLP Regulation and the UN Global Harmonised System (GHS). However, the lack of clarity with respect to how to apply these principles hampers the effectiveness of this method. It also leads to discrepancies in interpretation and acceptance of classification by Member States. The Commission is now taking steps to address this issue, including guidance on the harmonised application of the legal requirements.
Issues with mixture classification have also been raised by metal industry stakeholders in relation to metals and metal alloys e.g. the alloy used in Euro coins and the stainless steel-nickel-cobalt alloys used as medical implants. While the metal alloys are to be classified following the CLP chemical mixtures classification rules, this stakeholder group believes that it leads to metallic alloys receiving classifications that do not match their real hazard properties. They also believe that this situation could have negative consequences on metals recycling and thus on the realisation of circular economy with some unintended consequences in downstream legislation (e.g. the Toy Safety Directive, the Transport of Dangerous Goods Directive, the Industrial Emissions Directive). It should be noted that the Commission has already been made aware of these concerns and has started to address them, in particular through the bio-elution project (involving industry stakeholders) which is reviewing possible test methods for assessing the bioavailability/exposure to metals in alloys. Whether, and how, several other hazard classes such as persistent, bioaccumulative and toxic/very persistent and very bioaccumlative substances (PBTs/vPvBs), endocrine disruptors (EDs), and neurotoxicity are addressed by the EU chemicals legislation is further discussed in the Section 7 Coherence (for more details on specific substances, i.e. CMRs, PBTs/vPvBs, EDs please refer to Annex 7).
5.2.4Communication of hazards and risks to consumers and professional users and public authorities
A.What's the issue?
Communication of hazard, risk and safety information about chemical substances and mixtures to users, consumers, workers and public authorities is a key measure to promote the safe use of chemicals, to mitigate risks and to help users make informed product/substance related choices. Various communication measures exist across the legislative framework. Their effectiveness has a direct impact on the correct functioning of the EU chemicals framework and on achieving its objectives.
Please refer to Annex 5 Section 5.2.5 for more detailed description of rules regarding communication of hazards and risks and the related aspects.
B.What are the findings?
Conclusions
The requirements to communicate chemical hazards and risks to consumers, workers, and professional users via hazard pictograms, labels and safety data sheets is considered by most stakeholders to be generally effective and important. Some concerns have been raised by industry stakeholders that labels are becoming overloaded with information making it difficult for consumers to focus on the essential hazard information. A recent Eurobarometer survey suggests that one or two of the hazard pictograms are not well recognised or understood by a majority of consumers.
Within the framework of EU chemicals legislation, one of the primary mechanisms of hazard and risk communication is via pictograms and product labels for hazardous chemicals and mixtures, as prescribed by the CLP Regulation (and in line with the UN Global Harmonised System (GHS)). This means that that any changes agreed to at the GHS level (e.g. refinements to the wording of the hazard statements required on labels) are transposed into EU law via the CLP Regulation. There are also a small number of additional sector-specific labelling requirements (e.g. for cosmetics, toys, and detergents). In addition, the EU Ecolabel Regulation sets out rules for a voluntary labelling scheme.
A recent Eurobarometer survey indicated that 70% of EU citizens find information on the hazards of chemicals on the label useful. It also showed that there are varying levels of awareness and comprehension of the four (out of a total of nine) chemical hazard pictograms that were examined by the survey. While 'flammability' is well recognised and understood (92% of respondents have seen it before and 96% could correctly state its meaning), it is less the case for the 'environmental' hazard pictograms (47% of respondents have seen it before and 83% could correctly state its meaning), 'serious health hazard' pictograms (20% of respondents have seen it before and 69% could correctly state its meaning), and 'exclamation mark' pictograms (63% of respondents have seen it before and 17% could correctly state its meaning). Nevertheless, when they see one of the chemical hazard pictogram on an unfamiliar product, most respondents (76%) read the safety instructions (57% read the safety instructions on the product label, while 19% say they go further by reading the safety instructions on the product label and then trying to find further information from other sources). The Eurobarometer Survey also found that even in Member States where understanding of the issues surrounding chemical products is high, the comprehension of some of the hazard pictograms is relatively low.
At a more general level, another recent Eurobarometer survey found that less than half of the respondents (45%) feel well informed about the potential dangers of the chemicals contained in consumer products. However, again, this proportion varies considerably between Member States.
Respondents to SME Panel consultation expressed the following views:
·76% of respondents agreed or strongly agreed that the information currently required to be included on labels is necessary and appropriate.
·78% of respondents agreed or strongly agreed that the CLP hazard pictograms are generally representative of the actual hazard.
·63% of respondents agreed that consumers generally do not look beyond the label for hazard information and information on safe use.
·29% of respondents agreed or strongly agreed that consumers understand the CLP pictograms and information provided on labels regarding the safe use of chemicals (against 41% disagreeing or strongly disagreeing and 31% neither agreeing nor disagreeing).
·65% of respondents agreed or strongly agreed that employers and workers understand the CLP pictograms and information provided on labels regarding the safe use of chemicals.
In part, this is an issue of citizen education and awareness raising by Member States. Hazard communication to workers and professional users is considered to be more effective with a higher level of awareness, recognition and understanding of the pictograms than consumers; in part due to employee training.
Evidence also indicates that labels can become overloaded with information e.g. too much text, too long and not meaningful chemical names to non-professional users making it difficult for downstream users and consumers to focus on the essential hazard information, thus reducing the effectiveness of hazard communication. Too much text included on labels, especially when this is required to appear in multiple languages, thus restricting the understandability of the information. This could be overcome by increasing the use of digital tools to communication hazard information. 61% of respondents to SME panel consultation agreed or strongly agreed that providing information on chemical hazards to consumers should rely more on novel tools, such as QR-codes, apps and websites. Currently, however the legal (mandatory) requirements do not incentivise the use of more innovative techniques and digital tools and when it happens, industry is using digital tools on voluntary basis. While this may improve the understanding and management of hazards and risks, it can also lead to confusion between the CLP-required and the sector-initiated pictograms and labels.
Complementing product labelling, Safety Data Sheets (SDS) are a key communication tool for downstream industry users of hazardous substances and mixtures towards workers. Even though the CLP criteria are used to trigger the obligation to develop a SDS, provisions are in REACH. A SDS must provide information on all hazards covered by the CLP Regulation, as well as on whether a substance or mixture meets the criteria of persistent, bioaccumulative, toxic or very persistent and very bioaccumulative (PBT/vPvB) substances or on substances included in the Candidate List of substances of very high concern (SVHCs). These provisions were evaluated as part of the REACH evaluation which showed that there has been a continued increase in the information passed through the supply chain. However, the evaluation also pointed out a relatively high level of non-compliance and highlighted the potential for clarification and simplification especially for SMEs. Another factor to consider is the capacity of SMEs to perform the risk assessment at the workplace based on the exposure scenarios provided in the safety data sheets (SDS) due to the limited resources and expertise. ECHA together with industry organisations developed a set of tools to simplify and harmonise the elaboration of exposure scenarios for the chemical safety report and their incorporation in the SDSs.
The EU has established two alert systems to enable rapid exchange of information between Member States and the EU authorities in emergency situations when products, food or feed pose an immediate risk to health and safety of consumers. The Rapid Alert System for non-food dangerous products (RAPEX) is an effective tool for allowing public authorities to rapidly take appropriate risk mitigation measures for consumer goods (toys, textiles, cosmetics, etc.). Nevertheless, there is still room for further co-ordination of national market surveillance activities and authorities (i.e. customs), which could benefit from the measures included in the 'Goods Package'. In a similar way, the Rapid Alert System for Food and Feed (RASFF) provides food and feed national control authorities with an effective tool to exchange information about e.g. undesirable chemicals in food causing food poisoning, not labelled allergens, migration of chemicals from the food contact material into food such as formaldehyde, plasticizers, volatile organic compounds etc.
5.2.5Legislative gaps affecting the effectiveness
The evaluation found a number of legislative gaps that affect the effectiveness of the chemical legislation. A more detailed assessment is provided in the relevant sections in the remained of this document as well in the Annexes Section 5.2.6:
·combination effects (Relevance Section 8.1.2 1));
·exposure to substances in articles (Relevance Section 8.1.2 3));
·protection of vulnerable groups (Coherence Section 7.2.B) 2) b));
·endocrine disruptors (Coherence Sections 7.2. B) 1) b) and 7.2. B) 2) a) ii) as well as Annex 7.3).
5.2.6Application of the Precautionary Principle
A.What's the issue?
The precautionary principle is one of the three principles guiding environmental policy under the Treaty (article 191(2) of the TFEU). It allows for taking action when there is still a degree of scientific uncertainty about the risk. Whilst the precautionary principle has not been explicitly defined in EU legislation, the Commission Communication on the precautionary principle sets out steps to be followed in the decision making process. When applied in the chemicals policy area, this mechanism has two steps:
1.A scientific step, where the responsible scientific body (Agency or Committee) assesses if the uncertainties are bigger than those inherent to risk assessment of chemicals and if the consequences of those uncertainties could lead to a significant undesirable impact.
2.A risk management step, where the responsible risk management body (the Commission and the associated committees) decide what action, if any, is required. Options range from taking no action to precautious and/or restrictive (e.g. a ban of further use of a substance) measure, including gathering more data in order to reduce the level of scientific and risk assessment uncertainty.
The precautionary principle enables a rapid response to be given in the face of potential significant impacts to human, animal or plant health, and to the environment. In particular, where scientific data do not permit a complete evaluation of the risk, recourse to this principle may, for example, be used to stop distribution or order withdrawal from the market of products likely to be hazardous.
Whereas both the precautionary and prevention principles can be strictly divided conceptually, it is not always straightforward to separate them as clearly in their application. Some legal instruments based on a general preventive approach nonetheless integrate a precautionary approach for specific substances where risks to health and the environment or the thresholds needed to limit hazards are not identifiable (e.g. the Seveso III Directive aims at prevention, preparedness and response to accidents involving dangerous substances in industry in the EU, the Industrial Emissions Directive takes into account the whole environmental performance of a plant through granting a permit). The precautionary principle should not be confused with the element of caution that scientists apply in their assessment of scientific data e.g. generic risk management approach based measures and application of safety factors are examples of preventative action and not the application of precautionary principle.
Where scientific uncertainty is encountered, the challenge is in finding the correct balance so that the proportionate, non-discriminatory, transparent and coherent actions can be taken. Proportionality also covers examination of the benefits and costs of action/inaction. It is a question of how effectively the EU chemical risk assessment and management processes are working in terms of detecting and acting upon early warnings and avoiding late lessons versus taking over-precautious, unnecessarily restrictive measures and unwarranted recourse to the precautionary principle, as a disguised form of protectionism. Whatever is the measure decided, it remains subject to review, in light of new scientific data, and should allow assigning responsibility for producing the scientific evidence necessary for a more comprehensive risk assessment.
B.What are the findings?
Conclusions
The inherent uncertainty created by the difficulty in determining the exact level to which exposures to hazardous chemicals can be attributed to human health and environmental impacts (when this is just one factor amongst a number of confounding factors (lifestyles, genetic predisposition, habitat degradation, climate change, etc.)) presents particular challenges for the chemical risk management decision makers. Although the precautionary principle is explicitly taken into account in the design of various pieces of chemicals legislation, to date, it has actually been applied in very few instances in the chemicals policy area. Whilst it does not mean that actions must be taken systematically, a number of stakeholder groups expressed concerns that risk management decision makers err towards a wait-and-see approach whilst more data is gathered to reduce the level of uncertainty. The Bisphenol A case shows, however, that this is not always the case.
The precautionary principle is explicitly taken into account in the design of various pieces of chemicals legislation (e.g. those requiring safety assessments such as the Biocidal Products Regulation and the Plant Protection Products Regulation, the Water Framework Directive, the Persistent Organic Pollutants (POPs) Regulation and the Restriction of Hazardous Substances in Electrical and Electronic Equipment (RoHS Directive), as well as REACH (many persistent, bioaccumulative and toxic/very persistent and very bioaccumlative substances (PBTs/)vPvBs are regulated on precautionary basis)).
The following examples show cases where the precautionary principle was applied (non exhaustive):
·The “Community strategy for endocrine disruptors” adopted in 1999 and updated in 2001, 2004 and 2007.
·Ban of Bisphenol A (BPA) in polycarbonate infant feeding bottles in 2011.
·Setting lower specific migration limit for Bisphenol A for varnishes or coatings applied to materials and articles intended to come into contact with food in 2018.
A number of stakeholder groups including NGOs, trade unions, and some Member State Competent Authorities have raised concerns that in the assessment of chemicals, authorities often hesitate to introduce risk management measures in situations where the precautionary principle applies and prefer to wait and request additional data to reduce the level of uncertainty. The BPA case shows however that this not always the case. Indeed, while still facing uncertainties including about the potential replacement substances and their safety and effectiveness, the Commission has mandated EFSA to undertake a full re-evaluation of BPA on the basis of the results of anticipated new studies and scientific data. Following the principles established in the 2000 Communication mentioned above, the Commission will then decide what and if any further action is necessary to protect consumers.
5.2.7Balance and Mix Between the Risk Management Measures based on 'Generic' and 'Specific' Risk Considerations
A.What's the issue?
Risk management measures in the EU chemicals legislation are taken based on an assessment of the risks to human health or the environment associated with the exposures to hazardous chemicals. As described in more depth in Section 2.1.5 and Annex 8 Section 8.2.1, there are two basic approaches to risk management used, often in combination, in the EU chemicals acquis: one based on specific risk assessment (SRA) approach and one based on generic risk consideration (GRC). Under the GRC approach, exposure scenarios are assessed generically based on the hazard of a substance or mixture without considering specific exposure situations. Under the SRA both the hazard of and the potential specific exposure scenarios of humans and the environment to the substance or mixture in question are assessed at the same time.
B.What are the findings?
Conclusions
Generic and specific risk management approaches both have their role to play within the framework of EU chemicals legislation but the application of both approaches has room for improvement.
Findings of this Fitness Check show that both the GRC and SRA have their role to play in the EU chemical legislative framework and that the current balance between the use of generic and specific risk management approaches works well, each under particular circumstances.
|
|
Advantages
|
Drawbacks
|
|
Generic Risk Considerations (GRC)
|
Provide a clear signal to all the actors involved (enforcement authorities, industry and downstream users) on the types of hazardous substances which should be avoided
|
Automatically triggered risk management measures may lead to disproportionate outcomes and unintended (legal and/or socio-economic) consequences if a mechanism for derogation is absent or not appropriate
|
|
|
The outcome of the risk management decision making process is more predictable (compared to SRA)
|
Potential consequences of automatically triggered measures in downstream legislation might influence the upstream scientific debate leading to the classification
|
|
|
Might be more appropriate for substances of higher concern and where vulnerable populations are at risk and/or cannot be protected through e.g. training or protection equipment (e.g. children under the Toy Safety Directive)
|
Less appropriate where exposures are minimal or would not occur through the route of exposure of concern and therefore can lead to over-regulation for non-relevant routes of exposure
|
|
Specific Risk Assessments (SRA)
|
Allow more targeted and differentiated consideration of exposures and thus risks and therefore more appropriate identification of actual risks and of risk management measures
|
The process might be slower compared to GRC and often more costly
|
|
|
Allow more targeted consideration of costs and benefits of various risk management options
|
Predictability of risk management decisions can be more difficult
|
Table 2 Main comments received from stakeholders regarding the GRC and SRA application
Where a derogation mechanism is connected to the GRC approach (i.e. a derogation from e.g. an automatic restriction or ban if certain conditions are fulfilled, such as demonstration of negligible exposure), industry stakeholders stated that it helps to ensure that the risk management measure stipulated will not lead to disproportionate costs or unintended effects e.g. regrettable substitutions. The process of issuing derogations, including their specified limitations, requirements and justifications, was considered useful by various industry stakeholders as the flexibility is necessary to the implementation of legislation.
Respondents to the public consultation were invited to indicate to what extent they find that the chemicals legislation framework overall should be more oriented towards SRA, GRC or should remain as it is. The preferences of the different groups varied quite considerably. Industry and in particular bigger companies tended to prefer a more extensive use of SRA approaches while NGOs tended to have a higher preference for more GRC approaches. The most common response among Member State competent authorities was that the current application of GRC and SRA approaches within the framework of the EU chemicals legislation is well balanced and should remain as it is. Responses from citizens were mixed, providing equal support for more SRA and for more GRC approaches, but a majority of citizens (ca. 60%) did not know how to answer or did not provide an answer to the question. Respondents were also asked to provide comments on, or arguments for, their preference; these are summarised in Annex 5 Section 2.8.
During the FC+ Study workshop, participants agreed that both approaches have their merits depending on the case at hand. There was no conclusive agreement on which one is to be preferred.
During the 1st FC study workshop, one of the topics discussed was the appropriatness and impacts on the existing linkages between the CLP and the relevant pieces of downstream legislation affected by harmonised classifications under CLP and that trigger risk management requirements. The following views were expressed:
·Automatic triggers provide legal certainty and a quick, high level of protection (particularly for cumulative risks). The focus should be on when the use of hazardous chemicals should be allowed (for example, when exposure is controlled), rather than the other way around, meaning an automatic ban with possible derogations is preferable. Another participant noted that for some classifications there should be no derogation.
·Other participants, however, expressed severe criticism against hazard-based risk management measures, which was seen as giving the European industry a competitive disadvantage vis-à-vis the rest of the world. In this respect, some argued that any hazard should only trigger risk assessment, with risk management measures (RMMs) then identified based on this. Consequently, if there is a change in the hazard classification the RMM currently required should be re-assessed.
·There are also arguments in favour of a more mixed approach, which would allow for automatic triggers appropriate under some legislation (where justified) but not under other legislation.
6EFFICIENCY
6.1Evaluation question: what are the costs and benefits associated with the implementation of the legislative framework for chemicals? What are the key drivers for those costs and benefits? To what extent are the costs proportionate to the benefits?
In addition to examining the costs and benefits associated with the implementation of the EU legislative framework for chemicals, the analysis provided below also looks at whether the costs are proportionate to the benefits. Annex 6 and Annex 11 provide a more detailed overview of the costs and benefits identified by the Fitness Check.
6.1.1Costs and cost drivers
A.What's the issue?
The efficiency of the EU chemicals legislation in achieving its three core objectives is examined by analysing:
·the direct regulatory costs and the enforcement costs;
·drivers for these costs; and
·who is facing these costs.
Indirect costs and the costs of risk management measures triggered under the downstream legislation are not assessed here, but the Fitness Check does consider those processes and whether they are working properly (see Section 5 Effectiveness). Given the differences in the organisation of public administrations across the EU, enforcement costs imposed on public authorities at national level are analysed from a cost drivers' perspective (i.e. not providing monetised and quantified figures).
B.What are the findings?
Conclusions
The methodological, counterfactual and data challenges make it unfeasible to provide a quantified estimate of the overall costs of the EU chemicals legislation. However, an assessment of the CLP Regulation indicates the on-going annual regulatory costs to industry in the EU range from EUR 0.97 to 1.7 billion. Similarly, the annual regulatory costs for industry due to the Plant Protection Products Regulation are estimated at EUR 122-189 million. The annual regulatory costs for industry due to the Detergents Regulation are estimated at EUR 63.7-149 million). This would suggest that the overall regulatory costs of the EU chemicals legislation for EU industry are several billion euros per year. Quantitative and qualitative analysis suggests that regulatory costs have remained relatively stable over the last decade.
Depending on the piece of legislation, the main cost drivers are data generation (hazards, chemical uses, exposures, etc.), staff and worker occupational hygiene monitoring and control costs, as well as enforcement and monitoring costs for public authorities. SMEs are more affected than bigger companies by certain aspects of the EU chemicals legislation such as understanding and compliance with legal obligations. The overall pace of the risk assessment and risk management processes can also have significant implications for the costs borne by both industry and the public authorities (at both the EU and at Member States levels).
The regulatory costs assessed for the purposes of this Fitness Check cover:
·Direct regulatory costs affecting the EU chemicals industry, downstream users and, which may end up being passed onto consumers to greater or lesser extent. These costs correspond to regulatory charges, substantive compliance costs and administrative costs.
·Implementation and enforcement costs affecting public authorities both at the EU and Member State level.
The baseline used is a simple counterfactual of no EU or Member State chemicals legislation. As explained above (Section 4.2), it was challenging to quantify the overall cumulative costs of the EU chemicals legislation.
1)Direct regulatory costs
a)Overview
All stakeholders recognise that the costs of the chemicals legislation are significant, especially for SMEs with a perception of costs that varies depending on the stakeholder group i.e. industry, NGOs, public authorities, citizens.
An estimate was made of the cost of the EU legislation with a bearing six subsectors of the chemical industry during the period 2004-2014 (see Annex 6 Section 6.1.2 Table 12 for the list of pieces of legislation covered). When added up, the estimated average annual total direct cost borne was around EUR 8 billion, representing around 1.7% of their turnover and 9% of the value added.
Among the legislation packages, the emissions and industrial processes package represents approximately 33% of the regulatory cost (4% of the subsectors’ value added), the chemicals package (including REACH) 29% (3.5% of value added) and workers’ safety 24% (2.9% of value added). Whilst there are different estimates, quantitative and qualitative analysis suggests that regulatory costs have remained relatively stable over the last decade.
However, the figure of EUR 8 billion cannot be considered as an entirely accurate estimate of the cost of the chemicals acquis due differences of scope and in the methodology applied:
·The period covered (2004-2014) corresponds only partly to the one covered by this Fitness Check.
·Costs correspond to only six subsectors (organic and inorganic basic chemicals, plastics in primary forms, pesticides and agrochemical products, soaps and detergents, paints, varnishes and similar coatings and other chemicals products) and not all the industry and companies.
·Costs presented above also include regulatory costs for several pieces of legislation that are not in the scope of this Fitness Check. In addition, several other pieces of legislation although within the scope of this Fitness Check, were not covered by the abovementioned cumulative cost assessment attempt. Please see Annex 6 Section 6.1.2 Figure 13 for a full list of pieces of legislation covered.
·While the occupation safety and health (OSH) Framework Directive, per se, is not in the scope of this Fitness Check, it can be reasonably assumed that the costs related to occupational health and safety legislation in the chemicals sector derive primarily from the daughter regulations (the Chemical Agents Directive, the Carcinogens and Mutagens Directive, etc.) which are within the scope of the Fitness Check. That said, it should also be noted that the estimated occupational health and safety costs probably include costs of worker safety protection beyond specific risks posed by exposure to hazardous chemicals(e.g. falls from heights, electrocution, burns, etc.) which are substantive but are not within the scope of the Fitness Check.
·Regarding the emissions and industrial processes legislative package, it should be noted that the EU Emission Trading System (ETS) related legislation is not in the scope of this Fitness Check. In this legislative package, most of the monetary obligations are due to ETS. Therefore, the regulatory costs of emissions and industrial processes legislative package as assessed for the purposes of this Fitness Check can be estimated to represent EUR 2.6 billion (instead of EUR 3.1 billion).
Therefore, additional cost elements were gathered where possible and qualitative assessment is presented where providing reliable quantified figures was considered to be impossible.
b)Regulatory charges
Regulatory charges are the fees, levies or taxes imposed by the legislation, primarily faced by industry (see Annex 6 Section 6.1.2. A) table 13 providing a list of regulatory charges by piece of legislation). Fees and charges are, in general, set using the cost recovery principle i.e. they correspond to the actual cost of the work involved and services delivered.
While creating business opportunities for innovative and specialised SMEs, understanding and complying with the chemicals legislation remains a key challenge for them. Therefore, mitigating measures such as reduced fees have been introduced under some pieces of legislation (the CLP Regulation, the Biocidal Products Regulation). However, the SMEs fee reduction mechanism does not exist under all pieces of legislation (e.g. the Plant Protection Product Regulation, the Waste legislation, the Residues of Pesticides Regulation, the Export and Import of Hazardous Chemicals Regulation, the Detergents Regulation and the Fertilizers Regulation). The use of this tool remains uneven across the EU where it can be applied as in most cases it is up to Member States to define the level of fee reduction.
c)Substantive compliance costs
Substantive compliance costs can be divided into:
·One-off costs that are often borne by a particular regulated group e.g. manufacturers, having to adjust and adapt to the changes in legal rules. The transition from the Dangerous Substances Directive (DSD) and Dangerous Preparations Directive (DPD) to the CLP Regulation generated one-off costs estimated ex-post to range from EUR 0.9 - 2.2 billion. Such costs were generated mainly by the (re-)classification obligation and changes to be made in order to comply with the new labelling and safety data sheet requirements. Transition costs can also occur where substance specific risk management measures are taken e.g. a ban of a substance that is classified as carcinogenic, mutagenic or toxic for reproduction (CMR) which requires manufacturers to reformulate and, in some instances, to stop the manufacture of a particular product line altogether. Costs can be very low, for example, where a substitute is readily available, and significantly higher, where it is not, or where reformulation involves significant change to the production process.
·Recurrent costs that are sustained by the regulated stakeholders on regular basis. The importance of these costs depends on the overall complexity of legislation. The main cost drivers for recurrent costs are the obligation to generate and provide data for chemical hazard classification (including testing), the risk assessment step, in particular the exposure assessment element, as well as the implementation of risk management measures e.g. hazard communication through labelling. The costs of the classification of a substance are driven mainly by the CLP Regulation. Annual costs arising from the CLP Regulation are estimated to amount to EUR 1.3 billion (EUR 0.97-1.7 billion). Costs are often dependent on data availability and usability. Costs of data generation and risk and exposure assessment are often related to an authorisation/approval/renewal process e.g. under the Plant Protection Products and Biocidal Products Regulations and can be significant cost drivers. The total costs for the pesticides industry are estimated at approx. EUR 122-189 million per year. The regulatory charges (fees) represent a small share of the total costs for the industry Study supporting the REFIT Evaluation of the EU legislation on plant protection products and pesticides residues (Regulation (EC) No 1107/2009 and Regulation (EC) No 396/2005) p.126; not yet publicly available. The costs for pesticides maximum residue level (MRLs) procedures are estimated at around EUR 55 million per year for the industry. Annual costs that the detergents industry has incurred as a direct result of the Detergents Regulation are estimated to range between EUR 63.7 – EUR 149 million (appr. EUR 764 million – EUR 1.8 billion in total since 2005). Depending on the sector, compliance with occupational health and safety legislation, e.g. investments in workers’ health protection equipment, can also lead to significant costs.
"Understanding and keeping up-to-date with changes in legal requirements" was identified during the public consultation and the SME Panel Survey as a significant driver of costs by the highest number of companies (84% (147) of companies for the former and 45% of SME respondents for the latter), with the costs of risk management under the different legislation ranked second (73% or 127). Training staff to ensure compliance with legal requirements was also identified as important cost driver (61% (106) by respondents from Industry association and companies).
SME stakeholders underlined the fact that SME specific challenges are often linked to the availability of resources. For SMEs, it is difficult to find the time and money to attend workshops, webinars, conferences, etc. (especially if information is only available in English), and to find the necessary time to track, understand and implement the many and often complex requirements of the EU chemicals legislation and to keep up-to-date with changes to the requirements. From an authority and industry association perspective, it can be difficult to reach smaller companies. There are also differences in the support to SMEs provided by Member States. There was a general view amongst the SME stakeholders consulted that Member States need to do more.
d)Administrative costs
Administrative costs are those borne by businesses, citizens, civil society organisations and public authorities in complying with information obligations. They include:
·the obligation of reporting; and
·retrieving data on applications from downstream users and labelling (also discussed under the section on substantive regulatory costs above).
Estimates of the costs of reporting by Member States to the EU level were made as part of the “Fitness Check of Reporting and Monitoring of EU Environment Policy”. These varied by piece of legislation: for example, the CLP Regulation and the Asbestos Directive were between EUR 30 000 and 100 000 per annum; the Persistent Organic Pollutants (POPs) Regulation and the Regulation on Export and Import of Hazardous Chemicals were under EUR 30 000 per annum.
Another factor that could increase the administrative costs is the pace of the processes for the specific risk assessments. Under the EU chemicals legislation, the expected duration of the risk assessment procedure ranges between several months and several years e.g. the risk assessment and authorisation procedure for active substances in plant protection products and products lasts at least 12 months. The longest average duration of a risk assessment is attributed, according to the stakeholders consulted, to the Biocidal Products Regulation and to the Plant Protection Products Regulation. According to these stakeholders, the process of regulatory validation can take up to 10-15 years, due to delays both from the industry applicant in submitting missing data and from the evaluating authorities. For the Biocidal Products Regulation, one could note however that, in most cases, industry can place their substances/products on the market during the assessment period by authorities, which also allows them to recover some costs during that period.
2)Enforcement costs
Legal rules have to be monitored and enforced by public authorities to be effective which implies costs. It is not possible to provide quantified figures for costs of enforcement of the EU chemicals legislation at national level. These costs will vary across legislation and also depend on the regulatory option chosen (e.g. self-regulation, providing information and guidelines, market-based instruments, more or less stringent and prescriptive regulatory actions). Enforcement costs will also vary across Member States depending on the national administrative choices and the related functional costs.
The costs for public authorities include costs associated with:
·Implementation activities: participation in expert groups and scientific bodies, research and regulatory proposals, risk assessments, etc. is time- and resource-intensive. Therefore, the fact that many Member States are lacking resources leads to differences in their involvement in bringing forward harmonised hazard classification dossiers under the CLP Regulation, for example.
·Compliance monitoring and enforcement activities: costs will depend on the way in which this is organised at the national level. For example, data available from the REACH-EN-FORCE projects indicate that on average over 2 000 inspectors are trained on REACH and the CLP per annum in the EU, at an annual cost of around EUR 1.7 million.
For illustrative purposes, the overall costs for Member States generated by the Plant Protection Products Regulation for the approval and authorisation procedures are estimated at approx. EUR 44 million annually. The costs for the Residues of Pesticides Regulation (which sets maximum residues levels (MRLs) of pesticides on food products) procedures are estimated at around EUR 5 million annually for the 28 Member States.
At the EU level, the average annual costs to ECHA associated with implementing the CLP are approximately EUR 2.57 million. This figure is the cost of providing guidance, running helpdesks, overseeing committees and forums, etc. The total cost to ECHA of implementing the CLP over the period 2010 to 2016 was over EUR 22.8 million, equivalent to 17% of the combined REACH and the CLP budget. The total capital costs to ECHA of developing the Classification and Labelling Inventory (CLI) were around EUR 1 million, with an annual operating expenditure of around EUR 0.2 million.
The costs for MRLs procedures are estimated at around EUR 3 million for EFSA and the Commission.
6.1.2Benefits
A.What's the issue?
The efficiency of the EU chemicals legislation is the ratio of the benefits to the costs. Having looked at the costs, this section looks in a similar way at:
·What are the benefits of the EU chemicals legislation?
·How significant are these benefits and what are the key drivers?
·To whom do the benefits accrue?
Data, knowledge and methodological gaps mean it was not possible to arrive at a cumulative 'monetised' benefit estimate for the whole framework of the EU chemicals legislation. Nevertheless, certain components of the broader picture are presented below. It is important to recognise, however, that these benefit estimates represent just a portion of the overall health and environmental benefits of the EU chemicals acquis.
B.What are the findings?
Conclusions
The EU chemicals legislation has clearly led to significant benefits in terms of avoided health and environmental impacts. Some of the most notable benefits relate to reduced exposure to hazardous chemicals at the workplace known to increase the risk of cancer and cardiovascular disease. Key benefit drivers include avoided healthcare costs, avoided productivity losses (due to avoided lost working hours as a result of illness or premature death), avoided suffering and premature deaths, avoided remediation costs (including wastewater and drinking water treatment costs) and avoided degradation of environmental/eco-system services costs.
Significant benefits in terms of protecting human health and safeguarding the environment have been delivered over the last 50 years by the EU chemicals legislation to industry, to public authorities and regulators as well as to consumers and citizens and to society and the economy more generally.
Table 3
provides a list of the main categories of benefits and direct beneficiaries.
|
Benefits
|
Direct beneficiaries
|
|
Health
|
'Physical' benefits
|
Workers, consumers and citizens
|
Reduced morbidity and mortality health impacts (e.g. reduced number of cancers, cardiovascular diseases, allergies, reproductive illnesses, neurological diseases, etc.) from reduced exposures to hazardous chemicals. This includes avoided suffering and health effects through higher income (due to avoided lost earnings as a result of avoided illness) and longer life expectancy
|
|
|
Monetised benefits
|
Consumers and citizens
|
Avoided healthcare costs, avoided suffering (assessed through willingness to pay techniques), value of avoided life years lost due to premature death, productivity losses due to lost work hours as a result of illness and/or premature death
|
|
|
|
Industry
|
Avoided health costs and productivity losses; a less hazardous working environment can reduce the costs that companies face (healthcare costs, insurance costs, lost productivity, fines, etc.)
|
|
|
|
Member States
|
Reductions in the damage costs associated with chemical exposures (healthcare costs, environmental clean ups, etc.)
|
|
Avoided environmental damage
|
Society
|
Various ecosystem services, recreational values, increased fishing revenues and avoided water treatment costs
|
|
|
Industry
|
Reductions in the costs associated with environmental remediation and clean ups. Improved access to and reduced costs of clean water, etc.
|
|
|
Members States
|
Reductions in the costs associated with environmental remediation and clean ups.
|
|
Regulatory
|
Member States
|
Reductions of some of the burden faced by Member States, by enabling them to share efforts (and hence resources) at the European level in the implementation of the legislative framework
|
Table 3 Benefits of the EU chemicals legislative framework and direct beneficiaries
Some of the biggest, currently measurable, health benefits of the EU chemicals legislation are associated with reductions in the exposure to carcinogenic substances. However, one should keep in mind that, while the extent of cancer incidence due to occupational exposure has been extensively studied, the impacts from environmental exposure to carcinogens are harder to estimate. It is in an occupational setting where the link between exposure to certain chemicals and cancer is the most clear. Based on reductions in exposure to a group of 13 carcinogens since 1995 that have been targeted by EU occupational health and safety legislation, the total number of cancer deaths avoided across the EU is estimated to be around 1.4 million. Other examples include the estimated benefits from a reduced exposure to hexavalent chromium, phthalates, to pesticides and polychlorinated biphenyls (PCBs) (see
Table 4
below as well as Annex 6 Section 6.1.3. B) Table 15 for additional examples).
|
Benefits
|
Estimated Benefit Value (€)
for the EU
|
What's Included?
|
Time period
|
Legislation
|
|
Reduced poisoning incidents, occupational skin and respiratory diseases and occupational cancers
|
EUR 391 – 512 million/yr
|
·Avoided healthcare costs
·Avoided productivity losses (lost working hours and income)
|
2000-2008
|
·The Dangerous Substances and Preparations Directives
|
|
|
EUR 217 – 338 million/yr
|
|
Since 2008
|
·The CLP
|
|
Reduced exposures to hexavalent chromium at workplace
|
EUR 100 million/yr
EUR 4 billion in total
|
Avoided cancers:
·Avoided healthcare costs
·Avoided productivity losses (lost working hours and income)
·Avoided suffering/death
|
1995 - 2010
|
·The Carcinogens and Mutagens at Work Directive
·The Chemical Agents Directive
|
|
Reduced exposure to phthalates (DEHP; DBP) via a variety of consumer products
|
DEHP:
EUR 7 billion cumulatively from (i.e. approx. EUR 580 million/yr)
|
Reduced female/male reproductive disease:
·Avoided healthcare costs
·Avoided productivity losses (lost working hours and income)
|
1996 - 2008
|
·Legislation on consumer products (cosmetics (since 2005), food contact materials (2007), electrical equipment (2015), medical devices)
·The Water Framework Directive (priority substance since 2001)
·Pre-REACH Regulation (the Existing Substances Regulation) 1994-2006
|
|
|
DBP:
EUR 6.7 billion cumulatively (i.e. approx. EUR 560 million/yr)
|
|
|
|
|
Better control and management of plant protection products
|
EUR 15 – 50 billion/yr
|
Reduced environmental and pollination impacts:
·Value of eco system services
·Agricultural value of pollination services provided by pollinating insects
|
Since late 70s (legislation on water 1975 and 1979 legislation on pesticides)
|
The Plant Protection Products Regulation (PPPR)
|
|
Reduced pesticide contamination of surface and groundwater reserves
|
EUR 500 million/yr
|
Avoided drinking water treatment costs:
·Avoided cost of removing pesticides from water treated for drinking water supply
|
Since late 1970s (legislation on water 1975 and 1979 legislation on pesticides)
|
·The Plant Protection Products Regulation (PPPR)
·The Water Framework Directive and the EQS Directive
·The Drinking Water Directive
|
|
Reduced contamination by PCBs
|
Cumulative cost of EUR 0.4 - 1.9 billion/yr (EUR 20 – 90 billion in total)
|
Avoided clean-up costs association with PCB use in the past:
·Remediation and waste management costs excluding any health and environmental impact costs
|
1971 to 2018
|
·Classified under the CLP
·Directive 96/59/EC on the disposal of PCBs and PCTs (not within the scope)
·The POPs Regulation (2004)
·Hazardous Waste List
|
Table 4 Selected monetised environmental and health benefits of reduced hazardous chemical exposures
Regarding enhancement of the internal market, competitiveness and innovation objectives, these benefits are examined in Sections 5. Effectiveness and 9. EU added value. There have been positive impacts of the EU chemicals legislation in terms of an efficiently functioning internal market. Benefits in terms of innovation and positive impact on the EU industry's competitiveness are more complex.
More generally speaking, the EU chemicals legislation plays an important role in the shift towards a more circular economy. It also contributes directly to the achievement of the 2030 UN Sustainable Development Goals (SDGs).
Respondents to the public consultation agreed that the EU chemicals legislation and chemical-related legislation generate benefits from reducing the exposure of consumers and citizens to toxic chemicals, reducing the exposure of workers to toxic chemicals and reducing damage to the environment and ecosystems (see
Table 5
).
|
Group
|
Benefits identified by largest proportion of respondents by group
|
|
|
Top ranked
|
Second ranked
|
Third ranked
|
|
Group 1 (citizens)
|
Reducing the damage to the environment and to eco-systems (58%)
|
Reducing the exposure of consumers and citizens in general to toxic chemicals (54%)
|
Reducing the exposure of workers to toxic chemicals (54%) [equal second ranked]
|
|
Group 2 (industry)
|
Reducing the exposure of workers to toxic chemicals (85%)
|
Reducing the damage to the environment and to eco-systems (84%)
|
Reducing the exposure of consumers and citizens in general to toxic chemicals (79%)
|
|
Group 3 (public authority)
|
Reducing the exposure of consumers and citizens in general to toxic chemicals (95%)
|
Reducing the exposure of workers to toxic chemicals (92%)
|
Reducing the damage to the environment and to eco-systems (89%)
|
|
Group 4 (NGO/ others)
|
Reducing the exposure of workers to toxic chemicals (91%)
|
Reducing the exposure of consumers and citizens in general to toxic chemicals (80%)
|
Reducing the damage to the environment and to eco-systems (70%) = encouraging research and innovation, generating jobs and improving competitiveness (70%)
|
Table 5 Summary of the views of respondents by group to public consultation
Respondents to the public consultation also indicated additional benefits that are generated from:
·encouraging research and innovation, generating new jobs and improving competitiveness;
·stimulating competition and trade within the EU single market; and
·stimulating international trade between the EU and other countries.
Results of the SME Panel consultation suggest the impact of the CLP Regulation (and other EU hazard communication requirements) has been overall positive (increased access to classification data for substances and more consistent classification, safe use of chemicals by workers and consumers, preparedness for industrial accidents, increased awareness of the potential health and environmental impacts). The only exception is “changes in packaging requirements” where proportion of ‘neutral/no change’ responses was higher compared with proportion of responses suggesting positive impact (40% and 35% respectively; 10% negative impacts; 15% “Don’t know”).
6.1.3Are costs and benefits proportionate?
A.What's the issue?
Answering this evaluation question requires the assessment of the framework-wide costs and benefits (environmental, health, internal market, etc.) of the EU chemicals acquis to determine whether the costs are proportionate.
B.What are the findings?
Conclusions
The existing data and methodological limitations combined with the scope limitations of the Fitness Check (a framework-wide assessment and not a full, in-depth evaluation of each and every one of the more than 40 pieces of legislation covered) meant it was not possible to estimate of the overall costs and benefits of the EU chemicals acquis and therefore, to determine whether or not costs are proportionate. However, from the partial evidence that was available, it appears that both the costs and the benefits generated by EU chemicals legislation are significant.
It is not possible to provide a credible estimate of the cumulative benefits or costs of the EU chemicals acquis. This, coupled with the partial picture on the costs and benefits at the specific legislation level, means it was not possible to arrive at a single cost-benefit ratio and that it is impossible to draw any strong conclusions regarding the proportionality.
It appears from the analysis above that the benefits directly or indirectly generated by the EU chemicals legislation are significant while costs to companies and public authorities are also significant. These views are shared by different stakeholders although the perception of the importance of the costs and therefore of whether costs are proportionate to benefits varies amongst different groups and even within the same category.
Amongst Member States, the UK is the only country to have tried to provide an estimate of the costs and benefits of chemicals legislation. The environment ministry quantified the costs and benefits of 428 of its regulations affecting UK businesses, just over half of which were derived from EU or international legislation. The most positive cost-benefits ratio amongst the different policy area clusters was for regulations on ‘chemicals and genetically modified organisms’ with a ratio of 1:18.9 (with 82% of the costs coming from EU legislation).
6.2Evaluation question: what aspects of the functioning of the framework are the most efficient and what are the least efficient?
This sections looks at factors that affect the efficient functioning of the EU chemicals legislation beyond the sole cost-benefit point of view.
6.2.1Reliance on the CLP Regulation as the basis for hazard classification and labelling
A.What's the issue?
The CLP Regulation is the primary basis for identifying hazards, providing hazard classification across almost all other pieces of legislation as well as labelling and other risk and hazard communication measures. To what extent it is functioning efficiently is assessed regarding:
·its general architecture;
·resources and expertise available for putting forward harmonised classification dossiers; and
·communicating chemical hazard and risk information to consumers via labelling.
B.What are the findings?
Conclusions
The overall architecture of the CLP Regulation and many aspects of its practical implementation are operating efficiently. The CLP Regulation provides an efficient and harmonised approach to the hazard identification and classification of chemicals placed on the market in the EU. However, its full implementation and enforcement appears to be challenging. Moreover, resource and expertise constraints in a number of Member States reduce the overall efficiency, particularly with respect to harmonised classification. Whilst most of the CLP hazard pictograms are well recognised and understood by consumers, there are some inefficiencies in relation to the consumer labelling requirements under the CLP and some overlaps between the CLP, the Detergents Regulation and the Cosmetic Products Regulation.
The legal architecture of the CLP Regulation based on self-classification by duty holders and backed up by harmonised classification for substances of concern, provides a clear and consistent approach to identifying, characterising and classifying hazardous chemicals. It ensures that the science of chemical hazard assessment and classification is done separately but then fed into decision-making in the risk assessment and risk management decision steps in other, downstream pieces of legislation. Various stakeholders were of an opinion that maintaining the CLP system as purely hazard based is important. It allows classification of a wide range of chemicals without creating a disproportionate burden on administration while focusing resources of public authorities to the most relevant substances for public health and the environment. Furthermore, where no harmonised classification exists, self-classifications allows for faster evaluation by companies.
Harmonised classifications rely on the initiative of either companies or Member State authorities to create and submit a proposal to ECHA for a harmonised classification which is eventually adopted by the Commission. Resource and expertise constraints in a number of Member States hinder their ability to make these proposals. The fact that the workload in developing harmonised classification dossiers is shared unequally between Member State Competent Authorities is a factor that negatively affects efficiency. The pace of whole process – from proposal to final agreement - is a factor affecting the overall efficiency given that these are the cornerstone of the legislative framework (see Section 5.2.3).
There are inefficiencies in relation to consumer labelling under the CLP Regulation as highlighted above in terms of proportionality of costs for companies to change some aspects of labelling and the effectiveness of the communication. The CLP Regulation is amended every two years (via the Adaptation to Technical Progress (ATP)) in order to comply with the changes made at the UN Global Harmonised System (GHS) level. According to participants of one of the workshops, the EU approach of having an 18 month transitional period for applicability of GHS updates is generally perceived as being sufficient but the constant need to re-label is a cost. They also believed that minor changes have no real benefits but could have significant negative impacts due to re-labelling requirements. Moreover, according to these stakeholders, SMEs (downstream users) may have very little time to make labelling changes as suppliers upstream provide details late.
Regarding detergents’ labelling information received from AISE and other consultees suggests that there are also legislative overlaps between the Detergents Regulation, the CLP Regulation and the Cosmetic Products Regulation with regard to the labelling of allergens which creates unnecessary regulatory burden. The CLP Regulation sets out the hazard classification criteria and requirements for respiratory and skin sensitisation and requires the inclusion of skin sensitisers in the list of ingredients when they occur above certain thresholds. The Detergents Regulation also relies on the list of allergens identified under the Cosmetic Products Regulation. The Cosmetic Products Regulation does not refer to the CLP classification criteria for skin and respiratory sensitizers.
6.2.2Use and access to data
A.What's the issue?
Data generation was identified as one of the main cost drivers. How data is developed, used, and accessed affects the speed at which risk management measures can be implemented. The current mechanisms regarding data sharing and access to data are assessed based on:
·the extent to which they are flexible and facilitate the use of data across different pieces of legislation; and
·what is the contribution of the Good Laboratory Practice (GLP) Directive in facilitating data sharing.
B.What are the findings?
Conclusions
Considerable efforts have gone into improving the sharing of hazard and risk assessment related data on chemicals generated under different pieces of legislation and/or held in several different databases. However, unnecessary duplication of effort in data generation still occurs in some instances due to a lack of data sharing as a result of various related factors including confidentiality and intellectual property rights.
The review of the priority substances list under the Water Framework Directive is an example of how taking appropriate action can be delayed even when a potential risk can be identified based on hazard data, for example. This is because adequate exposure data are often not available to allow the risk assessment to be completed. Better links with risk assessments carried out under other legislation might help in such situations, i.e. better access to full risk assessments (including relevant exposure data). The Watch List mechanism was introduced a few years ago to allow exposure data from surface waters to be generated when otherwise not available. Faster feedback of monitoring data obtained under the Water Framework Directive to that other legislation may also facilitate the prompt introduction of additional measures where necessary.
The use of the GLP Directives has played an important and useful role in standardising quality requirements for test facilities and in ensuring repeatability and consistency in data generation.
The GLP Directives are one of the most efficient elements of the EU chemicals legislation. By standardising data quality requirements, they have helped to avoid double testing and thereby helped saving time and resources. In addition, the avoidance of double testing helps to avoid unnecessary animal tests. However, accepting for regulatory purposes only GLP compliant data would be counterproductive. Non–GLP data has a potential of being useful source of information (providing that the data is correctly referenced, reliable and robust) thus reducing the need to generate new data, additional costs and delays (see Section 5.2.1 Data, knowledge and information as well as Annex 5 Section 5.2.1 C) and Annex 6 Section 6.2.4).
Another aspect that plays a significant role in how efficient is the EU chemicals legislation, is access to data and data sharing. Problems are still encountered with data access and sharing between regulatory areas because of the lack of a centralised access point (e.g. when useful hazard and risk assessment data is sitting in regulatory clusters linked to particular agencies, scientific committees and/or legislative risk assessment processes for individual regulations and is not readily shared or available to other users), a lack of awareness of what exists in the different databases, the lack of efforts in investigating whether data can be used, and too restrictive access rights for use and re-use of data. It leads to a certain duplication of effort where the nature of assessment made is similar between different pieces of legislation and therefore can generate extra costs, as well as longer-than-necessary timeframes and lead to duplication of testing. This issue might also have negative consequences in cases when companies are seeking a derogation, as the timeframes can be relatively short in comparison with the time it takes for new and sufficient data to be gathered to prove safe use.
Difficulties encountered in updating the Water Framework Directive’s list of priority substances illustrate the potential efficiency gains for better data sharing. An additional ‘Watch List’ mechanism was put in place in 2013 in order to gather data to inform decision making on candidates for potential inclusion in the list of priority substances needs to be updated. This ‘Watch List’ was updated recently. Several substances from the first watch list are included in the updated version. This demonstrates how long it can take to gather the necessary exposure information. If adequate data is not available by the time that legislation (in this case, the priority substances list) needs to be reviewed, it can lead to delays in taking appropriate actions. Better links with risk assessments carried out under other pieces of legislation might help to avoid such situations, e.g. access to risk assessment (including exposure) data, etc.
During one of the workshops different participants believed that there are cases of duplication of effort in assessing hazards and risks because data cannot be shared, due to the above mentioned obstacles, leading to inefficiency and inconsistency. Information exchange should be improved also between the EU Agencies and scientific committees. According to these stakeholders, information gains from improved data sharing would be significant.
6.2.3Grouping approach vs. substance-by-substance approach
A.What's the issue?
The EU chemicals legislation is based on a substance-by-substance approach. Its efficiency is assessed based on potential benefits compared to increased use of grouping approach.
B.What are the findings?
Conclusions
The substance-by-substance approach is efficient in identifying the hazards of a specific substance and the risk from the situation in which it is used. However, as highlighted by different stakeholders, there is a need for greater flexibility and a more integrated and holistic view in assessing substances as groups. The substance-by-substance approach can limit in some cases the efficiency of the risk assessment process both in terms of protecting human health and the environment, as well as in terms of avoided costs to industry for further replacement by alternatives e.g. pre-empting industry's investment in substances that are likely to be banned subsequently. Approaches based on grouping chemicals of a similar hazard/risk nature together for risk assessment were supported by NGOs and some Member State authorities as a way of addressing this challenge.
The EU chemicals legislation is currently based on the substance-by-substance approach. It is often the most pragmatic approach to conducting risk assessments. Much of the hazard and exposure data needed are held by industry with assessments completed on single substances. Indeed, hazard data on chemicals are usually focussed on single substances rather than groups of chemicals and, equally, defined uses of chemical substances are also based on individual substances. Moreover, most OECD test guidelines and also alternative in-silico i.e. performed on computer or via computer simulation, approaches work on a substance-by-substance basis.
Although the substance-by-substance approach is effective in identifying the hazards of a specific substance and the risks from the situation in which it is used, stakeholders from all categories have highlighted the need for greater flexibility and a more integrated and holistic view in assessing substances as groups. The efficiency of the risk assessment process is limited by this, both in terms of protecting human health and the environment, as well as in terms of avoided costs to industry for further replacement by alternatives e.g. pre-empting industry's investment in substances that are likely to be banned subsequently. NGOs and some Member State authority stakeholders supported the use of chemical grouping approaches whereby chemicals of a similar hazard/risk nature are assessed collectively. However, further grouping of chemicals, if envisaged, would need to have been designed and integrated in the current framework without leading to longer decision-making processes (and would not be suitable in all contexts).
During one of the workshops views expressed by many different stakeholders were in favour of increased use of grouping approach. According to these stakeholders grouping would provide opportunities for efficiency, as it would prevent industry investing substantial resources and investments to replace a substance that would most likely be shortly banned. This is especially the case for SMEs, for which the processes can become extremely burdensome, compared to large companies. According to stakeholders, there is also potential for synergies between legislation e.g. grouping done under REACH could be used under other pieces of legislation. However, defining what the meaningful group of substances proves to be a challenge.
6.2.4Organisational efficiency of the EU Agencies and scientific committees
A.What's the issue?
At the EU level, risk assessments are conducted by a number of different agencies and scientific committees depending on the chemicals legislation in question. The organisation efficiency is assessed in terms of the speed of different processes and the coherence of their outcomes.
B.What are the findings?
Conclusions
Currently, difference agencies and committees are involved in providing scientific advice and risk assessments within the chemicals regulatory framework. The EU organisational efficiency regarding the overall process could be improved and simplified, therefore avoiding duplication of procedures and reducing the risk of diverging opinions.
Currently, different Agencies and Committees provide scientific advice and risk assessment without prejudice to the competencies conferred to another one. In most cases, delineation of areas of competencies is clear e.g. for cosmetics ECHA is doing the environmental risk assessment while the Scientific Committee on Consumer Safety (SCCS) is in charge of assessing risks for human health. In some other, there is a potential overlap e.g. substances assessed by the Scientific Committee for Occupation Exposure Levels (SCOEL) under the Occupational Safety (OSH) legislation and ECHA (REACH) or between the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) and/or the SCCS and ECHA or ECHA and EFSA.
Annex 8 Section 8.2.2 provides a list of the EU Agencies and Scientific Committees involved with hazardous chemical risk assessment (see Figure 18).
The SCCS and the SCHEER have generally been capable of addressing the Commission’s information needs related to the assessment of health risks on consumer products in a satisfactory manner. However, some variations between opinions, depending on the nature of the questions addressed and the availability of data and scientific literature on the subject have occurred. Since the independent risk assessment agencies have been created, the broader issue of how to ensure methodological consistence between them has appeared as prominent, as no institutional mechanism was any longer available to this aim. Therefore, while the internal coherence of their opinions and that of the opinions of the different scientific committees is fully satisfactory, the external coherence - i.e. with the opinions of other EU risk assessment bodies – presents some problematic aspects. Applicants in particular have raised concerns about the misalignment of methodological approaches of the scientific committees with those of the other EU risk assessment bodies, and expressed the need for more standardisation in this regard.
The Rules of Procedure governing the functioning of the SCCS and the SCHEER explicitly recognise the need to ensure good and effective cooperation between these two Committees as well as with other scientific bodies of the EU. This means identifying and solving at their earliest stage any potential conflicts or divergence of opinions, and the obligation to seek the convergence. The good and effective cooperation between the SCCS and the SCHEER is ensured via the establishment of the Inter-committee Coordination Group (ICCG) which deals with (amongst others) matters relating to harmonisation of risk assessment and diverging scientific opinions. The SCOEL has only the obligation to seek to ensure cooperation with other scientific bodies and committees. Differences in the strength of the obligation to seek the convergence is maybe a reason explaining why there have been cases of divergence of opinions between the RAC and the SCOEL while such cases have not (yet) occurred between the RAC and the SCHEER/the SCCS or between the SCHEER and the SCCS.
The majority of stakeholders consider the division of responsibilities and resources for the assessment of chemical risks to human health and the environment between ECHA's Risk Assessment Committee (RAC) for industrial chemicals (including biocides, also with ECHA's Biocidal Product Committee (BPC) involvement), EFSA for pesticides and food contact materials, and the European Medical Agency (EMA) for pharmaceutical products to be generally appropriate and efficient.
During one of the workshops participants considered that merging risk assessment committees may help to avoid conflicts in responses provided. At the very least there needs to be more communication between risk assessment committees assessing the same substances/mixtures. Different results are not always wrong, but for related topics, there should be consistency in the outcomes.
For the sake of improved coherence and efficiency, there may be opportunities to simplify the risk assessment setup by bringing the risk assessment activities currently done by some of these scientific committees together under the remit of ECHA or EFSA. It should be however noted that in some cases, the assessment done by the committees goes beyond assessing chemical risks e.g. for toys, risks can be chemical but also mechanical and other physical risks.
7COHERENCE
7.1Evaluation question: to what extent are the legal acts consistent in how they attempt to reach the stated objectives and can differences in the hazard identification and risk management of chemicals be justified?
A.What's the issue?
There are some differences in approaches with respect to hazard/risk assessment and risk management processes between some of the different pieces of EU chemicals legislation. In many instances, these differences reflect variations in legal scopes and objectives and thus different needs in terms of depth of analysis and evidence required to draw conclusions and decide upon any risk management measures that may be needed. Therefore, these differences do not necessarily imply incoherence. They illustrate the legislator’s intention to provide a framework that is tailored to the specific circumstances of the substances used and/or the likely hazards and exposure.
The assessment looks at the consistency in the way different pieces of legislation within the scope of this Fitness Check contribute collectively to achieving the primary policy objectives. Therefore, for the purposes of this Fitness Check, any differences identified were only considered to be a coherence issue where they affected the correct functioning of hazard/risk assessment and risk management procedures.
Where coherence with REACH and other pieces of legislation which are, in principle, outside the scope of this Fitness Check was considered important for a better understanding of the coherence issue, then this was also included in the analysis.
B.What are the findings?
Conclusions
Even though the objectives of different pieces of legislation within the scope of this Fitness Check are not always identical, the legal acts are generally coherent in how they attempt to reach the stated objectives, as illustrated by the use of similar underpinning legal mechanisms to do so.
The focus of the Cosmetic Products Regulation solely on human health aspects was identified as a legal gap by NGO stakeholders. While it may impact consumer ability to differentiate between products in terms of their environmental performance (due to the lack of labelling requirements on environmental hazards) and, therefore, to make better informed purchases, in principle, any potential environmental risks arising from cosmetic ingredients are addressed under REACH, for example, via authorisations or restrictions.
While many of the pieces of the legislation within the scope of this Fitness Check are underpinned by all three core policy objectives e.g. the CLP, the Biocidal Products Regulation, the Plant Protection Products Regulation, the Detergents Regulation, a number of others are underpinned by only one or two of them, for example (see also Annex 4 Table 1):
·Human health and the environment: the Seveso III Directive;
·The internal market and human health: the Cosmetic Products Regulation;
·The internal market and the environment: the Packaging and Packing Waste Directive;
·The environment: the Urban Waste Water Directive;
·Human health: the Drinking Water Directive, the Occupation Safety and Health (OSH) legislation (the Carcinogens and Mutagens at Work, the Chemical Agents and the Asbestos Directives);
·The internal market: the Fertilizers Regulation.
Moreover, some pieces of legislation have very specific objectives e.g. establish measures for the protection of animals used for scientific or educational purposes (the Laboratory Animals Directive); provide for a harmonised system for study audit and inspection of laboratories (the GLP Directives); and establish a voluntary ecolabel award scheme intended to promote products with a reduced environmental impact (the Ecolabel Regulation).
These variations in the scope and nature of objectives do not, per se, point to incoherence. They simply illustrate that there is a clear delineation between different priorities depending on the scope of the legislation.
NGOs and several Member States, however, highlighted that the Cosmetic Products Regulation focuses only on human health impacts and the internal market (while the Detergents Regulation covers all three core objectives including protection of the environment). As a result risks assessments carried out for cosmetic product ingredients do not consider the intrinsic environmental hazard properties and the environmental fate and risks of cosmetic products and their ingredients across the lifecycle of the product. For example siloxanes, triclosan, synthetic fragrances and UV filters might not constitute a significant health risk for consumers, however, the cumulated amounts of individual small dosage released into the environment when cosmetic products are washed off can be high and thus constitute a risk for the environment. The available evidence and stakeholder inputs to this Fitness Check were insufficient to identify the contributing factors and determine the significance of this gap in practice. It should, however, be noted that the environmental risks of substances used in cosmetic products should, in principle be addressed by REACH. For example, as of 2020 the placing on the market of siloxane substances D4 and D5 will be restricted for wash-off cosmetic products. REACH already restricts the use of nonylphenol, used as a surfactant, in cosmetic products. To date, 9 substances used in cosmetics have been identified under REACH as endocrine disruptors with adverse effects to the environment. However, the lack of labelling requirements for cosmetic products relating to environmental hazards impacts the ability of consumers to differentiate between products in terms of their environmental performance and make better informed purchases.
While the different pieces of legislation within the scope of this Fitness Check try to reach sometimes different objectives, the hazard and risk assessment and risk decision making procedures and mechanisms stipulated under the different pieces of EU chemicals legislation, are broadly consistent but do vary to some degree. Much of this variation is in line with the different scopes, focus, and objectives of the legislation in question and does not represent a framework-wide inconsistency. However, some variations pointed to a degree of incoherence.
Industry holds the main responsibility for generating the data necessary for required hazard and risk assessment of chemicals. In some instances, however, data is also generated at Member State level (under the Water Framework Directive and the Marine Strategy Framework Directive for example) as well as at the EU level (under the Water Framework Directive to set a list of priority substances, under the Industrial Emissions Directive for the revision of the Best Available Technique Reference Documents (BREFs) as well as by ECHA and the existing Scientific Committees).
There are considerable variations in the data requirements (for hazard and risk assessment) specified by the different pieces of EU chemicals legislation. While the lower data requirements come at a cost of potentially missing some hazardous properties and impacting human health and environmental protection as a consequence, these differences can, for the most part, be explained and justified on the grounds of differing likelihood of exposures (risks), of costs and proportionality and of laboratory animal welfare considerations.
Regarding information and testing requirements for CMRs, PBTs/vPvBs and EDs, differences in approaches to gathering data have been noted but they do not seem to lead to incoherence issues. In general, data requirements need to be systematically updated in order to ensure risk assessments and risk management decisions are being made based on the latest scientific knowledge and technology. This includes, for example, applying new or revised chemical test methods and guidelines. Where legislation is slow to adopt new or updated test guidelines, this can lead to a lack of systematically developed data and therefore affect the ability of risk assessment processes to reach robust conclusions.
In terms of data quality requirements, consistency is supported by the Good Laboratory Practice (GLP) Directives which were introduced to ensure integrity and quality of laboratory testing and studies. However, the exact wording of the data quality requirements often deviates between regulations (referring either to GLP, to the GLP Directives, or to the OECD principles). This can cause confusion for duty holders. However, Member States and industry stakeholders indicated that it is clear what types of hazard and risk assessment data need to be provided under the different pieces of legislation and, in general, how their quality and completeness will be assessed. In addition, some outdated or inconsistent provisions in the GLP Directives have been identified. This includes the undefined role of the EU chemicals agencies and a lack of clarity on how to treat GLP data from non-OECD/Mutual Acceptance of Data (MAD) countries, on the exact scope of the definition of chemicals and data quality requirement for physical hazard testing under the CLP Regulation.
The following views were expressed by different groups of stakeholders:
·Industry stakeholders and Member State stated that, in general, they find the data requirements to be coherent and clear.
·Animal rights organisations drew attention to the ban on animal testing under the Cosmetic Products Regulation and the fact that cosmetics ingredients can be subject to different data generation requirements. While for human endpoints all new data for cosmetics ingredients has to be developed using non-animal test methods to meet the requirements of the Cosmetic Products Regulation, for the cosmetics ingredients that are also used in other products or applications, animal testing may still be required under other regulations such as the Plant Protection Products Regulation, the Biocidal Products Regulation or REACH.
·All stakeholders were of the opinion that a greater harmonisation of data requirements would help ensure consistency, in particular for EDs. A number of stakeholders highlighted a lack of coverage in data requirements for a number of human health endpoints (e.g. sensitisers, EDs and immunotoxic and neurotoxic) in risk assessment processes across chemicals legislation.
During the public consultation, industry associations and companies as well as civil society representatives were of the opinion that some aspects of the EU chemicals legislation framework are internally inconsistent. Citizens and public authorities remained neutral (neither agreed nor disagreed) while a third of public authorities respondents also considered the EU chemicals legislation to be internally inconsistent. A more in-depth analysis based on further comments and position papers received by the Commission shows, however, that although such issues were indeed identified, they most often affect specific aspects of functioning of some pieces of legislation within the scope of this FC while not necessarily being relevant to the functioning of the overall framework. Therefore, the opinion that the EU chemicals legislation is internally inconsistent needs to be nuanced and taken with caution given also that the share of opinions neither agreeing nor disagreeing was significant. Moreover, these views are also in contrast to the generally positive opinions expressed by SMEs (SME Panel) on the overall internal coherence of the EU chemicals legislation.
7.2Evaluation question: what, if any, are the inconsistencies, contradictions, unnecessary duplication, overlap or missing links between different pieces of legislation? Are these leading to unintended results?
A.What’s the issue?
The coherence of hazard and risk assessment processes was assessed in terms of:
·the consistency of hazard identification via the CLP;
·whether all the relevant hazard classes are covered;
·whether legal criteria have been established for identification of all the relevant substances of potential concern;
·whether there are differences in classification criteria and approaches that could impact the hazard/risk assessment and risk management procedures;
·how the current legal provisions take into account vulnerable groups; and
·how the current legal provisions take into account risks posed by substances of specific concern.
Annex 7 provides more detailed assessment of the coherence of hazard/risk assessment and risk management procedures when dealing with specific substances such as carcinogenic, mutagenic or toxic to reproduction (CMRs), persistent, bio-accumulative, toxic and very persistent and very bio-accumulative substances (PBTs/vPvBs) and endocrine disrupting chemicals (EDs).
B.What are the findings?
Conclusions
The CLP Regulation ensures the coherence of chemical hazard assessment and classification at the EU level is in line with what is done at the international level (through the UN Global Harmonised System (GHS)). It acts as a horizontal reference point for great majority of the EU chemicals and chemicals-related legislation, thus ensuring a high degree of consistency of chemical hazard identification and classification. Regarding PBTs/vPvBs and terrestrial toxicity, which are currently not defined as separate hazard classes under the CLP or the GHS, the potential additional benefits of introducing these as new hazard classes under the CLP need to be further assessed.
Regarding EDs, the recently adopted Communication on endocrine disruptors announced further actions to ensure that citizens and the environment are protected from exposure to endocrine disruptors. Inter alia, the Communication announces that the Commission will launch a cross-sectoral Fitness Check to assess whether relevant EU legislation on endocrine disruptors delivers against this overall objective.
The current approach to allergens lacks coherence with respect to the provision of consumer information and to the assessment of risks to human health. It also creates overlaps in terms of labelling obligations.
There is no overarching approach to risk assessment for vulnerable groups. Reference to vulnerable groups is not systematic across the legislation and risks to these groups are not always addressed in a consistent manner across product/risk/sector specific legislation. Where such legal provisions do exist, risks are taken into consideration on a case-by-case basis with differences in definition and wording used. This could lead to different levels of protection for the same vulnerable group (e.g. children) between different pieces of legislation.
1)Hazard identification
In the EU, chemical hazard identification, assessment and classification is governed primarily by the CLP Regulation, which sets the criteria for a fairly comprehensive set of hazard classes. CLP hazard classification is the basis for chemical hazard classification in most other pieces of EU chemicals legislation. Furthermore, since the CLP is aligned to the UN Global Harmonised System (GHS), it also helps ensure coherence at international level.
During the public consultation, respondents were asked if the hazard classes in the CLP Regulation for environmental, physical and human health risks cover all relevant hazards (the views are summarized in
Table 6
). While there was a clear ‘yes’ response from industry associations and companies, the most common reply from civil society representatives was ‘no’. Public authorities responded mainly ‘yes’ regarding human health risks. Regarding environmental risks, while the predominant reply was ‘yes’, one third of respondents disagreed. Responses from citizens were mostly ‘don’t know’.
|
Do the hazard classes in the CLP Regulation cover all relevant hazards?
Predominant replies to the public consultation (question 29)
|
|
|
Environmental risks
|
Physical risks
|
Human health risks
|
|
Citizens
|
‘I don’t know’ (45%)
|
‘Yes’ (45%)
‘I don’t know’ (45%)
|
‘I don’t know’ (45%)
|
|
Industry
|
‘Yes’ (82%)
|
‘Yes’ (85%)
|
‘Yes’ (86%)
|
|
Publics authorities
|
‘Yes’ (44%)
‘No’ (34%)
|
‘Yes’ (71%)
|
‘Yes’ (63%)
|
|
NGOs and other civil society organizations
|
‘No’ (56%)
|
‘Yes’ (70%)
|
‘No’ (53%)
|
Table 6 Extent to which respondents agreed that all relevant hazards are covered: replies from the public consultation
Under some pieces of legislation and for one or two particular hazard classes, there are elements of hazard identification that are not directly linked to the hazard classes and criteria prescribed by the CLP Regulation. Examples include the hazard classes of persistent, bio-accumulative, toxic (PBT) and very persistent and very bio-accumulative substances (vPvB), terrestrial toxicity and endocrine disruptors.
a)PBTs/vPvBs
Criteria for PBT/vPvB hazard identification are stipulated in REACH Annex XIII. The Plant Protection Products Regulation adopted the criteria set out in REACH Annex XIII before its revision in 2011, whilst the Biocidal Products Regulation refers directly to REACH criteria and, therefore, remains consistent with latest updates to REACH Annex XIII. This creates a potential for inconsistent PBT/vPvB hazard determinations between the two regulations. To date, only one known case of an inconsistent PBT determination has arisen and it concerns the substance acetamiprid. This was not identified as 'Persistent' under the Plant Protection Products Regulation and therefore re-approved for 15 years. However, it was identified and classed as 'very persistent' under the Biocidal Products Regulation. Being also ‘toxic, it was identified as a candidate for substitution and only approved for 7 years.
Under REACH, the obligation to perform a chemical safety assessment applies only to substances placed on the market in quantities of 10 tonnes or more per year. This means that, for instance, some substances with PBT/vPvB properties may potentially be missed. If harmonised PBT/vPvB criteria were established under the CLP Regulation, it would result in the obligation to classify and label substances fulfilling these criteria before placing them on the market regardless the tonnage. A proposal was made by the EU in 2009 to include PBT/vPvB hazard classes and criteria in the UN GHS which would then be reflected in CLP. However, the UN GHS expert sub-committee concluded that the existing hazard classes i.e. hazardous to aquatic environment, would capture any substances with PBT or vPvB hazard properties and ensure that they are appropriately classified and labelled. There is also the option to create additional hazard classes for PBT/vPvB directly within the CLP that would apply only within the EU. However, the potential benefits of introducing this new hazard class under the CLP Regulation needs to be further assessed. The CLP Regulation (in line with the UN GHS Environmental hazards ‘building block’) covers only aquatic toxicity. As is the case for PBTs/vPvBs, in order for the CLP to include criteria for terrestrial toxicity, it would either require amending the UN GHS or the EU amending the CLP without changes made at the UN GHS level. An attempt to include a terrestrial toxicity hazard class within the UN GHS was made in 2006 by Spain but did not receive the support of the relevant UN GHS Sub-committee of experts.
The fact that the CLP does not contain harmonised criteria for terrestrial toxicity does not mean that these hazards are not identified and assessed. In principle, registrants under REACH are required to consider whether or not their substance might present a risk to the terrestrial compartment and, if so, to include this in the risk assessment done for substances which are placed on the market in quantities exceeding 10 tons per year. In practice, however, the lack of a defined hazard class under the CLP combined with the challenge for ECHA of checking the veracity of many thousands of registration entries under REACH means there is a potential for chemicals that are toxic to the terrestrial environment to be overlooked. Terrestrial toxicity is, however, explicitly and carefully addressed under the Plant Protection Products and the Biocidal Products Regulations and to some extent, by the Industrial Emissions Directive and the Seveso III Directive. Further evidence needs to be gathered to determine the extent and significance of these potential gaps in chemical hazard identification and classification.
b)EDs
The legislative measures constituting the EU legal framework regulating chemicals have been developed at different points in time and have, in certain cases, different objectives.
·Under the Plant Protection Products Regulation and the Biocidal Products Regulation, the Commission set similar scientific criteria for the determination of endocrine-disrupting properties in 2017 and 2018, respectively. A common ECHA/EFSA guidance document drafted with support from the Joint Research Centre (JRC) has been established for the identification of endocrine disruptors in the context of these Regulations.
·Several other pieces of legislation contain provisions on how to address endocrine disrupors, such as the legislation on chemicals in general (REACH), medical devices related legislation and water-related legislation. Requirements vary depending on the specific legislation.
·Food contact materials legislation, the Cosmetic Products Regulation, the Toy Safety Directive and the occupational safety and health (OSH) legislation do not contain specific provisions for endocrine disruptors. This does not, however, prevent the identification and assessment of substances with endocrine disrupting properties on case-by-case basis.
This has resulted in different approaches to endocrine disruptors and has raised questions about the level of coherence of the EU legal framework in terms of regulating endocrine disruptors.. The absence of horizontal criteria for the identification and classification of EDs has also been criticized by a number of different stakeholder groups including both NGOs and industry, as well as national authorities and was identified as an area for action in the EU's 7th Environment Action Programme.
The Commission considers that there should be a coherent approach to the identification of endocrine disruptors across all relevant Union legislation, based on the broadly accepted definition of the World Health Organisation. The recently established criteria for pesticides and biocides constitute a first step in that direction but EU legislation in other fields does not yet contain such criteria. However, since no single regulatory evaluation completed to date has covered all the different vertical and horizontal aspects of addressing endocrine disruptors in EU chemicals legislation, the Commission has committed to undertaking a cross-cutting assessment of the current situation. This will assess whether relevant EU legislation on endocrine disruptors delivers against the objectives to protect human health and the environment by minimising exposures to these substances. It will also pay particular attention to those areas where legislation does not contain specific provisions for endocrine disruptors, such as toys, cosmetics and food contact materials. In addition, attention will be paid to the consistency and intensity of actions to protect vulnerable population groups that are particularly sensitive to endocrine disruptors, such as the foetus or adolescents.
2)Risk management
a)Of the known adverse effects on human health and the environment
The majority of currently known adverse effects on human health and the environment are covered. However, some inconsistencies occur regarding risk management decisions for EDs, PTBs/vPvBs and substances fulfilling the classification criteria for Specific Target Organ Toxicity (STOT). Regarding neurotoxicity, immunotoxicity and allergens, further evidence needs to be gathered to determine the extent and significance of these potential gaps in chemical risk management.
I.CMRs
Approaches to the risk management of CMRs are generally coherent, as they are in principle prohibited for use in professional and consumer products based on harmonised classifications under the CLP Regulation. One issue, however, is that of non-threshold CMRs i.e. where a no-effect level (a defined exposure level below which no health impact can be detected or observed) cannot be established. Since, by definition, a non-threshold CMR creates a potential risk at any level of exposure, it becomes important to define what the acceptable level of risk is. In accordance with the conclusion of the REACH Review, there is currently no consensus within the EU on defining the acceptable level of risk.
II.EDs
Under the Biocidal and the Plant Protection Products Regulations, EDs are given the same priority as CMRs cat. 1 and are subjected to generic risk considerations, i.e. they should not be approved except if negligible risk from exposure or negligible exposure to the substance can be demonstrated or if specific derogations apply. Under the Biocidal Products Regulation EDs are also automatically banned from use in consumer products on the basis of generic risk considerations. Other legislation, such as that on food contact materials, cosmetics, toys or protecting workers at the workplace, does not contain specific provisions for EDs. However, substances with ED properties are subject to case-by-case regulatory action on the basis of the general requirements of the legislation.
NGOs and civil society representatives, as well as some Member State authorities consider the regulatory action taken so far to be inadequate, and have called for stricter and broader EU measures. The issue is recognised in the Commission’s recently adopted Communication on endocrine disruptors which underlines that the EU strategic approach on EDs for the years to come should be based on the application of the precautionary principle and aim (amongst others) at minimising overall exposure of humans and the environment to endocrine disruptors, paying particular attention to exposures during important periods of development of an organism, such as foetal development and puberty.
III.PBTs/vPvBs
Substances that are identified as PBTs/vPvBs can be addressed under REACH, the Plant Protection Products and the Biocidal Products Regulations, and the Water Framework Directive. However, there are some inconsistencies regarding how these pieces of legislation:
·Take into account socio-economic aspects: Under REACH and the Biocidal Products Regulation, in contrast to the Plant Protection Product Regulation, a socio-economic analysis, including an analysis of alternatives, is also required as an input to the risk management decision process (e.g. for authorisations, restrictions, etc.). The cost-effectiveness and proportionality of measures are to be taken into account under the Water Framework Directive, when taking decisions on measures against the pollution of water for the priority substances (Annex X) some of which are PBT/vPvBs.
·Apply (or do not apply) exclusion criteria:
oComparing the Plant Protection Product Regulation and the Biocidal Products Regulation, the main difference lies in the possibility to obtain a derogation from the automatic ban for the use of active substances identified as PBTs/vPvBs. Under the Biocidal Products Regulation, their use in products is prohibited unless conditions for derogation are met (negligible risk, essential to control serious danger for human/animal/environmental health, disproportionate negative impact on society when compared to the risks; availability of alternatives is also considered). Under the Plant Protection Product Regulation, active substances identified as PBTs/vPvBs cannot be approved and there is no possibility to obtain a derogation.
oSubstances used in cosmetics, food contact material, toys and medical devices are regulated as regards PBT/vPvB-properties under REACH. Such substances can be restricted if there is an unacceptable risk to the environment arising from their use in these product types. If a PBT/vPvB-substance is added to the authorisation list under REACH, an authorisation to use them can only be granted if the socio-economic benefits outweigh their risk and if no alternatives are available.
Some inconsistencies were identified with respect to the legislation covering medicinal products for human use and veterinary medicinal products. An assessment of PBTs/vPvBs properties of the emissions into the environment from veterinary medicinal products, while not mandatory, can still be performed on the basis of various guidance documents. If risks linked to PBTs/vPvBs properties of a substance are identified, it is not clear what impact this will have, if any, on the authorisation of the veterinary medicinal products that include the substance. For medicinal products for human use, the outcome of the environmental risk assessment (e.g. the PBTs/vPvBs assessment) is not considered in the benefit/risk analysis, and as such it does not serve as a basis for refusal of the marketing authorisation (see Annex 7 Section 7.2.5). The European Commission adopted recently an EU strategic approach to pharmaceuticals in the environment. The actions announced include considering the findings of this and recent REACH Review as regards links with the medicinal products legislation in relation to environmental protection. This could, among other things, help to clarify the PBT/vPvB requirements. Expanding environmental monitoring and knowing more about the concentrations of pharmaceuticals in the environment would allow environmental risk assessments to be improved and measures to be more focused.
IV.STOTs (Single Target Organ Toxicity substances)
Under the Biocidal Products Regulation, substances classified as STOTs under the CLP Regulation are subject to risk management measures based on generic risk considerations (i.e. automatically prohibited from use by the general public). This, however, is not the case under the Plant Protection Products Regulation. There are no equivalent provisions under other product specific legislation within the scope of this Fitness Check.
Regarding the occupational safety and health (OSH) legislation, in principle, risks to the safety and health of workers arising from any chemical agent - even those not classified as hazardous under the CLP Regulation but which potentially pose an occupational health or safety risk - needs to be assessed by the employer. In the case of activities involving the potential exposure to several different hazardous chemical agents, the combined risk of these exposures should also be assessed. In both instances, hazards and risks relating to single target organ toxicity can be addressed where this is identified as a potential source of risk. These risk assessments then constitute the basis for taking preventive risk management measures at workplace.
V.Neurotoxicity and immunotoxicity
NGOs and some Member State authorities pointed out a potential gap for some "new emerging endpoints" e.g. neurotoxicity, immunotoxicity. These hazard aspects present health and environmental risks of a similar level of concern to those associated with CMRs, PBTs/vPvBs and EDs but are not always explicitly addressed by the EU framework of chemicals legislation e.g. these do not consitute a hazard class under the CLP Regulation.
In principle, neurotoxicity can be addressed via the STOT hazard class under CLP and via related pieces of legislation such as REACH, the Biocidal Product Regulation and the Plant Protection Products Regulation. However, in practice, expert stakeholders indicated that testing for neurotoxicity is rarely undertaken despite the availability of internationally recognised test methods.
With respect to immunotoxicity, there are currently no internationally recognised test methods to identify substances with this hazard characteristic. It requires further research and development for legislation to be able to address the potential adverse effects on human health.
VI.Allergens
There is currently no common definition of what constitutes an allergen, i.e. a substance that may cause allergic reaction. The CLP Regulation sets out the hazard classification criteria and requirements for respiratory and skin sensitisation. The Cosmetic Products Regulation does not rely on the CLP classification criteria for the identification of skin or respiratory sensitizers. Instead, it simply refers to substances that can cause allergic reaction. Such substances are identified by the Scientific Committee on Consumer Safety (SCCS). While there is no specific list of allergens under the Cosmetics Regulation, substances which can cause allergic reaction can be restricted, used only under certain specific conditions or banned. References to fragrance allergens regulated by the Cosmetics Regulation are also included in the Detergents Regulation and the Toy Safety Directive. There are no specific legal provisions for allergens other than fragrances under these two pieces of legislation.
There are differences in the number of allergens that are regulated under different pieces of legislation; this may be appropriate given the different scopes of the legislation, but reasons for the differences are not clear.
The lack of a harmonised approach to allergens is considered by a number of stakeholders to have negative implications for the single market, competitiveness and innovation, and for ensuring a high level of protection of human health. It also impacts the communication of chemical hazards to consumers and their ability to make informed purchases. Regarding toys in particular, a number of stakeholders have noted that allergens are an issue too softly regulated under the Toy Safety Directive and suggested that other allergens that are not specifically fragrance allergens should also be regulated.
There are legislative overlaps between the Detergents Regulation and the CLP Regulation with regard to the labelling of allergens. According to AISE and other consultees, multiple regulations dealing with the labelling of detergents products (the CLP, the Cosmetic Products Regulation and the Detergents Regulation) create unnecessary regulatory burden and there is a clear opportunity for streamlining labelling requirements.
b)For vulnerable groups
The analysis shows that not all pieces of legislation within the scope of this FC take into account risks to vulnerable groups. Where such risks are taken into consideration, the definition of vulnerable populations covered varies as there is no horizontally applicable definition of 'vulnerable group'. This means that risks for such groups are addressed on case-by-case basis through product/risk/sector specific legislation taking into consideration circumstances, products or environments of chemical exposure that could lead to different level of protection across the legislation. The following pieces of legislation (non-exhaustive) refer to 'vulnerable groups':
·The Toy Safety Directive (children under 14 years of age);
·The Occupation Safety and Health (OSH) legislation (young workers; pregnant workers);
·The Plant Protection Products Regulation (pregnant and nursing women, the unborn, infants and children, the elderly and workers and residents subject to high pesticide exposure over the long term);
·The Residues of Pesticides Regulation (children and the unborn, vulnerable consumers);
·The Biocidal Products Regulation (pregnant and nursing women, the unborn, infants and children, the elderly and, when subject to high exposure to biocidal products over the long term, workers and residents);
·The Regulation on Food Additives (not mentioning 'vulnerable groups' as such but prohibiting the use of food additives in foods for infants and young children).
·The Cosmetic Products Regulation (specific assessment for cosmetic products intended for use on children under the age of three, and particular attention is to be paid to the microbiological specifications of cosmetic products intended to be used on children under three years of age, on elderly people or on persons with compromised immune responses; for derogation to CMR 1A/B ban, particular account to be taken of vulnerable population groups by the Scientific Committee on Consumer Safety (SCCS) in its evaluation).
NGO stakeholders indicated that clear definitions of children and vulnerable groups are missing in most pieces of the EU chemicals legislation and therefore the current approach is not consistent. They also indicated that the notion of 'vulnerable groups' should be broader and include other categories such as citizens with low income and/or socio-economic status. Moreover, NGOs highlighted the fact that in some cases risk management measures taken under one piece of legislation to protect a vulnerable group are not complemented under another where the exposure could be similar. For example, certain phthalates are banned in the use of toys under REACH while they are allowed in other products such as carpets, textiles or furniture to which children can be exposed to.
Such differences can be partly explained by differences in legal scope. For example, workers safety legislation, by definition, will not apply to children as the child labour is prohibited while the Toy Safety Directive in principle will not cover risks posed to adults. However, it is not clear:
·Why the risk assessment carried out under the Cosmetic Products Regulation take into account exposure of children under three years of age to substances classified as CMR 1A and 1B while the Toy Safety Directive also covers risks to children from CMRs of category 2.
·Why the Plant Protection Products Regulation and the Biocidal Products Regulation take into consideration pregnant and nursing women, and the unborn while the Pregnant Workers Directive only covers risks to pregnant worker herself but excludes consideration of risks to the unborn child.
The recently adopted Communication ‘Towards a comprehensive European Union framework on endocrine disruptors’ highlights the fact that particular attention needs to be paid to the consistency and intensity of actions to protect vulnerable population groups that are particularly sensitive to endocrine disruptors, such as the foetus or adolescents. In this regard, it can be noted that the current legal provisions do not specifically identify adolescents as ‘vulnerable group’ (except under the Young People at Work Directive).
The main potential implications are during the risk assessment step and with potential knock on consequences for the decisions on risk management measures. However, the assessment done for the purposes of this Fitness Check did not come to a conclusion on the extent of the issue and if, in practice, risks to vulnerable populations are not sufficiently well addressed and managed because of these legislative gaps and inconsistencies.
8RELEVANCE
8.1Evaluation question: to what extent do the objectives of the legislative framework for chemicals meet the current needs?
In this section the relevance of the policy objectives, as well as the risk management approaches of the EU chemicals legislation is assessed. The analysis examines whether there are any mismatches between the existing chemicals policy objectives and the current situation. It revisits the underlying drivers and assumptions that were considered when designing and implementing the various components of the EU chemicals acquis to assess whether or not these remain valid and whether new drivers have emerged that have not yet been accommodated.
8.1.1Do the original needs still exist or are parts of the chemicals legislative framework now redundant?
A.What's the issue?
The relevance of the legislative framework is evaluated here in terms of whether or not the issues and needs that triggered the introduction of the legislation still exist and are still relevant. In order to answer this question, it is necessary to link it to the original objectives and their alignment with the priorities that have emerged progressively since the adoption of the different pieces of EU chemicals legislation.
B.What are the findings?
Conclusions
The original needs in terms of protecting human health and the environment from the risks of hazardous chemicals, of enhancing the functioning of the internal market and of promoting innovation and competitiveness continue to exist. As such, the three core objectives of the EU chemicals legislation continue to be relevant. The basic components and approaches applied within the EU chemicals acquis to assess and manage the hazards and risks of chemicals also remain relevant.
1)Ensuring a high level of protection of human health and the environment
A recent assessment of the cumulative health and environmental benefits of chemicals legislation identified the continued need for risk assessment and risk management measures in order to protect human health and the environment from exposures to hazardous chemicals. Taking into account the growing volumes and complexity (number, structure/composition, form (e.g. nanomaterials), etc.) of chemicals and supply chains as well as the ever increasing number of products and uses that involve chemicals, identifying and managing the risks of hazardous chemical exposures to humans and the environment remain highly relevant. A significant share of the volume of chemicals produced are classified as hazardous to human health (+60%) and/or the environment (+40%). This share has largely remained the same over the last decade. Furthermore, the imports of consumer goods and other articles into the EU have tripled between 2000 and 2015 creating additional challenges for managing the risks associated with the presence of hazardous substances in articles.
There was broad consensus amongst stakeholders that the basic components and approaches of the EU chemicals acquis remain appropriate and relevant in reaching environmental and health objectives. It was pointed out a number of times by different stakeholders that the EU approach to identifying and managing chemical risks is considered a benchmark by other countries and regions in the world.
2)Ensuring the functioning of the Single Market and enhancing the competitiveness and innovation of EU industry
The EU chemicals acquis is still relevant regarding its basic goals of enhancing the functioning of the internal market and of promoting the competitiveness and innovation of the EU chemicals sector and related downstream sectors. Horizontal rules on basic information, packaging and health and environmental safety are prerequisites for a well-functioning and transparent market, including relatively equal (reciprocal) access to information, fair and equal competition and informed consumer choices.
The legislation in the scope of this Fitness Check has facilitated intra-EU trade (i.e. EU companies selling in the EU single market rather than only in their home country market) through the harmonisation of regulatory requirements as evidenced by the increase in the share of intra-EU trade of total EU chemicals sales.
In terms of international competitiveness, in 2016 the EU chemical industry represented 15.1% of the global market, behind China (39.6%) but ahead of the United States (14.2%). Although the European share of global sales has decreased (it was 32.5% in 1996), the EU chemicals industry remains internationally competitive as evidenced by the increase in exports to non-EU countries (around EUR 100 billion in 2006 and EUR 146.2 billion in 2016). Moreover, the EU is the largest chemicals exporting market in the world.
The EU is frequently cited as a global leader in terms of the development and implementation of chemicals policy. Where the EU acts on restricting the use of hazardous chemicals, other countries and regions often follow. Therefore, the potential for the EU chemicals legislation to act as a driver of innovation in the chemicals sector and related downstream sectors remains relevant, particularly in view of the EU commitment to achieving the UN Sustainable Development Goals (SDGs) and the United Nations Strategic Approach to Chemicals Management (SAICM) objective of shifting towards a more sustainable design and use of chemicals. Another illustration of this is in controls on the use of persistent organic pollutants (POPs), where international action has frequently followed on from EU action.
8.1.2Have new needs emerged in relation to the health and environmental risk management of chemicals? If yes, what are they?
A.What's the issue?
As science continues to evolve and new data become available regarding the links between exposures to hazardous chemicals and the impacts on human health and the environment, a number of concerns have emerged during the last 10-20 years that are still either only partially, or not at all, addressed by the existing framework of EU chemicals legislation. The most important of these are discussed below.
B.What are the findings?
Conclusions
Some gaps remain within the framework of EU chemicals legislation, namely, how to address combination effects, how to better understand and address impacts on the environment, biodiversity and eco-system resilience, and how to gather knowledge and better manage the risks related to the use of hazardous substances in articles. Concerns regarding the former have been acknowledged and steps have been taken to improve the existing methodology and risk assessment approach. Gathering knowledge about substances in articles is particularly important as the EU is in the process of shifting towards a more circular economy.
1)Combination effects
Humans and the environment may be simultaneously exposed to multiple chemicals by a single route or multiple routes, a situation referred to as ‘combined exposure’. The term 'unintentional chemical mixtures' is sometimes used to refer to the combined exposure to multiple chemicals from different sources. The adverse effects or toxicity of a combination of different substances might be more severe than, and/or different from, the individual substances involved. It is a cross-cutting issue relevant for a number of topics assessed under this Fitness Check (e.g. endocrine disruptors, substances in articles, etc.).
Effects from combined exposures are documented for a limited selection of substances in human biomonitoring studies as well as in animal studies. Several such studies indicate the occurrence of a growing number of different hazardous chemicals in human blood and body tissue, including in pregnant women and new-born infants. Chemicals identified include pesticides, biocides, pharmaceuticals, heavy metals, plasticisers and flame retardants . There are, however, still considerable knowledge gaps regarding human and environmental exposures to combinations of chemicals. In part, this reflects insufficient attention to combination effects in screening and human and environmental biomonitoring programs.
Risk assessment processes implemented within the framework of EU chemicals legislation are not expressly designed to identify and assess potential human health and environmental risks of different hazardous chemicals acting in combination. Intentional mixtures, i.e. products composed of a defined mixture of different chemical substances such as glue, paint and detergents are subject to hazard classification and risk assessment and classification under e.g. REACH and the CLP. By contrast, the combination effects of 'unintentional mixtures' formed e.g. during production processes, in the products themselves, in the human body or in the environment are often complex, varying and unknown and therefore currently difficult to risk assess. Moreover, the potentially high number of combinations of chemicals implies that actual physical testing of these is less feasible.
Estimation of effects of typical combined exposure to a known selection of chemicals in the working environment for a particular occupational group is generally easier to achieve and very relevant due to the high levels of exposure. However, workers are also citizens and consumers who can also be exposed to hazardous chemicals through other routes outside of the workplace e.g. food, drinking water and the external environment. While being an important reality these exposure factors are currently not easy to factor in.
The issue and risk assessment challenge of combination effects is recognised by the Commission. Efforts are underway to better address this issue. An example is EFSA's development of combination effect assessment methods and guidance for pesticides, based on Cumulative Assessment Groups (CAGs). This identifies compounds that exhibit similar toxicological properties in a specific organ or system, assuming that pesticides causing the same toxic effects in tissues, organs and physiological systems can produce joint, cumulative toxicity, even if they do not have similar modes of action. EFSA published recently a Guidance document describing harmonised risk assessment methodologies for combined exposure to multiple chemicals for all relevant areas within EFSA's remit, i.e. human health, animal health and ecological areas.
2)Impacts on environment, biodiversity and eco-system resilience
Impacts of hazardous chemicals on biodiversity and eco-systems contribute, together with other stressors, to the reduction of 'eco-system resilience' i.e. the ability to resist damage and to recover. This can lead to rapid declines in animal populations (see Annex 5 Sections 5.1.1 B) and 5.1.1 C)) and, ultimately, to extinctions. Other consequences include sub-lethal effects such as reductions of fertility, impaired feeding patterns and lost ability of orientation which, over time, lead to the weakening of populations. Publications found that exposures to hazardous chemicals, in particular pesticides, in combination with other factors, are leading to significant reduction of insect populations with effects in the food chain, in particular for bird populations, many of which are in decline in the EU.
Although the potential of some hazardous chemicals to cause harm is recognised and considered in the regulatory context, their role in the complex interaction with other environmental stressors and the actual contribution – compared to the other stressors – to the effects seen in the environment is less well understood. Current standard test and assessment methods typically do not focus on these long term, large scale and complex environmental effects.
3)Substances in articles and Circular Economy aspects
Hazardous substances are included in articles and may be released at any lifecycle stage, resulting in exposures and potential risks for humans and for the environment. This is true for articles newly produced or already placed on the market. Access to information on the chemical content of articles is, therefore, important for risk management across all stages of the product lifecycle, including its end-of-life and for potential recovery into secondary raw material cycles, as well as for appropriate labelling and informed consumer choices.
There is a general lack of information about the presence of hazardous substances in articles e.g. the possible presence of chemicals of concern such as flame retardants in plastics used in construction, automotive, aviation, furniture and electronics applications. This lack of information renders it difficult for:
·Regulators to carry out overall risks assessment, determine the scale of risks, and to choose regulatory risk management measures.
·Economic operators and consumers to make well-informed purchasing decision about articles containing or not hazardous substances.
·Waste treatment operators to separate and treat end-of-life articles in a manner that prevents contamination of recycled materials.
Moreover, new chemicals are continuously placed on the market whilst others are forbidden or gradually phased out when it is discovered that they pose a risk. This means that products legally produced today may contain a substance that later on may be banned. When the product becomes waste and is then recovered, the banned substance may still be contained in the recovered material as so-called legacy substance. For example, certain brominated flame retardants that are persistent, bio-accumulative and toxic have been reportedly found in recycled plastic products including toys and kitchen utensils.
Furthermore, an assessment conducted by ECHA across 27 Member States and reported in 2018 found a considerable number of non-compliance cases with existing rules on hazardous chemical restrictions or bans in articles. The non-compliance rate was higher for articles 'of unknown origin' and for articles originating from China.
An existing horizontal approach to information on chemicals content in articles is based on REACH requirements for the notification and provision of information on the content of substances of very high concern (SVHCs) to professional users and consumers (Article 7 and 33 respectively). Provisions also exist in the Biocidal Products Regulation concerning the labelling of articles treated with biocides. Communication of information to consumers (article 58(3), (4), (5) and (6) of the Biocidal Products Regulation) is done in a similar way to REACH. In parallel to these legal requirements, there are a range of voluntary business-driven initiatives to manage and make available information on chemicals in articles mainly aimed at supply chain communication. However, the coverage of article types and businesses is still limited.
Civil organisations and NGOs, as well as some Member State authorities have identified the lack of chemical safety criteria in the General Product Safety Directive (GPSD) and consider this to be a major gap within the horizontal legislative framework for consumer products. Examples of categories of articles which are not covered by any specific EU product legislation addressing chemical exposure include materials in contact with drinking water, construction materials/products, furniture, clothing and textiles, child care articles and sports and playground equipment and surfaces.
The GPSD, however, was not designed to set out specific chemical safety criteria but to manage the risks of products in general. According to the GPSD, all articles ('consumer products') placed on the market, must be safe and comply with its provisions. In areas where no EU legislation or standards exist, the compliance with the GPSD safety requirement is determined according to other reference points such as national standards, Commission recommendations, codes of practice, etc. When measures are taken against unsafe products found on the market, national market surveillance authorities notify this to other Member States and the Commission through the Rapid Alert System for dangerous non-food products (RAPEX).
As the EU shifts towards a more circular economy, one of the commitments of the Circular Economy Action Plan in 2015 was to develop a strategic approach on chemicals in the circular economy. It covers assessing and addressing the health and environmental risk aspects associated with information about substances in articles as the recycling and re-use of products and materials in general will become increasingly relevant.
The scale of the problem is significant. The Commission has recognised the issue and proposed a number of priority areas and options for action as a part of its work on the interface between chemicals, waste and product policies. This includes accelerating work to identify possible ways to make chemicals easier to trace in recycled streams. In relation to this, the recently revised Waste Framework Directive provides the legal basis for the establishment of an ECHA-managed database on the presence of SVHCs in consumer goods ('articles') with access provided to waste treatment operators as well as consumers upon request.
The inclusion of circular economy considerations into chemicals risk management will require a transformation of the life cycle stages and timescales considered in risk assessment, with assessors needing to consider not only the ‘first’ life of a product, but also the ‘second’, ‘third’ and all potential future lives, moving to a new form of life-cycle assessment. In order to adapt hazard and exposure scenario assessments accordingly, more information and data will need to be gathered on substance uses and releases from articles, which are currently often not available.
8.2Evaluation question: to what extent does the current legislative framework for chemicals take into account health, environmental, social and economic consequences that are relevant to citizens and stakeholders?
The ability of the EU chemicals acquis to remain relevant and fit for purpose is dependent, among other things, on the ability of EU and Member State policy makers to take into account and address the concerns and issues raised by different stakeholders in a balanced, open and well-justified manner. A number of requirements and processes for ensuring proper stakeholder engagement are built into both the overall EU regulatory system, such as the Better Regulation programme, as well as into individual pieces of legislation. This includes active communication to citizens and other stakeholders about the hazards and risks of chemicals. This section assesses the adequacy and continuous relevance of these processes.
8.2.1Taking into account the concerns of citizens and other stakeholders
A.What's the issue?
Public and stakeholder consultation is integral to well-informed decision-making and to improving the quality of law-making. It is also vital that citizens and other stakeholders feel they can make their concerns known and that these are heard and addressed.
B.What are the findings?
Conclusions
The current EU chemicals legislation includes numerous mechanisms for ensuring that concerns of citizens and other stakeholder groups are known and addressed including health, environmental, social and economic consequences. By and large, stakeholders appreciate the level of consultation that is undertaken although some stakeholders, notably NGOs but also industry, feel that their voice, whilst heard, is not always addressed.
In the design, development, implementation and update of EU chemicals legislation, there are multiple opportunities for different stakeholders to have access to the hazard/risk assessment information and considerations and to express their views. It can be done through both formal and informal processes. This includes online public consultations, workshops, targeted stakeholder interview processes, etc., during the life of a piece of legislation, starting from ex-ante impact assessment for newly proposed (or to be revised) legislation to ex-post evaluation of existing legislation as it was done for this Fitness Check (regarding the stakeholder consultation activites carried out for the purposes of this Fitness Check please see Section 4.1.2 and Annex 2). The Commission also conducts regular citizen surveys via its Eurobarometer service e.g. the two recent surveys on citizen views of chemical safety and on the environment which included a focus on the impact of chemicals. Many of these elements are managed as an integral part of the Commission's Better Regulation programme.
Stakeholders can also provide expert input to the policy making and implementation processes through the various expert groups created by the Commission. For example, CARACAL is an expert group which advises the European Commission and ECHA on questions related to REACH and the CLP Regulations. CARACAL is composed of representatives of Member States competent authorities for REACH and the CLP, representatives from competent authorities of European Economic Area and European Free Trade Association countries as well as a number of observers from non-EU countries, international organisations and stakeholders. In a similar way, expert groups were established to ensure cooperation between Member States, stakeholders and the Commission and to ensure consistent implementation of the legislation within the EU for toys safety, detergents, cosmetics and medical devices, fertilizers and others.
These different processes help ensure that socio-economic consequences of relevance to different stakeholder groups such as impacts on businesses, especially SMEs, and consumers, increases in administrative costs, human health and environmental impacts, etc., are properly taken into account. It also helps to identify and avoid potential unintended consequences of changes in legislative requirements e.g. chemicals legislation can have an impact on recycling activities when content/concentration limits are set for a specific substance.
The following aspects were highlighted by different stakeholder groups related to their participation in the decision making process:
·NGO stakeholders expressed some frustration about the lack of transparency and ability to provide input to the risk assessment processes conducted by the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) and the Scientific Committee on Occupational Exposure Limits (SCOEL).
·Industry stakeholders reported a lack of consultation on opinions under the Scientific Committee on Consumer Safety (SCCS). They claim that the SCCS allows little to no stakeholder participation in practice while in principle the committee procedure allows to call upon stakeholders to provide further scientific information as and when required, and the potential for public consultation is available.
·The working groups under EFSA that formulate the opinions for approval of active substances have been criticised by stakeholders for their lack of stakeholder input. The main mechanisms for such input are there but they are not considered to be sufficient. It has been suggested by one stakeholder that although the procedures for scientific opinions are appropriate, because the process is not always transparent, it is not entirely clear to either industry or civil society how a decision is made.
·Member State authorities have highlighted the lack of representation of Member States in the working groups on chemicals and particularly in the SCOEL. However, the SCOEL more generally has been put forward as providing a good level of stakeholder participation in their processes even though this is not outlined in their rules of procedure in contrast to the other committees. More generally, authorities have noted that participation of stakeholders (excluding Member States) is dependent on the committee concerned.
Respondents to the public consultation were asked to identify if they thought that all relevant chemical hazard and risk assessment/management considerations are taken into account, including combined effects of chemicals, impacts on vulnerable groups, impacts on jobs and competitiveness, etc. ‘No’ was the most common reply from all groups of stakeholders. Based on the detailed comments received, the main topics considered to be insufficiently taken into account are combination effects (all stakeholder groups), jobs, competitiveness and cost-benefit and socio-economic analysis (industry stakeholders) and new science and data (citizens, industry and NGOs).
The recent OECD Regulatory Policy Outlook 2018 report ranked the Commission as one of the top performers amongst OECD countries and institutions in terms of stakeholder engagement for both primary and secondary law and for impact assessment and ex-post evaluation. The OECD used a number of parameters that included oversight and quality control, public access to information on planned consultations, comment received by stakeholders during the consultation phase or replies to consultation comments.
8.3Evaluation question: to what extent are the current procedures transparent and robust enough to enable decisions related to hazard identification, risk assessment and risk management to be relevant and evidence-based?
Key principles and objectives of the EU's Better Regulation programme include ensuring that decision-making is open and transparent, that citizens and stakeholders can contribute throughout the policy and lawmaking process and that EU actions are based on evidence and on a clear understanding of the policy/regulatory impacts.
8.3.1Transparency of procedures
A.What's the issue?
The ability of different stakeholders, including industry, NGOs, academics, experts and citizens to gain access to the data used for, and to be part of, the decision-making process especially during key stages of hazard and risk assessment/management processes is essential for the effective chemicals risk management.
B.What are the findings?
Conclusions
The general decision making process in the chemicals policy area has been continuously improved in line with the Commission's Better Regulation principles. Overall, the different stakeholders groups are satisfied with the ability to gain access to the hazard/risk assessment/management decision-making process although both industry and NGOs expressed a degree of frustration in some particular cases e.g. access to certain steps of the harmonised classification process and to the studies and data used as a basis for certain risk assessment/management decisions by the different risk assessment agencies and scientific committees.
Respondents to the public consultation were asked to indicate their level of satisfaction regarding the transparency of procedures (the overall EU legislative framework). Public authorities together with industry stakeholders were the most satisfied while NGOs and others, and citizens assigned lower scores indicating lower level of satisfaction.
During one of the workshops, participants expressed the following views:
·In general, the participants agreed that transparency has increased with, for example, the publication of meeting documents, draft opinions and opinions of committees etc. Nevertheless, this transparency may be more evident to those people who regularly deal with the assessment procedures (e.g. experts) than to those who do not (SMEs, downstream users, trade unions). As regards SME participation in the processes, the issue of language (many of the hazard/risk assessment processes are conducted soley or primarily in english) being a barrier to participation was raised, as was the issue of a lack of resources, which is also relevant to NGOs and their ability to be represented in different fora.
·Overall, expert groups were perceived as a good model for ensuring transparency, because stakeholders can participate as observers or experts. There were some concerns about the transparency of scientific committee selection processes (e.g. decisions on nominations and potential conflicts of interest).
Regarding in particular the CLP related processes:
·All stakeholder groups (industry, NGOs, government authorities, other civil society representatives, etc.) consider the Harmonised Classification and Labelling (CLH) process to be well understood.
·They also stated that the process in place up to the point when ECHA's Risk Assessment Committee (RAC) opinion (pre-regulatory phase) is issued is, in principle, transparent. However, the lack of communication between the companies providing data for the CLH dossier and the Member State authorities can result in a lack of clarity as to what information was taken into account during the decision making. This is exacerbated by the fact that the raw data/full studies underlying an opinion or CLH decision are not published.
·ECHA's efforts to further improve transparency on what stage a particular substance is at within the various regulatory processes have been well received by stakeholders.
·In contrast to the pre-regulatory and RAC processes, some stakeholders, notably industry, expressed concerns about transparency and stakeholder involvement during the risk management decision making phase i.e. after the risk assessment opinion has been issued by RAC. It should be noted, however, that this lack of transparency results, in part, from industry submitting other (e.g. socio-economic) information into the process and therefore, to some extent, unnecessery duplication of efforts.
·In addition, long time periods for arriving at the final risk management decision can lead to questions over the objectivity and predictability of the process from both industry and NGOs.
Despite considerable progress, transparency of risk analysis remains an important issue under the General Food Law (GFL) as recognised in its 2018 ex-post evaluation. In terms of perception as regards risk assessment in the context of authorisation dossiers, EFSA is bound by strict confidentiality rules and by the legal requirement to primarily base its assessment on industry studies, laid down in the GFL Regulation and in the multiple authorisation procedures in specific EU food legislation e.g. the Plastics Food Contact Materials Regulation. These elements lead civil society to perceive a certain lack of transparency and independence of EFSA with impacts on the trust in EFSA's scientific work by the general public. Risk communication has not always been effective with consequent impacts on consumers' trust and acceptance of risk management decisions.
Member States also expressed some concerns related to the transparency of the processes under the Plant Protection Products Regulation. In particular, they noted that there can be a lack of communication between EFSA and the Member State Rapporteur when concluding on the harmonised classification of an active substance. In this regard, in April 2018 the Commission made a proposal which aims to improve the disclosure of data contained in the dossiers submitted by industry for active ingredient approval under the Plant Protection Products Regulation and ensuring the involvement of EFSA in pre-submission meetings.
8.3.2Robustness of procedures
A.What's the issue?
The robustness of chemical risk assessment and decision making is dependent on the relevance and reliability of the underlying science and data. The necessary requirements, procedures, processes and capabilities need to be enshrined and applied within the framework of EU chemicals legislation to ensure decisions are based on relevant and reliable science and data.
B.What are the findings?
Conclusions
Numerous requirements, mechanisms and safeguards have been incorporated into the framework of EU chemicals legislation to ensure that risk management decisions are based on sound science and evidence. Their application is considered to be generally effective but more can be done to ensure all relevant evidence (e.g. peer-reviewed academic studies) is available for the assessment and decision-making processes.
The EU legal framework on chemicals is generally well designed to make science and evidence based decisions. In particular:
·The Cosmetic, Detergents, Biocidal Products, Plant Protection Products and Fertilizers Regulations are considered to take adequate account of scientific and technical developments. No significant issues have been identified in terms of the existence of mechanisms to adapt these pieces of legislation to new developments.
·Cosmetic, detergents, biocidal and plant protection products legislation put in place appropriate assessments based on state of the art methods. Moreover, even though there is no legally binding frequency for undertaking a review of risk assessment requirements and other procedures under these pieces of legislation, mechanisms are in place for these purposes (through updating Annexes in the case of cosmetics and detergents or through updating Annexes, Implementing Regulations, or guidance documents used for the approval of active substances for biocides and pesticides).
·The overall approach of data generation under the CLP (consideration of all available data and possibility to use alternative methods to fill data gaps) is considered to be adequate and reducing the level of uncertainty.
·The Fertilizers Regulation (currently under revision) lacks specific data requirements and the risk assessment process is not deemed sufficient to ensure that risk assessment is based on the latest state of the art methods.
·The responsible agency and scientific bodies take into account the latest scientific finding for classification, risk assessment and risk management decision making purposes.
The robustness of these procedures has also led to progressive improvement of the state of knowledge and to closing knowledge gaps.
As explained in Section 5. Effectiveness (as well as in Annex 5 Section 5.2.1 C)) more can be done to ensure all relevant evidence (e.g. peer-reviewed academic studies) is available for the assessment and decision-making processes and that available data in key databases held by ECHA and EFSA is made available and re-used instead of requesting new, duplicative data to be generated.
9EU VALUE ADDED
9.3.1Evaluation question: what is the added value of regulating the risk management of chemicals at an EU level rather than at national level?
A.What's the issue?
The principle of subsidiarity requires that legislating at the EU level should occur only when and where there is evident added-value of doing so, i.e. where necessary and more effective. This section looks at whether there is added value in regulating chemicals at the EU level as opposed to solely at the national level and, if there is one, what this added value is.
B.What are the findings?
Conclusions
The harmonisation of chemicals legislation at the EU level has proved important and largely successful in terms of the protection of human health and the environment as well as the functioning of the internal market. The sharing of knowledge and resources and the application of common rules and standards across the EU has resulted in significant positive economic, health and environmental impacts that would not have been possible to achieve on the basis of legislation at the Member State level alone. The EU chemicals legislation is also the reference point for international standards in several areas which helps to reduce potential trade frictions as well as address transboundary chemicals related issues.
In line with the subsidiarity and proportionality principles, the approach of the EU chemicals legislation guarantees that decisions are taken as closely as possible to the citizen and where necessary and more effective, at the EU level. The core pieces of chemicals legislation such as the CLP, the Plant Protection and Biocidal Products Regulations collectively provide for a harmonised framework (based on article 114 of the TFEU). Some other pieces of legislation in the chemicals legal framework, e.g. the waste legislation and other pieces of environmental legislation (based on Article 192 of the TFEU) and the occupational safety and health (OSH) legislation (Article 153 TFEU) establish a system of basic principles, rules and requirements which must be transposed into national law while leaving room for Member States to be more stringent or go further in their implementation. These pieces of legislation typically include objectives such as the protection of human health and the environment, often with some further specifications of what aspects of environment and natural resources are of particular importance.
Risks to human health and the environment stemming from exposure to hazardous chemicals are similar across the EU. Harmonised rules, procedures, requirements, definitions, criteria etc. allow for a comprehensive risk assessment of exposure to hazardous chemicals. This approach helps ensure an equal level of protection of human health and the environment across the EU while also taking account of variations in local conditions. It also helps ensure that the same amount of information about chemical risks and hazards is made available to public authorities, citizens, consumers, chemicals industry and downstream users across the EU.
Regulating the risk management of chemicals at the EU level also increases efficiency. Hazard and risk assessment processes often require a high level of scientific expertise and therefore can imply high costs for public authorities, especially in smaller Member States. By harmonising and coordinating the hazard and risk assessment processes at the EU level (combined with the principle of reversed burden of proof and self-classification or self assessment of conformity by industry), the EU chemicals legislation helps avoid duplication of effort between Member States. This results in cost savings for public authorities as workload and expertise are shared and it reduces the administrative burden and complexity for the companies that operate in many different Member States. It also contributes to improving the state of knowledge, quality and availability of data needed for risk management decision making.
A system that guarantees the safety of products placed on the EU market and often produced via complex and global value chains is needed in order to protect consumers' interests and to secure their trust both in European companies and those who produce outside the EU. Such a system is established by the EU chemicals legislation. The Eurobarometer survey showed that EU citizens consider products manufactured in the EU to contain safer chemicals than those imported from outside the EU. This indicates a higher level of confidence in the EU regulatory framework for manufactured products compared to regulatory regimes abroad. The EU chemicals legislation is, potentially, a driver of innovation although currently available evidence does not allow a clear conclusion to be drawn about whether or not the legislation is fostering innovation and substitution.
EU chemicals legislation has become a benchmark for development of chemical risk management rules, both at the international level as well as in other countries and regions. European companies also benefit from perception of quality of EU products in non-EU country markets which has brought important advantages in terms of international trade.
The EU chemicals legislation has also helped to decrease the barriers to, and costs, of intra-EU trade by limiting the application of multiple and potentially diverging national rules with limited territorial coverage and existing only in the applicable national language(s). Regulating the risk management of chemicals at an EU level also plays a role in preventing unfair competition between Member States e.g. based on low standards for working conditions.
Stakeholders (industry, NGOs) as well as national authorities were of an opinion that the harmonised community-wide approach in the chemicals legal framework is paramount to achievement of its core policy objectives and that there is clearly an added value in taking action at the EU level versus a situation with 28 different sets of chemicals legislation and standards at Member State level. This includes the facilitation of sharing of knowledge, data, expertise and methodology as well as pooling of resources between Member States, EU institutions and other stakeholders.
The transparency of the process and equal, reciprocal access to information and the quality of information have improved considerably through the implementation of the current legislation, as also highlighted by different stakeholders.
10CONCLUSIONS
The different pieces of chemicals-related EU legislation adopted since late 1960s have, over time, effectively become a legal framework. All of them have been amended, updated or replaced at least once. Many of the Directives have also been repealed, codified or have become Regulations (see Annex 4 Table 2). Yet, how all these pieces work together has never been assessed. This Fitness Check is the first evaluation of most of this complex and extensive legal framework. It is important to note, however, that the REACH Regulation as well as the pharmaceutical and veterinary products legislation are outside the scope of the Fitness Check. This presented a number of challenges, particularly in terms of disentangling costs and benefits estimates where REACH is often an integral part of the policy mix that is responsible for the costs and benefits of exposure reductions.
The framework’s fitness for purpose was assessed against the core policy objectives of ensuring a high level of protection of human health and the environment, ensuring a well-functioning internal market and enhancing EU business’ competitiveness and innovation in an effective, efficient and coherent manner. The focus was primarily on the chemical hazard assessment, risk assessment and risk management decision processes. The Fitness Check also paid attention to the framework’s relevance and capability to respond to stakeholder concerns and future challenges such as the transition towards a more circular economy.
The Fitness Check takes into consideration the findings presented in the related 'Interface' Communication. Together with the findings of the REACH Evaluation, it helps to provide a complete picture in term of taking stock of the current EU’s chemicals legislation. Furthermore, a number of ongoing legislation-specific evaluations (see Annex 4 Table 4) will complement the findings of this Fitness Check, especially regarding the state of play, implementation and enforcement of the legislation as well as costs and benefits generated. All these different evaluations will help ensure that future improvements and refinements made in these policy areas are well-founded, coherent and well-focused.
A comprehensive and generally well-functioning framework
The Fitness Check evaluation found that, overall, the EU framework of chemicals legislation is fit for purpose in terms of meeting the core policy objectives of ensuring a high level of protection of human health and the environment, ensuring the efficient functioning of the internal market while enhancing competitiveness and innovation. These core policy objectives remain highly relevant as well as the framework’s basic components and its current approach. The added value of policy action at the EU level is high.
Although a range of on-going and emerging health and environmental concerns related to the exposure to hazardous chemicals remain (see Section 5.1.1), the EU chemicals legislation has clearly led to significant benefits in terms of reduced and avoided negative health and environmental impacts for regulated hazardous substances and in terms of the efficient functioning of the internal market (see Section 6.1.2). Where these benefits can be reliably monetised, the outcomes are often significant. For example, the benefits of reduced poisoning incidents, occupational skin and respiratory diseases and occupational cancers amount to an estimated EUR 217 – 338 million per year. As another example, the better control and management of plant protection products have resulted in reduced negative impacts on ecosystem, including pollination services and have thus generated estimated benefits of EUR 15 – 50 billion per year. In addition, estimated benefits of EUR 500 million per year result from avoided costs of removing pesticides from drinking water supplies (see Section 6.1.2
Table 4 Selected monetised environmental and health benefits of reduced hazardous chemical exposures
. The level of harmonisation achieved across the EU has played a significant role. In addition, the EU is considered as a frontrunner in terms of chemicals innovation. The EU remains the largest chemicals exporting market in the world and is internationally competitive.
Key benefit drivers include avoided healthcare costs, avoided productivity losses (due to avoided lost working hours as a result of illness or premature death), avoided suffering and premature deaths, avoided remediation costs (including wastewater and drinking water treatment costs) and avoided degradation of environmental/eco-system services.
The EU chemicals legislation has decreased the barriers to, and costs, of intra-EU trade by limiting the application of potentially diverging national rules with limited territorial coverage and existing only in the applicable national language(s). The EU chemicals legislation is also the reference point for international standards in several areas, which helps to reduce potential trade frictions as well as address transboundary chemicals related issues.
Generic and specific risk management approaches both have their role to play within the framework of EU chemicals legislation. Regarding the balance between the two, the preferences of different stakeholder groups vary considerably, with industry having a tendency to prefer a more extensive use of the specific approach, NGOs tending to have a higher preference for the generic approach, and many Member States expressing satisfaction with the current balance. There is room for improvement in the application of both approaches, particularly in terms of:
·speeding up the identification and risk assessment of hazardous chemicals; and
·ensuring the concerns of, impacts on and implications for different stakeholder groups are properly identified and taken into consideration in order to avoid any disproportionate or unintended consequences.
Achieving the core objectives of the EU chemicals legislation is predicated on sound scientific knowledge and robust and comprehensive data that is reliable, comparable and reproducible. Within the EU, the quality and the availability of data needed to perform a risk assessment and to manage risks has improved considerably. The EU’s knowledge base on chemical hazards and risk is now an important asset. Much of this improvement in data reflects the shift of responsibility from EU and Member State authorities to industry for generating the necessary data for hazard and risk assessment. It has, therefore, been primarily resourced and underpinned by industry assuming the responsibility of ensuring the safe use of chemicals placed on the market. The significant investment in the establishment of independent regulatory EU chemicals agencies (EFSA, ECHA, EMA) and specific scientific committees has also been instrumental in the improvement of data quality and its availability as well as to provide expertise and support to the EU decision making process.
The CLP Regulation was identified as one of the most efficient aspects of the functioning of the EU chemicals legislative framework, as it allows hazard classification of a wide range of chemicals without creating a disproportionate administrative burden for public authorities while focusing their resources on the most relevant substances for human health and environmental protection. The clear separation of hazard assessment and hazard classification from the risk assessment and risk management decision making steps is an important cornerstone of the framework’s effectiveness and should be safeguarded. The CLP Regulation ensures the coherence of hazard assessment and classification at the EU level with what is done at the international level (through the UN Globally Harmonised System (GHS)).
The scope and stringency of the hazard and risk assessment processes stipulated within the EU chemicals legislation are tailored to different needs under different pieces of legislation. After more than 50 years of continuous efforts and improvements, the linkages between the different pieces of EU chemicals legislation are now generally well established and functioning reasonably well. The level of transparency and stakeholder involvement built into the various hazard and risk assessment/management processes has improved over time and is considered by the majority of stakeholders to be good.
Burden reduction and simplification
The overall regulatory costs of the EU chemicals legislation for EU industry are estimated to be approximately several billion euros per year (see Section 6.1.1).
Depending on the piece of legislation, the main cost drivers for industry are data generation (hazards, chemical uses, exposures, etc.), staff training, biomonitoring of workers and monitoring of operational conditions, and control costs. For public authorities the main costs are generated by enforcement and monitoring activities.
While it was not possible to establish an overall quantified cost-benefit ratio for the framework of EU chemicals legislation and thus conclude on their proportionality, the evidence clearly indicates that both the costs and the benefits generated by the EU chemicals legislation are significant. The Fitness Check, however, identified a number of opportunities for burden reduction and simplification, both for companies and for Member State authorities, the most significant of these being as follows:
·Small and Medium Sized Enterprises (SMEs): SMEs are more affected than bigger companies by certain aspects of the EU chemicals legislation such as understanding and compliance with often interlinked legal obligations or opportunities to actively take part in decision making processes. Increased involvement of these stakeholders in the decision making processes can be improved and will help ensure that all interests at stake are properly taken into consideration. This will also improve their understanding of their legal obligations and thus provide for a predictable, fair and trusted environment that continues to ensure a high level of protection of human health and the environment and a well-functioning internal market.
·Data sharing: the availability and quality of data have improved and are generally good. However, some difficulties in data sharing across legal clusters are still encountered. This affects mainly industry that needs to generate data for regulatory purposes. These difficulties occur for a variety of reasons, including general data confidentiality rules and intellectual property rights but also because of the lack of a centralised access point or the lack of awareness of what exists in the different databases. It leads to a certain duplication of effort where the nature of assessment made is similar. This can generate extra costs, as well as longer-than-necessary timeframes and lead to duplication of testing. A comprehensive mapping of the existing information and an assessment of how to optimise the use of the available information are needed.
·Hazard communication to consumers: not all the opportunities to improve and simplify the communication of chemical hazards and safety information towards consumers have been seized e.g. the opportunities offered by digital technologies such as Q-R codes have not yet been assessed. The communication of hazards to consumers via pictograms and labels can also be improved e.g. labels overloaded with information and difficult to read with some duplication of certain information due to overlaps in legal requirements or because of the need to include hazard statements in all EU languages.
·Predominant substance-by-substance approach to risk assessment and management: using grouping approaches to identify and risk assess groups of chemicals with similar hazard and risk profiles as means of speeding up the risk management decision process and avoiding regrettable substitutions (that can be costly both to industry and to the society in general in terms of health and environmental impacts) warrants further attention.
·Risk of duplication of efforts by different EU agencies and scientific committees: these bodies provide the Commission with scientific advice and hazard/risk assessments. There are opportunities for simplifying their current setup and streamlining their activities thus making the functioning of the framework more efficient (i.e. avoiding duplication of efforts) and more reliable (i.e. reducing the risk of potentially diverging outcomes of hazard/risk assessments).
·Lack of clarity with respect to how to apply the CLP bridging principles method for the classification of mixtures: the clarification on how to apply these principles to mixtures will improve the effectiveness of this method. It will also avoid discrepancies in interpretation and acceptance of hazard classification by Member States. Actions taken so far by the Commission to address this issue, including guidance on the harmonised application of the legal requirements, need to be pursued.
Needs for improvement
Even though the objectives of legislation within the scope of this Fitness Check are not always the same, the legal acts are generally coherent in how they attempt to reach the stated objectives, as illustrated by the use of similar underpinning legal mechanisms to do so. One of the mechanisms used is the reverse burden of proof by industry complemented by the use of self-assessment of conformity. Where the outcome of the risk assessment is to be checked by a public authority thus determining whether, for example, a product can be used or placed on the market or an activity can be pursued, the quality of risk assessments done tends to be good. For the pieces of legislation where the underpinning mechanism relies on the presumption of compliance with the existing rules, information from enforcement activities carried out is scarce and therefore does not allow to conclude on the quality of the self-assesment of conformity carried out.
The current state of knowledge regarding exposure to hazardous chemicals needs to be further improved. Because industry and public authorities may be unaware of many uses of hazardous chemicals and there is only limited information available about the overall volumes of hazardous chemicals emitted/released into the environment, their capacity to develop realistic, acceptable and robust exposure scenarios can be hampered. Exposures to hazardous chemicals are known to or are strongly suspected of play a role in impacts on human health and the environment e.g. cancers, reproductive diseases, respiratory sensitisation, declines in insect and bird populations and water and soil pollution. In this regard, a number of on-going exposure situations to hazardous chemicals warrant further attention (e.g. endocrine disrupting chemicals and hazardous chemical exposures of the aquatic and terrestrial compartments). However, there are still uncertainties regarding the extent to which the negative trends can be attributed to exposure to hazardous chemicals rather than to other factors such as lifestyle. This hampers the legislator’s capability to provide with certainty the most appropriate answers. This also renders the practical application of the precautionary principle in the area of chemicals risk assessment and management particularly challenging for the decision makers.
Although the precautionary principle is explicitly taken into account in the design of various pieces of chemicals legislation, to date, it has actually been applied in very few instances under the various pieces of EU chemicals legislation. The appropriate use of the precautionary principle is an important element in helping to ensure on the one hand the protection of human health and the environment and the avoidance of potential costly future impacts and remediation, and on the other hand the avoidance of disproportionate or unnecessary risk management costs. The EU efforts in collecting human health exposure data need to be pursued. More data on hazardous chemical uses and their fate need to be collected. So far the Commission has funded the European Human Biomonitoring Initiative (HBM4EU). However, a similar initiative for animals, plants and eco-systems is currently lacking although the Commission’s development of the Information Platform for Chemical Monitoring (IPCHEM) can contribute to addressing this gap.
Several other important areas for future improvement or which warrant further assessment include:
·Better understanding of the impacts of hazardous chemicals on the environment, biodiversity and eco-system resilience. This includes the assessment of the benefits of introducing additional hazard classes in the CLP Regulation (e.g. persistent, bio-accumulative, toxic and very persistent and very bio-accumulative substances and terrestrial toxicity, endocrine disruptors).
·Better understanding and management of the potential human health and environmental risks associated with exposures to substances in articles (e.g. consumer products). The issue of substances in articles is particularly important as the EU is in the process of shifting towards a more circular economy. This implies the need for better traceability of substances of concern in articles (and communication of this to consumers and end-users), since such a shift will involve considerable changes in the way materials and articles are produced, used and disposed of. This warrants consideration of how to manage the health and environmental risks associated with the hazardous substances that pass through several cycles of production, use, and recycling.
·Addressing combination effects of different hazardous chemicals as well as the combined exposure via different routes.
·Improving consistency in identifying and managing the risks posed by allergens.
·Ensuring consistency and intensity of actions to protect vulnerable population groups, including those that are particularly sensitive to endocrine disruptors, such as prenatal and young infants, adolescents, etc.
·Gathering more evidence regarding neurotoxicity, immunotoxicity and respiratory sensitization to determine the extent and significance of potential weaknesses in the risk assessment of substances with these properties.
·Addressing the inconsistencies that occur regarding risk management decisions for endocrine disruptors, persistent, bio-accumulative, toxic and very persistent and very bio-accumulative substances and substances fulfilling the classification criteria for specific target organ toxicity. In some but not all pieces of legislation, they are subject to risk management measures based on generic risk considerations.
·Addressing risks posed by endocrine disruptors. The need for a coherent approach to the identification of endocrine disruptors across all relevant Union legislation approach, based on the broadly accepted definition of the World Health Organisation is a key element of the recently adopted EU strategy on endocrine disruptors.
·Boosting substitution of the most hazardous chemicals with less hazardous chemicals or non-chemical solutions where alternative substances or technologies are more sustainable and economically and technically viable. Efforts of supporting and encouraging research and innovation to catalyse the shift towards more sustainable chemicals need to be accelerated and pursued.
·Improving the reliability and consistency of the industry self-classifications of chemical hazards under the CLP Regulation. The current concerns need to be further investigated as these affect the value of the Classification and Labelling Inventory as a hazard communication tool.
11LIST OF ANNEXES
Annex 1 Procedural information
Annex 2 Synopsis report: stakeholder consultation activities
Annex 3 Methods and analytical models
Annex 4 Information regarding the legal scope of the Fitness Check, its supporting studies and other relevant sources of information
Annex 5 Effectiveness
Annex 6 Efficiency
Annex 7 Coherence of hazard/risk assessment and risk management procedures (CMRs, PBTs/vPvBs, EDs)
Annex 8 EU Approaches to Chemicals Risk Management: updated
Annex 9 Glossary
Annex 10 Evaluation questions
Annex 11 Overview of costs – benefits identified in the Fitness Check
 EUROPEAN COMMISSION
EUROPEAN COMMISSION