EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document 52021XC0407(04)
Communication from the Commission concerning the visual appearance of the label on EU fertilising products referred to in Annex III to Regulation (EU) 2019/1009 of the European Parliament and of the Council 2021/C 119/01
Communication from the Commission concerning the visual appearance of the label on EU fertilising products referred to in Annex III to Regulation (EU) 2019/1009 of the European Parliament and of the Council 2021/C 119/01
Communication from the Commission concerning the visual appearance of the label on EU fertilising products referred to in Annex III to Regulation (EU) 2019/1009 of the European Parliament and of the Council 2021/C 119/01
C/2021/726
OJ C 119, 7.4.2021, p. 1–50
(BG, ES, CS, DA, DE, ET, EL, EN, FR, HR, IT, LV, LT, HU, MT, NL, PL, PT, RO, SK, SL, FI, SV)
|
7.4.2021 |
EN |
Official Journal of the European Union |
C 119/1 |
COMMUNICATION FROM THE COMMISSION
concerning the visual appearance of the label on EU fertilising products referred to in Annex III to Regulation (EU) 2019/1009 of the European Parliament and of the Council
(2021/C 119/01)
INTRODUCTION
Pursuant to Article 4(3) of Regulation (EU) 2019/1009 of the European Parliament and of the Council (1) (the ‘Fertilising Products Regulation’ or the ‘FPR’), the Commission shall publish a guidance document for manufacturers and market surveillance authorities with clear information and examples concerning the visual appearance of labels referred to in Annex III to that Regulation.
A task force of representatives of EU Member States and industry stakeholders, representing all the Product Function Categories (PFCs) falling under the scope of the FPR, was created by the Commission in July 2019 in order to support its services (DG GROW/D2) in fulfilling this task. The mandate of this task force was to write a first draft of this document.
This document was shared and discussed with members and observers of the Commission Expert Group on Fertilising Products in 2019 and 2020.
This document is not legally binding and seeks only to provide useful guidance to stakeholders including manufacturers and market surveillance authorities. Only the Court of Justice of the European Union is competent to authoritatively interpret Union law.
This guidance document provides explanations on the practical implementation of the labelling requirements set in Annex III to the FPR. It includes examples of labels for the different PFCs of EU fertilising products. These examples are purely indicative. The position of each part, as well as the colours used in this guidance document are not mandatory. It is up to the manufacturer to decide where to place and how to format the information on the label, while respecting the requirements in the FPR.
Unless otherwise provided in this guidance document or no colours are used at all, the following colour codes are used in the label examples:
|
— |
In blue: general requirements, |
|
— |
In orange: specific requirements for each PFC, |
|
— |
In black: other information that has to be provided on the label, |
|
— |
In green: indicated nutrients. |
CONTENTS
| Introduction | 1 |
|
1. |
Overall rules on labelling in the core text of the FPR | 5 |
|
1.1. |
What does mandatory labelling information cover? | 5 |
|
1.2. |
Is it possible to provide voluntary information on the label? Where could this voluntary information appear? | 5 |
|
1.3. |
Is it possible to put information on the packaging, outside the label (i.e. batch n°, CE mark, notified body’s number, quantity)? | 5 |
|
1.4. |
Is there a minimal/maximal size for the label/the font? Is there a proportional size to respect? | 5 |
|
1.5. |
In what language(s) should a label be written? | 6 |
|
2. |
General labelling requirements in Annex III of the FPR | 6 |
|
2.1. |
How to write the designation of the claimed function? | 6 |
|
2.2. |
How to express the quantity of the EU fertilising product? | 6 |
|
2.3. |
How to provide information on the general application rates? | 7 |
|
2.4. |
How to provide information on storage conditions? | 7 |
|
2.5. |
What does the functionality period of products containing a polymer belonging to CMC 9 mean? | 8 |
|
2.6. |
How to provide the information on risk management? | 8 |
|
2.7. |
What does ‘ingredients’ mean and how to label them? | 9 |
|
2.8. |
How to label the function of products with two or more functions? | 10 |
|
2.9. |
Is it possible to use different wording for the requirements in points 4, 5, 6 and 9 in Part I of Annex III? | 10 |
|
2.10. |
Is it possible to use pictograms based on good practices? How to manage the interaction with the CLP Regulation? | 10 |
|
2.11. |
In which cases can the manufacturer express the nutrient content in elemental form? | 11 |
|
2.12. |
How to refer to the organic matter instead of organic carbon? | 11 |
|
2.13. |
Example for general labelling requirements and visual appearance | 11 |
|
3. |
Specific labelling requirements for PFC 1 Fertiliser | 12 |
|
3.1. |
Is it necessary to label the content of all nutrients present in a fertiliser? | 12 |
|
3.2. |
When the regulation does not define minimum content for secondary nutrients (PFC 1 (A) and PFC 1 (B)), how to label the content of these nutrients? | 12 |
|
3.3. |
When the content of nitrogen (N) or phosphorus pentoxide (P2O5) has to be indicated as it is above 0,5 % by mass, how should this information be provided? | 12 |
|
3.4. |
Can the term ‘mineral’ be used instead of or in addition to the term ‘inorganic’ in the designation of the product? Where should the term ‘mineral’ be labelled? | 12 |
|
3.5. |
Does ammoniacal nitrogen (NH3) refer to ammonium nitrogen (NH4 +) for PFC 1? | 12 |
|
4. |
Specific labelling requirements for PFC 1(A) Organic Fertiliser | 13 |
|
4.1. |
Example of a label | 13 |
|
4.2. |
How to declare organic nitrogen and the origin of organic matter? | 14 |
|
4.3. |
At which precision level should mandatory information for PFC 1(A) be declared? | 14 |
|
4.4. |
Should ammoniacal nitrogen be declared even if it is not present in the product? | 14 |
|
4.5. |
Is it possible to declare organic matter instead of organic carbon? | 14 |
|
4.6. |
Where to include the information related to the date of production? | 14 |
|
5. |
Specific labelling requirements for PFC 1(B) Organo-Mineral Fertiliser | 15 |
|
5.1. |
Example of a label | 15 |
|
5.2. |
How to declare organic nitrogen and the origin of organic matter? | 16 |
|
5.3. |
Should a specific form of nitrogen (N), phosphorus (P) or potassium (K) be declared even if it is not present in the product? | 16 |
|
5.4. |
How to provide pertinent information about the possible air quality impacts of the release of ammonia from the fertiliser use, and an invitation to users to apply appropriate remediation measures when urea (CH4N2O) is present in the product? | 16 |
|
5.5. |
How to declare the ‘low cadmium content’? | 16 |
|
5.6. |
At what precision can micronutrients be declared? | 16 |
|
6. |
Specific labelling requirements for PFC 1(C) Inorganic Fertiliser | 17 |
|
6.1. |
PFC 1 (C)(I): Inorganic Macronutrient Fertiliser | 17 |
|
6.1.1. |
Example of a label | 17 |
|
6.1.2. |
What is the minimum number of decimals that should be indicated on the label? | 18 |
|
6.1.3. |
How to provide pertinent information about the possible air quality impacts of the release of ammonia from the fertiliser use, and an invitation to users to apply appropriate remediation measures when urea (CH4N2O) is present in the product? | 18 |
|
6.1.4. |
How to declare the ‘low cadmium content’? | 18 |
|
6.2. |
PFC 1(C)(I)(a): Solid Inorganic Macronutrient Fertiliser | 18 |
|
6.2.1. |
Example of a label | 18 |
|
6.2.2. |
Example for granulometry | 18 |
|
6.2.3. |
In what way can granulometry and physical unit be indicated on the label? Is it allowed to reference more than one sieve when indicating the granulometry of a product? | 19 |
|
6.2.4. |
How is a ‘coating’ defined? | 19 |
|
6.2.5. |
How to declare the functionality period of the coated fertiliser? | 19 |
|
6.2.6. |
How to declare the type of coating agent? | 19 |
|
6.2.7. |
How to draw the label for mined fertilisers? | 20 |
|
6.3. |
PFC 1(C)(I)(b): Liquid Inorganic Macronutrient Fertiliser | 20 |
|
6.4. |
PFC 1(C)(II): Inorganic Micronutrient Fertiliser | 21 |
|
6.4.1. |
PFC 1(C)(II)(a): Straight Inorganic Micronutrient Fertiliser | 21 |
|
6.4.2. |
PFC 1(C)(II)(b): Compound Inorganic Micronutrient Fertiliser | 21 |
|
6.5. |
PFC 1(C) complete label example | 22 |
|
7. |
Specific labelling requirements for PFC 2 Liming Material | 24 |
|
7.1. |
Examples of a label | 24 |
|
7.2. |
Regulatory reference, explanation and voluntary additions | 26 |
|
8. |
Specific labelling requirement for PFC 3 Soil Improver | 27 |
|
8.1. |
PFC 3(A) Organic Soil Improver | 27 |
|
8.1.1. |
Examples of a label | 27 |
|
8.1.2. |
Regulatory reference, explanation and voluntary additions | 28 |
|
8.2. |
PFC 3(B) Inorganic Soil Improver | 29 |
|
8.2.1. |
Example of a label | 29 |
|
8.2.2. |
Regulatory reference, explanation and voluntary additions | 30 |
|
9. |
Specific labelling requirements for PFC 4 Growing Medium | 30 |
|
9.1. |
Examples of a label | 30 |
|
9.2. |
Regulatory reference, explanation and voluntary additions | 32 |
|
10. |
Specific labelling requirements for PFC 5 Inhibitors | 32 |
|
10.1. |
PFC 5(A) Nitrification Inhibitor | 32 |
|
10.2. |
PFC 5(B) Denitrification Inhibitor | 33 |
|
10.3. |
PFC 5(C) Urease Inhibitor | 33 |
|
11. |
Specific labelling requirements for PFC 6 Plant Biostimulant | 34 |
|
11.1. |
Examples of a label | 34 |
|
11.1.1. |
PFC 6(A) Microbial Plant Biostimulant | 34 |
|
11.1.2. |
PFC 6(B) Non-Microbial Plant Biostimulant | 36 |
|
11.2. |
How to label the physical form of the product? | 37 |
|
11.3. |
How to provide the relevant instructions related to the efficacy of the product, including soil management practices, chemical fertilisation, incompatibility with plant protection products, recommended spraying nozzles size, sprayer pressure and other anti-drift measures? | 37 |
|
11.4. |
How to include a statement regarding the fact that micro-organisms may have the potential to provoke sensitising reactions? | 37 |
|
11.5. |
How to provide the production and expiry date and where to place it on the label? | 37 |
|
11.6. |
Specific instructions for Microbial Biostimulants | 37 |
|
12. |
Specific labelling requirements for PFC 7 Fertilising Product Blend | 37 |
|
12.1. |
Examples of a label | 37 |
|
12.2. |
How to express labelling requirements for PFC 7? | 44 |
1. OVERALL RULES ON LABELLING IN THE CORE TEXT OF THE FPR
1.1. What does mandatory labelling information cover?
|
Labelling requirements |
|
|
Articles 6 and 8: name, registered trade name or registered trademark and the postal address of manufacturer/importer, as well as a type number, batch number or other element allowing the identification of the EU fertilising product |
Annex III General and specific labelling requirements |
|
Article 11: ‘repackaged by’/‘packaged by’ + name, registered trade name or registered trademark and the postal address Articles 17 and 18: CE marking and identification number of the notified body (if applicable) |
|
|
— |
These are mandatory requirements. |
|
— |
For manufacturers, the words ‘produced by’ can be applied on a voluntary basis before the requirement of Article 6(6). |
|
— |
For packers, it is possible to add the ‘id code’ provided by the national authority in addition to the requirements of Article 11. The number of the notified body has to be put on the labels only for EU fertilising products having had their conformity assessed through Module A1 and Module D1 as provided in Annex IV to the FPR. |
1.2. Is it possible to provide voluntary information on the label? Where could this voluntary information appear?
Yes, it is possible to provide voluntary information other than that defined in the Regulation (for example, the FPR lays down rules to label ‘poor in chloride’ as a voluntary information). In accordance with point 8 in Part I of Annex III to the FPR, voluntary information shall, among other things, not mislead the end user and shall relate to verifiable factors.
1.3. Is it possible to put information on the packaging, outside the label (i.e. batch n°, CE mark, notified body’s number, quantity)?
The label should not be interpreted as a strict physical unit. What needs to be covered by a label is all the mandatory information that has to be affixed on or to accompany the EU fertilising product.
|
— |
In case of a product with packaging, the labelling information can appear on the package itself and/or a document affixed to the package. |
|
— |
For a bulk product, the labelling information is included in an accompanying document or a leaflet. |
Therefore, if the practice of the economic operators is to affix the batch number, the quantity, the CE mark or any other mandatory information on the package, it fulfils the requirements of the FPR.
1.4. Is there a minimal/maximal size for the label/the font? Is there a proportional size to respect?
The regulation does not establish any rules related to the size for the label/the font. It is up to the manufacturer to decide which size of the label to use, and ensure that information is clear, understandable, legible and intelligible.
1.5. In what language(s) should a label be written?
Each Member State decides what language has to be applied for its national market.
Some Member States accept a written and signed agreement from a customer dealing with products for professional use which would accept to receive a product labelled in another language than the official one(s) for that Member State (for example, in English). The economic operator is advised to verify with the Member State in which a product is placed on the market whether such an agreement is acceptable. The national authorities competent for fertilising products are listed at:
https://ec.europa.eu/docsroom/documents/35205
2. GENERAL LABELLING REQUIREMENTS IN ANNEX III OF THE FPR
2.1. How to write the designation of the claimed function?
The designation of the claimed function has to be written with the objective of supplying end-users and market surveillance authorities with a sufficient level of information, without misleading them. A manufacturer can reduce the length of the designation of a product to the minimum necessary of the respective sub-category as long as the above is fulfilled. If this approach is applied, the PFC index corresponding to the respective sub-category as listed in Part I of Annex I to the FPR must be indicated.
Therefore, taking into consideration the above, the following examples could be used:
First option: it is possible to use the full name designation related to the product function as written in Part I of Annex I for PFCs 1 to 6.
For example:
|
— |
Compound inorganic micronutrient fertiliser |
|
— |
Compound solid inorganic macronutrient ammonium nitrate fertiliser of high nitrogen content |
|
— |
Liquid organo-mineral fertiliser |
Second option: it is possible to use the PFC index (with the letters in upper or lower case as applicable) + a shortened designation.
The following table shows some examples:
|
Full name designation |
PFC index + shortened designation |
Condition |
|
Compound Inorganic micronutrient fertiliser |
PFC 1(C)(II)(b) – Mineral micronutrient fertiliser |
Shortened designation is only applicable if the conditions in point 4 in PFC 1 in Part II of Annex III are fulfilled |
|
Compound solid inorganic macronutrient ammonium nitrate fertiliser of high nitrogen content |
PFC 1(C)(I)(a)(ii)(A) – Mineral fertiliser with ammonium nitrate of high nitrogen content |
Shortened designation is only applicable if the conditions in point 4 in PFC 1 in Part II of Annex III are fulfilled |
|
Liquid organo-mineral fertiliser |
PFC 1(B)(II) – Organo-mineral fertiliser |
N.a. |
Any function of a fertilising product can be claimed only when a successful conformity assessment has proven such function, including for products for which more than one function is claimed (see point 2 in Part I of Annex III). More details are given under sub-section 2.8.
2.2. How to express the quantity of the EU fertilising product?
Except for growing medium, the regulation does not lay down specific rules on the expression of the quantity. Thus, the quantity can be expressed in mass (t, kg or g) or volume (m3, L or mL). It is recommended to only use units from the ‘International System of Units’.
It is recommended to express the quantity by net mass for a solid fertilising product, and by net mass and/or volume for a liquid fertilising product.
For growing medium, special requirements are set in PFC 4 in Part II of Annex III. On a voluntary basis the quantity can be indicated by additional measurements to those required.
2.3. How to provide information on the general application rates?
As fertilisation recommendations may be crop, site, soil or climate specific, it may be justified for manufacturers and other economic operators to use a relatively general recommendation for the application rate, including maximum levels of application.
A manufacturer can choose to adapt the information regarding the application rate depending on the end-user. A distinction could be made between the following categories:
|
— |
Consumer use (i.e. private households, week-end gardeners), |
|
— |
Professional use (i.e. public domain, farmers), |
|
— |
Industrial use (i.e. use of substances as such or in preparation at industrial site, Business-to-Business). |
Following the abovementioned distinction, it is recommended for economic operators wanting to follow this approach to adapt the information regarding application rates as follows:
|
— |
Consumer use market: detailed information concerning the application rates per crop should be shown. |
|
— |
Professional use market: the label should show general application rates and a reference sentence such as ‘Contact Company X or company’s X distributor for more specific recommendations’. |
|
— |
Industrial market: the label should state a reference sentence (for example): ‘This product is not intended for direct application/use without further processing.’ |
In addition, it is suggested to add a sentence inviting farmers to follow good fertilisation practices:
‘These product application rates are recommendations. We recommend to the farmers to seek counsel from their adviser to adjust the recommendations to their particular situation and to avoid over-fertilisation.’
or
‘Farmers are encouraged to avoid nutrient losses and to take official recommendations into account while drawing fertilisation plans.’
Note: it is possible to provide voluntary information in addition to the mandatory requirements. For example, it is possible for an economic operator to sell a product to an industrial customer with the label prepared for a professional customer.
2.4. How to provide information on storage conditions?
It is under the responsibility of the manufacturers to define the storage conditions according to their knowledge of the product and based on good practices. The key objective should be to store the product without losing the quality and guaranteed content of the product under safe conditions. Pictograms reflecting good practices can be used as long as they are clear and not misleading.
Information about storage conditions may cover among others the following aspects:
|
— |
Storage period |
|
— |
Storage environment (open/roof/closed; covered; dry etc.) |
|
— |
Storage temperature/moisture |
|
— |
Stacking |
|
— |
Incompatibility with other materials |
|
— |
‘Please also refer to information provided in Material Safety Data Sheet (MSDS)’ (if it is provided). |
2.5. What does the functionality period of products containing a polymer belonging to CMC 9 mean?
The functionality period of a polymer belonging to ‘Component Material Category (CMC) 9: Polymers other than nutrient polymers’ may be decided by the manufacturer. It defines both how rapidly the polymer must degrade and how frequent applications the use instructions may provide for. If the claimed functionality period is short, the use instructions may provide for frequent application, but then the actual biodegradation should also be fast. By contrast, if the claimed functionality period is longer, the biodegradation may be slower, but then the application frequency in the use instructions must also be longer, since point 1(f) of Part I of Annex III stipulates that the period between two applications must be at least as long as the claimed functionality period i.e. re-application during the functionality period is not allowed.
A general sentence can be added on the label. If considered useful, a pictogram identifying the maximum duration of the functionality period can be added, as suggested below. The pictogram should be completed by a text such as the below recommendations. In the second example, where the functionality period is expressed as a range, it is important that the user instructions preventing re-application refers to the longest possible period covered by the range.

‘Re-application during the functionality period is not allowed. Contact company or company’s distributor for more specific recommendations.
www.website.com’

‘Re-application after less than 8 weeks is not allowed. Contact company or company’s distributor for more specific recommendations.
www.website.com’
In addition, if the product contains a polymer with the purpose of binding material, a sentence informing the user that the product cannot be in contact with the soil is required.
2.6. How to provide the information on risk management?
In case of products classified under Regulation (EC) No 1272/2008 of the European Parliament and of the Council (2) (the ‘CLP Regulation’), additional labelling requirements must be respected. For more information, refer to sub-section 2.10.
In other cases, it is the responsibility of the manufacturer to supply pertinent information enabling to manage risks. Pictograms (except CLP hazard pictograms if the product is not classified) can be used as long as they are clear and not misleading.
A generic sentence such as ‘To avoid risks to human health and the environment, please comply with the recommended use instructions of this fertilising product’ can be used.
According to points 4, 5 and 6 in Part I of Annex III to FPR, in the following specific cases, add the sentences mentioned below:
|
— |
Where the EU fertilising product contains derived products in the meaning of the animal by-products regulation, except manure, ‘Farmed animals shall not be fed, either directly or by grazing, with herbage from land to which the product has been applied unless the cutting or grazing takes place after the expiry of a waiting period of at least 21 days’. |
|
— |
Where the EU fertilising product contains ricin, ‘Hazardous to animals in case of ingestion’. |
|
— |
Where the EU fertilising product contains unprocessed or processed cocoa shells, ‘Toxic to dogs and cats’. |
2.7. What does ‘ingredients’ mean and how to label them?
Ingredients should be considered as any kind of material(s) (such as raw materials, substances, mixtures, bulky volume-building components, etc.) intentionally used for/added to the fertilising product during manufacturing, or substances intentionally obtained by chemical reaction within the production process of the product. In some cases, ingredients may contain impurities, which should be excluded from the list of ingredients.
For materials obtained by chemical reaction, only the reaction product must be declared (for example, ammonium nitrate, urea) and not the precursors.
In accordance with the FPR, all ingredients above 5 % by product weight shall be provided in descending order by the percentage of the dry weight.
Further to the obligation of declaring all ingredients above 5 % by product weight, economic operators may decide to label ingredients that are below 5 % by product weight. When doing so, and in order to avoid confusing mandatory and voluntary labelling, these ingredients should be listed as additional information and not in the section of ‘ingredients’, where only ingredients above 5 % by product weight are expected to be referenced.
According to the FPR, there is no labelling obligation to declare the actual percentage of each ingredient in the final formulation of the fertilising product.
For substances and mixtures covered by the CLP Regulation, the identification has to comply with all the requirements of this Regulation. Hence, for a mixture, its trade name and the identity of the substances contributing to the classification according to Article 18(3) of the CLP Regulation have to be given in the list of ingredients.
For natural materials, it is possible to use mineral names (for example, Sylvinite, Langbeinite) in addition to the names used in accordance with Article 18 of the CLP Regulation, and the corresponding identification number of the material (CAS number or EC number) if available.
To avoid very long lists on the label itself, it is recommended to describe the CMCs of the ingredients by using a footnote or a shortened CMC reference.
Example for an organo-mineral fertiliser:
|
— |
CMC by footnote Cocoa shell1, Feather meal2, Superphosphate concd.3 CAS n° 65996-95-4, Potassium chloride3 CAS n°7447-40-7, Magnesium oxide3 CAS n°1309-48-4, Castor cake1, Bone meal2, Urea3 CAS n° 57-13-6 With: 1 Plants, plant parts or plant extracts; 2 Derived products within the meaning of Regulation (EC) No 1069/2009; 3 Virgin material substances and mixtures. |
|
— |
Shorten CMC reference Cocoa shell (CMC 2: Plants, plant parts or plant extracts), Feather meal (CMC 10: Derived products within the meaning of Regulation (EC) No 1069/2009 of the European Parliament and of the Council (3)), Superphosphate concd. CAS n° 65996-95-4 (CMC1: Virgin material substances and mixtures), Potassium chloride CAS n° 7447-40-7 (CMC 1), Magnesium oxide CAS n°1309-48-4 (CMC 1), Castor cake (CMC 2), Bone meal (CMC 10), Urea CAS n° 57-13-6 (CMC 1) In the specific case of fertilising products containing composts and/or digestate, it is recommended to complete the list of ingredients with the raw materials used. |
Example:
|
— |
Compost CMC 3 (Green-Compost) |
|
— |
Digestate CMC 5 (Dried digestate from manure, energy crops and bio-waste) or Digestate CMC 5 (Solid fraction digestate from energy crops and bio-waste from plant origin) |
2.8. How to label the function of products with two or more functions?
The label must bear the designations as indicated in Annex I to the FPR corresponding to the product’s claimed functions. Only the designations of PFC for which there is a successful conformity assessment shall be claimed. In that case, the manufacturer is free to choose the order of appearance of the different (2 or more) designations on the label. These functions can be separated by a dash or a word such as ‘and’ or ‘with’.
Examples:
|
— |
Straight solid inorganic macronutrient fertiliser – Liming material |
|
— |
Straight solid inorganic macronutrient fertiliser with Liming material |
|
— |
Straight solid inorganic macronutrient fertiliser and Liming material |
If the product is a PFC 7, and a combination of a PFC 6(A) and PFC 6(B), the general recommendations described above apply.
The mentioning of PFCs index numbers is not mandatory, see for more details sub-section 2.1.
2.9. Is it possible to use different wording for the requirements in points 4, 5, 6 and 9 in Part I of Annex III?
Rewording the requirements in points 4, 5 and 6 in Part I of Annex III is not allowed by the FPR.
For point 9 in Part I of Annex III, a similar wording to ‘low in chloride’ may be used.
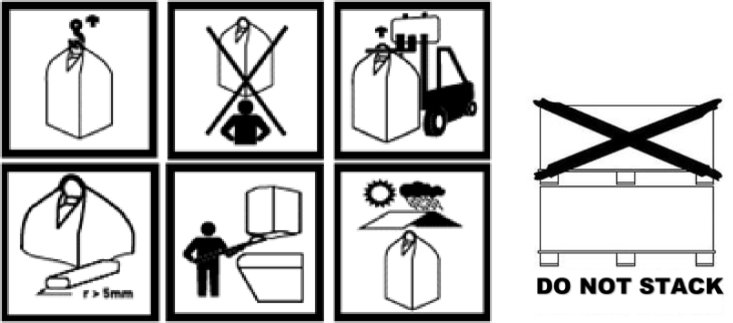
2.10. Is it possible to use pictograms based on good practices? How to manage the interaction with the CLP Regulation?
It is possible, on a voluntary basis, to inform the user on storage conditions or management of effects on health and environment with pictograms based on good practices, even if the product is not under the scope of the CLP Regulation.
If the CLP Regulation applies, the label of the product must bear all the labelling requirements set by it (hazard pictograms, signal words, hazard and precautionary statements, Unique Formula Identifier when applicable, additional requirements for consumer use and so on), including storage conditions and managements of risks. Additional information (ex.: pictograms on good practices) could be labelled in accordance with Article 25 of the CLP Regulation. They must not replace, deflect or contradict the mandatory labelling elements requested by the CLP Regulation.
In case of use of pictograms, it is important to avoid double labelling in accordance with Article 25 of the CLP Regulation.
Example:
2.11. In which cases can the manufacturer express the nutrient content in elemental form?
The manufacturer can express the nutrient content requested by the FPR in elemental form instead or in addition to the oxidised form in accordance with the conversion factors defined in point 10 in Part I of Annex III. For more information, see Section 3 of this guidance document.
2.12. How to refer to the organic matter instead of organic carbon?
The information requested by the FPR may refer to organic matter instead of, or in addition to organic carbon (Corg), in accordance with the following conversion factor:
organic carbon (Corg) = organic matter × 0,56
If both are used, the organic matter can be put beside to organic carbon (Corg) into brackets, or in the voluntary information section.
2.13. Example for general labelling requirements and visual appearance

A detailed label frame including all PFCs and references to the FPR labelling requirements is provided in the Annex to this guidance document.
3. SPECIFIC LABELLING REQUIREMENTS FOR PFC 1 FERTILISER
3.1. Is it necessary to label the content of all nutrients present in a fertiliser?
In accordance with point 1 in PFC 1: Fertiliser in Part II of Annex III, the nutrients declaration is a voluntary declaration and the manufacturers decide which nutrients they want to declare – as long as the requirements in relation to the minimum quantity specified in Annex I are met, except for:
|
— |
Nitrogen (N) or phosphorus pentoxide (P2O5) which have to be indicated as soon as they are above 0,5 % by mass (for more details see sub-section 3.3), |
|
— |
Micronutrients present in the minimum content specified in Annex I, which shall be declared if they are intentionally added to an inorganic or an organo-mineral fertiliser. |
If a nutrient is declared, all the FPR requirements in relation to the nutrient declaration have to be met.
3.2. When the regulation does not define minimum content for secondary nutrients (PFC 1 (A) and PFC 1 (B)), how to label the content of these nutrients?
It is under the responsibility of the manufacturer to declare content of secondary nutrients, taking into account the tolerances which must be applied to them.
3.3. When the content of nitrogen (N) or phosphorus pentoxide (P2O5) has to be indicated as it is above 0,5 % by mass, how should this information be provided?
The indication of the content of nitrogen (N) or phosphorus pentoxide (P2O5) can be a range of values and is shown as part of the label just below the nutrient declaration, and clearly separated by a line or by another labelling information. See the label frame provided as an example sub-section 2.13 of this guidance document. A generic sentence such as ‘the product contains…’ can be used to provide this indication.
3.4. Can the term ‘mineral’ be used instead of or in addition to the term ‘inorganic’ in the designation of the product? Where should the term ‘mineral’ be labelled?
Yes, it is possible to replace the term ‘inorganic’ with ‘mineral’ for the fertiliser that belongs to PFC 1(C) as long as the conditions stated in point 4 in PFC 1: Fertiliser in Part II of Annex III to the FPR are fulfilled. If so, in order to comply with point 1(a) of Part I in Annex III, the manufacturer has to add the PFC index of the respective sub-category to which the product belongs (i.e. PFC 1 (C) (I) (a) (ii)).
Example:
|
— |
Mineral Macronutrient Fertiliser (PFC 1 (C)(I)(a)(i)) |
|
— |
Mineral Macronutrient Fertiliser – PFC 1 (C)(I)(a)(i) |
|
— |
PFC 1 (C)(I)(a)(i): Mineral Macronutrient Fertiliser |
3.5. Does ammoniacal nitrogen (NH3) refer to ammonium nitrogen (NH4 +) for PFC 1?
Yes.
4. SPECIFIC LABELLING REQUIREMENTS FOR PFC 1(A) ORGANIC FERTILISER
4.1. Example of a label
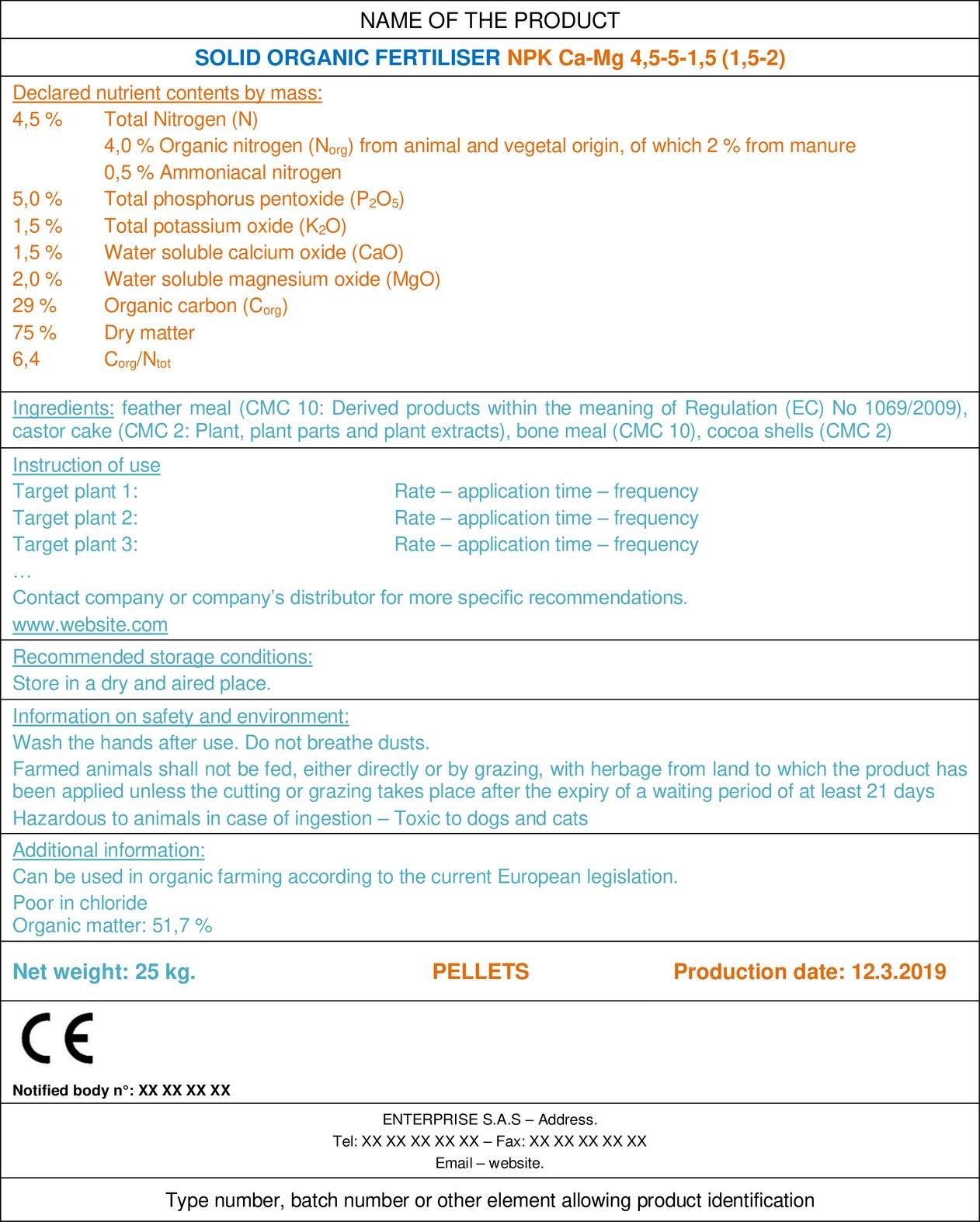
4.2. How to declare organic nitrogen and the origin of organic matter?
It is under the responsibility of the manufacturer to provide pertinent information on the origin of the organic matter in an organic fertiliser. He or she is also responsible for providing any relevant information necessary to manage risks to the environment. For the sake of the user’s compliance with the Nitrates Directive, the declaration of organic nitrogen should therefore at least mention:
|
— |
‘X % organic nitrogen from animal origin, of which Y % from manure’ if the product contains only animal raw material providing organic nitrogen, |
|
— |
‘X % organic nitrogen from vegetal origin’ if the product contains only vegetal raw material providing organic nitrogen, |
|
— |
‘X % organic nitrogen from animal and vegetal origin, of which Y % from manure’ if the product is a mix of animal and vegetal raw material providing organic nitrogen. |
4.3. At which precision level should mandatory information for PFC 1(A) be declared?
This sub-section is particularly relevant for information elements such as the organic carbon and the dry matter content.
The manufacturer is free to define the precision level for the abovementioned information which is most pertinent for the user. For organic carbon content and dry matter content, it is recommended not to go beyond one decimal, as going beyond would not be in accordance with the precision of current analytical methods.
4.4. Should ammoniacal nitrogen be declared even if it is not present in the product?
Ammoniacal nitrogen has to be declared only if it is present in the final product.
4.5. Is it possible to declare organic matter instead of organic carbon?
In accordance with point 11 in Part I of Annex III, it is possible to refer to the organic matter instead of or in addition to the organic carbon (Corg). It is important to respect the following conversion factor:
Corg = organic matter × 0,56
If both are used, the organic matter can be put next to organic carbon (Corg) into brackets, or in the voluntary information section.
4.6. Where to include the information related to the date of production?
The production date is the date on which the product manufacturing process is completed. It is up to the manufacturer to determine the date on which the manufacturing of the product is completed. In case, because of the manufacturing or storage system, the exact production date is not known to the manufacturer, the date of production can be understood as the date when the product is packed. The exact location of the production date on the label/packaging can vary depending on what suits best the product concerned, as long as all the information appears on the label. Thus, it is possible to use so called tracing, i.e. a reference to one single place on the label where the date is indicated. It is up to the economic operator to use the format of his/her choice to indicate the date (letters or numbers) as long as it is a full date (day/month/year). This information has been put in black colour on the label example.
5. SPECIFIC LABELLING REQUIREMENTS FOR PFC 1(B) ORGANO-MINERAL FERTILISER
5.1. Example of a label

5.2. How to declare organic nitrogen and the origin of organic matter?
It is under the responsibility of the manufacturer to provide pertinent information on the origin of organic matter in the Organo-mineral Fertiliser. He or she is also responsible for providing any relevant information necessary to manage risks to the environment. For the sake of the user’s compliance with the Nitrates Directive, the declaration of organic nitrogen should therefore at least mention:
|
— |
‘X % organic nitrogen, from animal origin, of which Y % from manure’ if the product contains only animal raw material providing organic nitrogen;, |
|
— |
‘X % organic nitrogen, from vegetal origin’ if the product contains only vegetal raw material providing organic nitrogen, |
|
— |
‘X % organic nitrogen, from animal and vegetal origin, of which Y % from manure’ if the product is a mix of animal and vegetal raw material providing organic nitrogen. |
5.3. Should a specific form of nitrogen (N), phosphorus (P) or potassium (K) be declared even if it is not present in the product?
Specific forms or solubility of nutrients have to be declared only if present in the final product.
5.4. How to provide pertinent information about the possible air quality impacts of the release of ammonia from the fertiliser use, and an invitation to users to apply appropriate remediation measures when urea (CH4N2O) is present in the product?
The label of all fertilising products marketed according to the FPR and containing urea must refer to the potential air quality impact due to the release of ammonia from the fertiliser use and invite users to take appropriate remediation measures. This statement should be preferably close to or underneath the nutrient declaration, or in the section concerning safety and environment.
The statement may be of general nature, for example, along the following lines:
‘This fertiliser contains urea, which can release ammonia and have an impact on air quality. Depending on local conditions, appropriate remediation measures must be taken.’
or
‘This fertiliser contains urea, which can release ammonia and have an impact on air quality. Depending on local conditions, appropriate remediation measures must be taken. The manufacturer of this fertiliser has already taken the remediation measure of incorporating a urease inhibitor.’
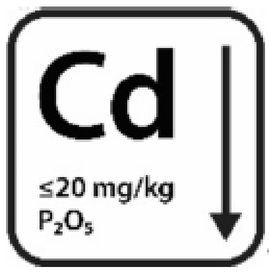
5.5. How to declare the ‘low cadmium content’?
When the product displays a cadmium content equal to or lower than 20 mg/kg phosphorus pentoxide (P2O5), it is possible to declare that the product is low in cadmium content. It is recommended to put this statement in ‘the Additional information’ part of the label. There are various ways to declare this statement, either by text and/or using a pictogram. Should a pictogram be used, it should contain the chemical symbol Cd, but no symbols representing other product features.
5.6. At what precision can micronutrients be declared?
The manufacturer should respect the decimals as referred in the FPR for micronutrients. For more details, see sub-section 6.1.2.
6. SPECIFIC LABELLING REQUIREMENTS FOR PFC 1(C) INORGANIC FERTILISER
6.1. PFC 1 (C)(I): Inorganic Macronutrient Fertiliser
6.1.1. Example of a label
Proposal for nutrient declaration for an inorganic macronutrient fertiliser with micronutrients including link to mineral fertiliser statement:
|
SOLID INORGANIC MACRONUTRIENT FERTILISER NPK (Ca, Mg, S) mineral fertiliser with micro-nutrients, 16-9-12 (+3 +2 +15) / 16-3,9-10 (+2,1 +1,2 +6) Or MINERAL FERTILISER (PFC 1(C)(I)(a)) NPK (Ca, Mg, S) fertiliser with micro-nutrients, 16-9-12 (+3 +2 +15) / 16-3,9-10 (+2,1 +1,2 +6) Or MINERAL FERTILISER (PFC 1(C)(I)(a)) NPK (Ca, Mg, S) complex (4) fertiliser with micro-nutrients, 16-9-12 (+3 +2 +15) / 16-3,9-10 (+2,1 +1,2 +6) Or MINERAL FERTILISER (PFC 1(C)(I)(a)) NPK (Ca, Mg, S) complex fertiliser 16-9-12 (+3 +2 +15) / 16-3,9-10 (+2,1 +1,2 +6) with micro-nutrients
To be used only where there is a recognised need. Do not exceed the application rate. |
Remark: this label example is only showing part of the mandatory labelling (applicable to this category of fertiliser). For an example in full detail, please see the example in sub-section 6.5.
6.1.2. What is the minimum number of decimals that should be indicated on the label?
The FPR is not providing guidance on the number of decimals to be used. The author of the label should keep it legible for the user and therefore it is suggested:
|
— |
To limit it to zero or one decimal for the declaration of macronutrients (N-P-K-Ca-Mg-Na-S), except for those for which minimum declarable quantity values are already defined with one or more decimals in Annex I to the FPR. |
|
— |
To respect, as much as possible, the number of decimals as referred to in the Regulation for the declaration of micronutrients. If needed (for example, to meet tolerance limits) one additional decimal, as referred to in the FPR for micronutrients can be used. |
6.1.3. How to provide pertinent information about the possible air quality impacts of the release of ammonia from the fertiliser use, and an invitation to users to apply appropriate remediation measures when urea (CH4N2O) is present in the product?
The label of all fertilising products marketed according to the FPR and containing urea must refer to the potential air quality impact due to the release of ammonia from the fertiliser use and invite users to take appropriate remediation measures. This statement should be preferably close to or underneath the nutrient declaration, or in the section concerning safety and environment.
The statement may be of general nature, for example, along the following lines:
‘This fertiliser contains urea, which can release ammonia and have an impact on air quality. Depending on local conditions, appropriate remediation measures must be taken.’
or
‘This fertiliser contains urea, which can release ammonia and have an impact on air quality. Depending on local conditions, appropriate remediation measures must be taken. The manufacturer of this fertiliser has already taken the remediation measure of incorporating a urease inhibitor.’
6.1.4. How to declare the ‘low cadmium content’?
When the product displays a cadmium content equal to or lower than 20 mg/kg phosphorus pentoxide (P2O5), it is possible to declare that the product is low in cadmium content. It is recommended to put this statement in the ‘Additional information’ part of the label. There are various ways to declare this statement, either by text and/or using a pictogram. Should a pictogram be used, it should contain the chemical symbol Cd, but no symbols representing other product features.

6.2. PFC 1(C)(I)(a): Solid Inorganic Macronutrient Fertiliser
6.2.1. Example of a label
Please refer to example provided under sub-section 7.1.
6.2.2. Example for granulometry
See below in sub-section in paragraph 6.2.3.
6.2.3. In what way can granulometry and physical unit be indicated on the label? Is it allowed to reference more than one sieve when indicating the granulometry of a product?
The determined sieve(s) is(are) to be defined by the manufacturer depending on the product.
The information in relation to granulometry and physical unit should be provided, preferably grouped on the label. Additional information concerning granulometry can be voluntarily given by the manufacturer, as long as it is compliant with the FPR.
Moreover, it should be allowed to indicate more than one form of the physical unit, as for stability reasons, for example, a combination of more than one physical unit can be present.
Example: Mandatory granulometry and physical unit label descriptions for an inorganic solid macronutrient fertiliser:
|
Granulometry: Powder. 90 % of the product passes through sieve of 1 mm. Granulometry: Granules. X % of the product passes through sieve of Y mm. |
Example: Alternative granulometry and physical unit label descriptions for an inorganic solid macronutrient fertiliser to be compliant to requirements in point 2 of PFC 1(C)(I)(a) in Part II of Annex III:
|
Granulometry: Combination of powder and prills. X % of the product passes through sieve of 1 mm and the remaining Y % through sieve of Z mm. Granulometry: Granules. 95 % of the product has a granular size between 2,0–4,5 mm. |
6.2.4. How is a ‘coating’ defined?
The specific information concerning coated fertilisers should preferably be grouped as much as possible on the label. Information concerning coated fertilisers that must be provided refers to:
|
— |
The functionality period of the coated fertiliser, |
|
— |
The type of coating agent as referred to in point 4 of PFC 1(C)(I)(a) in Part II of Annex III. |
6.2.5. How to declare the functionality period of the coated fertiliser?
See recommendations above under Section 2.5.
6.2.6. How to declare the type of coating agent?
With respect to the coated solid inorganic fertilisers the brand name of the coating agent(s) and the percentage of fertiliser coated by each agent should be indicated. Within the FPR, coating agent is a polymer or sulphur controlling water penetration into nutrient particles and thus the release of nutrients. This information should be followed by the markings: ‘The rate of nutrient releases can vary according to the temperature of the substrate. An adjustment of fertilisation may be necessary.’ In case the fertiliser is coated or partially coated with sulphur as a coating agent the first marking should be rephrased as: ‘The rate of nutrient release can vary according to the temperature of the substrate and the biological activity’.
Example covering all mandatory information as regards coated fertilisers:
|
An X-Y months product. 100 % of the product is coated with BRANDNAME® coating. The rate of nutrient release can vary according to the temperature of the substrate. An adjustment of fertilisation may be necessary. Re-application after less than Y months is not allowed. |
6.2.7. How to draw the label for mined fertilisers?
Mining is the extraction of valuable minerals or other geological materials from the earth, usually from an orebody, lode, vein, seam, reef or placer deposit. These deposits are natural sources of the minerals, which are used as inorganic fertilisers themselves or as raw materials to produce (some) inorganic fertilisers.
Due to the natural origin of those mined fertilisers the content of naturally occurring impurities (minerals not important for the product) can vary in the product during the mining process. However, as impurities should not be included in the list of ingredients (see sub-Section 2.7 of this guidance document for more information), only the mined product (mined mineral) itself should be seen as an ingredient and thus indicated in the ingredient section on the label.
Some mined fertilisers have been known by their mineralogical name for years. Therefore, when listing them in the ingredients section on the label, it is possible to use mineral names (for example, Sylvinite, Langbeinite) in addition to the names used in accordance with Article 18 of the CLP Regulation, and the corresponding identification number of the material (CAS number or EC number) if available.
Example: List of ingredients on the label for mined fertiliser (naturally occurring langbeinite): Ingredients: Langbeinite (Potassium magnesium sulphate) CAS 14977-37-8 (Virgin material substances and mixtures)
6.3. PFC 1(C)(I)(b): Liquid Inorganic Macronutrient Fertiliser
Proposal for nutrient declaration for a liquid inorganic macronutrient fertiliser with micronutrients including link to mineral fertiliser statement:
|
LIQUID INORGANIC MACRONUTRIENT FERTILISER NPK (Ca, Mg, S) fertiliser with micronutrients, 16-9-12 (+3 +2 +15) / 16-3,9-10 (+2,1 +1,2 +6) Or LIQUID MINERAL FERTILISER (PFC 1(C)(I)(b)) NPK (Ca, Mg, S) fertiliser with micronutrients, 16-9-12 (+3 +2 +15) / 16-3,9-10 (+2,1 +1,2 +6) Or LIQUID MINERAL FERTILISER (PFC 1(C)(I)(b)) NPK (Ca, Mg, S) fertiliser 16-9-12 (+3 +2 +15) / 16-3,9-10 (+2,1 +1,2 +6) with micronutrients
Micronutrients are completely water soluble: 0,01 % Boron (B), as sodium salt; 0,020 % Copper (Cu), complexed by HGA; 0,30 % Iron (Fe), 0,26 % as sulphate, 0,04 % chelated by EDTA; 0,05 % Manganese (Mn), as sulphate; 0,006 % Molybdenum (Mo), as sodium salt; 0,008 % Zinc (Zn), as sulphate To be used only where there is a recognised need. Do not exceed the application rate. |
Remark: this label example is only showing part of the mandatory labelling (applicable to this category of fertiliser). For an example in full detail, please see the example in sub-section 6.5.
6.4. PFC 1(C)(II): Inorganic Micronutrient Fertiliser
6.4.1. PFC 1(C)(II)(a): Straight Inorganic Micronutrient Fertiliser
Proposal for nutrient declaration for a straight inorganic micronutrient fertiliser including link to mineral fertiliser statement:
|
STRAIGHT INORGANIC MICRONUTRIENT FERTILISER mineral micronutrient fertiliser Or STRAIGHT INORGANIC MICRONUTRIENT FERTILISER mineral micronutrient fertiliser, 5,3 % Fe Or MINERAL MICRONUTRIENT FERTILISER (PFC 1(C)(II)(a)
To be used only where there is a recognised need. Do not exceed the application rate. |
Remark: this label example is only showing part of the mandatory labelling (applicable to this category of fertiliser). For an example in full detail, please see the example in sub-section 6.5.
6.4.2. PFC 1(C)(II)(b): Compound Inorganic Micronutrient Fertiliser
Proposal for nutrient declaration for a compound inorganic micronutrient fertiliser including link to mineral fertiliser statement:
|
COMPOUND INORGANIC MICRONUTRIENT FERTILISER mineral micronutrient fertiliser in solution Or COMPOUND INORGANIC MICRONUTRIENT FERTILISER mineral micronutrient fertiliser in solution, 0,2 % B, 0,52 % Cu, 2,3 % Fe, 0,5 % Mn, 0,06 % Mo, 0,8 % Zn Or MINERAL MICRONUTRIENT FERTILISER IN SOLUTION (PFC 1(C)(II)(b) Micronutrients are completely water soluble: 0,2 % Boron (B), as sodium salt; 0,52 % Copper (Cu), as sulphate, complexed by HGA; 2,30 % Iron (Fe), 1,04 % chelated by EDTA; 0,5 % Manganese (Mn), as sulphate; 0,06 % Molybdenum (Mo), as sodium salt; 0,8 % Zinc (Zn), as sulphate. or
To be used only where there is a recognised need. Do not exceed the application rate. |
Remark: this label example is only showing part of the mandatory labelling (applicable to this category of fertiliser). For an example in full detail, please see the example in sub-section 6.5.
6.5. PFC 1(C) complete label example


7. SPECIFIC LABELLING REQUIREMENTS FOR PFC 2 LIMING MATERIAL
7.1. Examples of a label
Example 1

Example 2

7.2. Regulatory reference, explanation and voluntary additions
Examples of voluntary additions on the label in section ‘additional information’:
— (5)
Since 2014, liming materials have been labelled according to the criteria set in Regulation (EC) No 2003/2003 as amended by Commission Regulation (EU) No 463/2013 (6). To ensure some consistency in the labelling information and to provide users with familiar information, a reference to the labelling according to this regulation may be provided in the section ‘additional information’ on a voluntary basis.
Alternatively, a reference to product denomination according to standard EN 14069 (7) can be placed voluntary on the label of the liming material. This European Standard specifies the standard and premium requirements of products of natural origin and products from industrial processes to be used as liming materials in agriculture.
—
Annex III to the FPR requires declaration of reactivity and method of determination of reactivity.
In existing commercial practices, three methods are recognized for the determination of the reactivity of liming materials:
|
(a) |
Determination of the reactivity of carbonate and silicate liming materials with hydrochloric acid; |
|
(b) |
Determination of product effect by soil incubation; |
|
(c) |
Determination of the reactivity by automatic titration method with citric acid. |
Annex I to the FPR sets minimum requirements for reactivity with reference to the hydrochloric acid or incubation tests. In some EU Member States the reactivity of liming materials is measured using another test: the citric acid method (as currently described in standard EN 16357 (8)). However, this method is not included in Annex I to the FPR and, therefore, cannot be used to prove compliance with the requirements therein.
The specific labelling requirements for PFC 2 in Annex III do not specify a mandatory reference to one of two tests that are included in Annex I. For labelling purposes, the manufacturer therefore has the possibility to choose among any available measuring tests the one that suits the product best and is of highest value to the user, and declare accordingly the reactivity of his/her product.
8. SPECIFIC LABELLING REQUIREMENT FOR PFC 3 SOIL IMPROVER
8.1. PFC 3(A) Organic Soil Improver
8.1.1. Examples of a label
Example 1: for the labelling of a 100 % peat organic soil improver to be used for instance as an amendment for blueberry cultivation:

Example 2 for labelling of a bulky compost soil improver:

8.1.2. Regulatory reference, explanation and voluntary additions
National regulations, both on the use of the product or on compliance with the requirements for placing it on the national market, may be added on a voluntary basis as long as they are clear to the user and separated from the FPR label.
Possible statements about compliance with the FPR include:
|
|
‘The product fulfils the requirements set for PFC 3(A) (Organic Soil Improver) in Part II of Annex I and for CMC 3 (Compost) in Part II of Annex II to the FPR.’ |
|
|
‘The product fulfils the requirements of Council Regulation (EC) No 834/2007 (Organic production and labelling of organic products).’ (9) |
|
|
‘The production process and the product has been externally controlled according to Module D1: Quality Assurance of the Production Process as described in Part II of Annex IV to the FPR.’ |
8.2. PFC 3(B) Inorganic Soil Improver
8.2.1. Example of a label

8.2.2. Regulatory reference, explanation and voluntary additions
Annex I of the FPR does not provide efficiency criteria or parameters for inorganic soil improvers, meaning that no product specific labelling requirements need to be provided. In the absence of harmonized criteria and their corresponding standards, product suppliers are invited to provide information on efficiency of the product in the section ‘additional information’.
9. SPECIFIC LABELLING REQUIREMENTS FOR PFC 4 GROWING MEDIUM
A PFC 4 product consists of a single bulky (volume-building) component or a mix of bulky (volume-building) components (for example: peat, wood fibers, coconut coir, compost, expanded perlite).
9.1. Examples of a label
Example 1: the labelling of a mineral wool growing medium

Example 2: growing medium consisting of only bulky (volume-building) components
A growing medium cannot contain fertilisers, liming materials, plant biostimulants or products belonging to other PFCs. This type of growing medium (PFC 4) is placed on the market for exceptional applications where the addition of products belonging to other PFCs is not essential. It will also serve as the basis for Fertilising Product Blends (PFC 7) containing other PFCs. Any Growing Medium (PFC 4) blended with one or more products of any other PFC (for example fertiliser, liming material, plant biostimulants) is a PFC 7. An example is given in Section 12 on the labelling requirements for PFC 7.

Remark: This label frame is given as a general, indicative example of the label structure.
9.2. Regulatory reference, explanation and voluntary additions
National regulations may be added on a voluntary basis as long as they are clear to the user and separated from the FPR label.
10. SPECIFIC LABELLING REQUIREMENTS FOR PFC 5 INHIBITORS
10.1. PFC 5(A) Nitrification Inhibitor
Example:

10.2. PFC 5(B) Denitrification Inhibitor
At the moment no denitrification inhibitors are commercially available on the EU market. The general label layout should be similar to the layout for a nitrification and/or urease inhibitor.
10.3. PFC 5(C) Urease Inhibitor
Example:

11. SPECIFIC LABELLING REQUIREMENTS FOR PFC 6 PLANT BIOSTIMULANT
11.1. Examples of a label
11.1.1. PFC 6(A) Microbial Plant Biostimulant
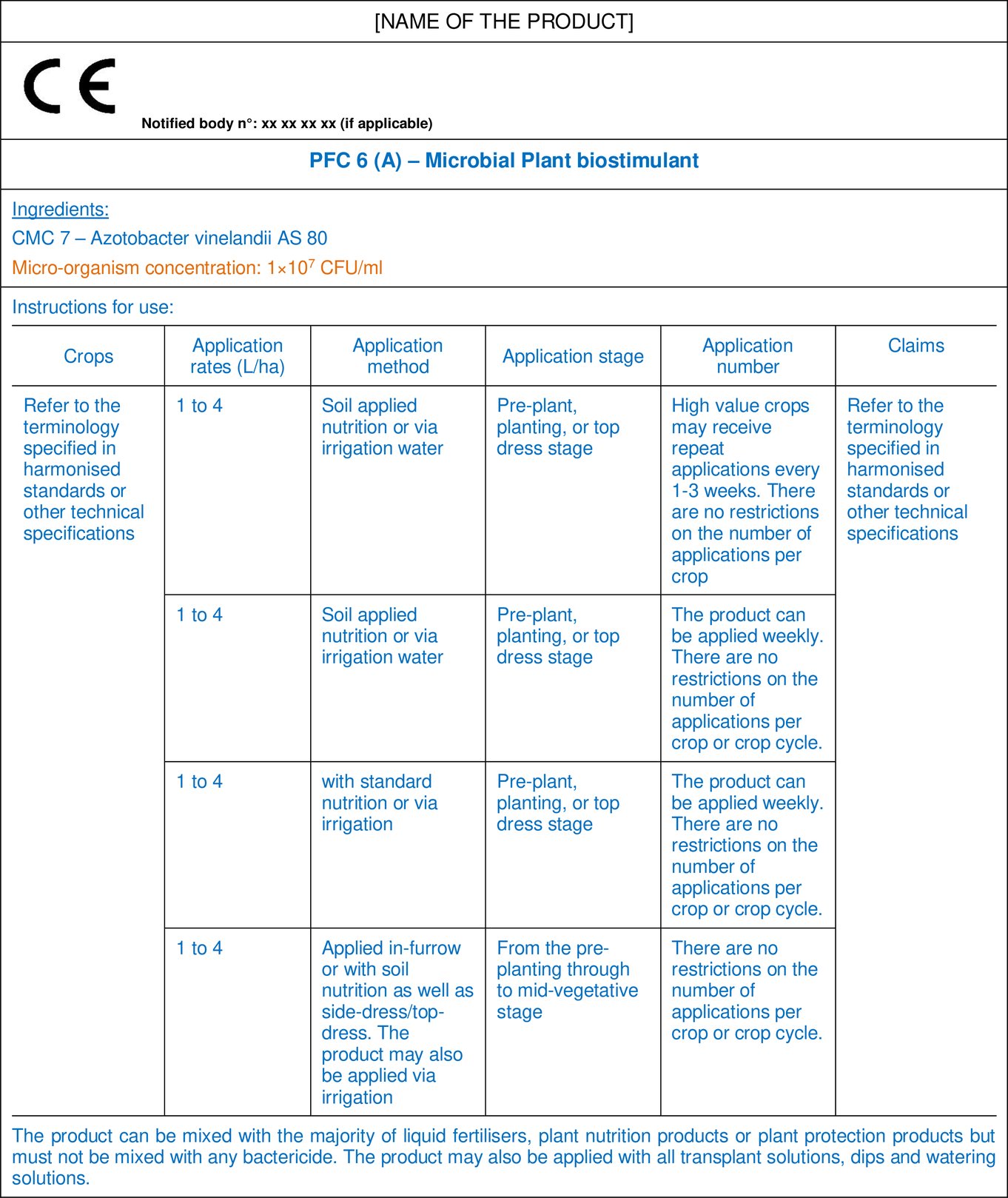

11.1.2. PFC 6(B) Non-Microbial Plant Biostimulant

11.2. How to label the physical form of the product?
The physical form (liquid or solid) should be indicated.
11.3. How to provide the relevant instructions related to the efficacy of the product, including soil management practices, chemical fertilisation, incompatibility with plant protection products, recommended spraying nozzles size, sprayer pressure and other anti-drift measures?
The instructions of use can be provided in a table format, as indicated in the examples in sub-section 11.1, including information such as crops, application rate, application method, application stage, application number and claims. The claimed effects should correspond to the ones indicated in the biostimulant definition, namely: nutrient use efficiency, tolerance to abiotic stress, quality traits, or availability of confined nutrients in the soil or rhizosphere. These should preferably be complemented by the claimed effects identified in harmonised standards for biostimulants.
11.4. How to include a statement regarding the fact that micro-organisms may have the potential to provoke sensitising reactions?
The label shall contain the following phrase: ‘Micro-organisms may have the potential to provoke sensitising reactions’. This phrase should be included within other hazard phrases in the label section ‘Information on Safety and Environment’.
11.5. How to provide the production and expiry date and where to place it on the label?
The production and expiration date should be provided on the label. The determination of the product expiry date should be up to the manufacturer. The production and expiry date can also be located directly on the package or on a folded leaflet (in case of a bulk product).
11.6. Specific instructions for Microbial Biostimulants
Within the part of the label ‘Declaration of content’ all intentionally added micro-organisms shall be indicated. Where the micro-organism should have several strains, the intentionally added strains should be indicated. The microorganism concentration is to be expressed as the number of active units per volume or weight, or in any other manner that is relevant to the micro-organism, for example, colony forming units per gram (cfu/g).
12. SPECIFIC LABELLING REQUIREMENTS FOR PFC 7 FERTILISING PRODUCT BLEND
As stated in the FPR, all the labelling requirements applicable to all component EU fertilising products apply to the fertilising product blend. For a better understanding, labelling requirements specific to each PFC are identified below by a colour code in the labelling examples.
12.1. Examples of a label
The following examples assume that the blending does not lead to a change of nature of each of the component of the respective fertilising product blends.
Example 1: Labelling of a fertilising product blend composed of 2 EU fertilising products from the same PFC (an already EU compliant PFC 1 (C) in light blue with another already EU compliant PFC 1 (C) in dark blue)

Example 2: Labelling of a fertilising product blend of 2 claimed functions: mixture of an already EU compliant PFC 1 (C) (inorganic fertiliser) in blue with another already EU compliant PFC 5 (inhibitor) in orange

Example 3: Labelling of a fertilising product blend of 3 claimed functions: PFC 4 (growing medium) in red with a PFC 1 (C)(I) (Compound Solid Inorganic Macronutrient Fertiliser) in blue and a PFC 2 (liming material) in orange
As explained in the Section 9, any growing medium blended with one or more other PFC (for example fertiliser, liming material, biostimulants) is a fertilising product blend.


Example 4: Labelling of a fertilising product blend of 3 claimed functions: PFC 1(C) (inorganic fertiliser) in blue + PFC 2 (liming material) in orange + PFC 6(B) (non-microbial plant biostimulant) in red

Example 5: Labelling of a fertilising product blend of 2 claimed functions: PFC 6(B) (non-microbial plant biostimulant) in red and PFC 1(B) (organic fertiliser) in blue

12.2. How to express labelling requirements for PFC 7?
As specified in Annex III to the FPR, labelling requirements of all component EU fertilising products apply to the fertilising product blend. They shall be expressed in relation to the final product.
If a labelling requirement applies to only one component EU fertilising product, it also applies to final fertilising product blend. In other words, a labelling requirement, which is relevant for a component, is also relevant for the entire blend.
As a general rule, labelling requirements of component EU fertilising products should be expressed for the final fertilising product blend.
If minimum content or concentrations are required for a specific component EU fertilising product of a fertilising product blend, they do not apply to the blend.
Example: The nutrient content of a fertilising product blend of which 10 % is a solid organic fertiliser with 4 % of total nitrogen (N) and 12 % of total potassium oxide (K2O), as declared nutrients, will be expressed for the final product blend as such:
|
— |
0,4 % total nitrogen (N) |
|
— |
1,2 % total potassium oxide (K2O) |
The minimum content requirement of 1 % of total nitrogen for solid organic fertilisers does not apply to the fertilising product blend.
If a labelling requirement doesn’t provide any useful information when expressed for the final fertilising product blend, or if it is not possible to express it for the final fertilising product blend, then it is expressed for the specific component EU fertilising product concerned. In that case, the percentage of the component EU fertilising product in the fertilising product blend is indicated.
Example: The labelling of reactivity of a fertilising product blend containing a liming material would be declared as follows:

If a labelling requirement is common to several component EU fertilising products, but has different ways of expression, both labelling requirements are mentioned on the label of the final fertilising product blend and expressed for each PFC respectively.
Example: Granulometry can be expressed as % by mass of product passing through different sieves (through a 1,0 mm sieve for liming materials and through a determined sieve for solid inorganic fertilisers that can be different than 1,0 mm).
Granulometry for a fertilising product blend containing a liming material and a solid inorganic fertiliser could be labelled as follows:
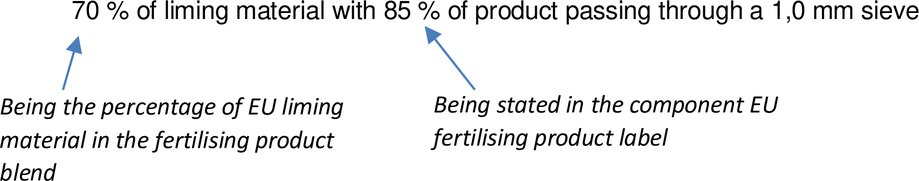
If an expiry date applies for one component EU fertilising product, it will also apply for the final fertilising product blend. The expiration date should be adapted according to the final fertilising product blend and cannot be later than the one applicable to the component EU fertilising product.
If this requirement applies to several components of the EU fertilising products, the most restrictive date applies.
If a notification body number is present on one or more component EU fertilising products label, it has also to be put on the label of the final fertilising product blend with the reference of the component EU fertilising product.
Example: Fertilising product blend composed of EU fertilising product which went through Module D1

Notified body number: 0123 (inhibitor)
The number of the notified body has to be put on the labels only for fertilising products having had their conformity assessed through Module A1 and Module D1.
(1) Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003 (OJ L 170, 25.6.2019, p. 1).
(2) Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 (OJ L 353, 31.12.2008, p. 1).
(3) Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by-products Regulation) (OJ L 300, 14.11.2009, p. 1).
(4) Only applicable for those fertilisers that fit the definition of complex (each physical unit contains all the declared nutrients in their declared content).
(5) Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 relating to fertilisers (OJ L 304, 21.11.2003, p. 1).
(6) Commission Regulation (EU) No 463/2013 of 17 May 2013 amending Regulation (EC) No 2003/2003 of the European Parliament and of the Council relating to fertilisers for the purposes of adapting Annexes I, II and IV thereto to technical progress (OJ L 134, 18.5.2013, p. 1).
(7) EN 14069:2017, Liming materials – Denominations, specifications and labelling.
(8) EN 16357:2013, Carbonate liming materials – Determination of reactivity – Automatic titration method with citric acid.
ANNEX
Example of a full label frame (for illustration purposes)
|
Section and Subsection |
References and details |
||||||||||
|
PFC designation |
Annex III – Part I: General Requirements (point 1.a-b) PFC 1 to 6 PFC 7: Designations of all PFCs claimed |
||||||||||
|
PFC 1 (point 4) for PFC 1 C under conditions |
||||||||||
|
relevant typology for PFC 1(C)(II)(a) – Annex III – Part II – PFC 1(C)(II)(a) – point 1 as referred to in the table under PFC 1(C)(II)(a) in Part II of Annex I |
||||||||||
|
Declaration of nutrients |
In PFCs (Annex III – Part II) |
||||||||||
|
Fertiliser: Content of nutrients may be declared only where they are present in the minimum quantity specified in Annex I for the relevant PFC (PFC 1 Point 1) Organic fertiliser: PFC 1 (A) (points a-b-c) Organo-mineral fertiliser: PFC 1 B (point 1.a-b-c) Inorganic fertiliser:
|
||||||||||
|
When fertilisers contain inhibitors PFC 1 (point 3.a) |
||||||||||
|
Inorganic fertiliser: PFC 1(C)(I)(a) under conditions (PFC 1 (C)(I)(a) point 1) |
||||||||||
|
Content |
Annex III – Part II |
||||||||||
|
Nutrient forms and solubilities… |
||||||||||
|
Organic fertiliser: PFC 1 (A) (point d) Organo-mineral fertiliser: PFC 1 (B) (point 1.d) Inorganic fertiliser:
|
||||||||||
|
Organic fertiliser: PFC 1(A) (point d) Organo-mineral fertiliser: PFC 1 (B) (point 1.d) Inorganic fertiliser:
|
||||||||||
|
Organic fertiliser: PFC 1 (A) (point (d)(v)) Organo-mineral fertiliser: PFC 1 (B) (point 1(d)(v)) organic carbon (Corg) = organic matter × 0,56 |
||||||||||
|
Organic fertiliser: PFC 1 (A) (point d.vi) Organo-mineral fertiliser: PFC 1 (B) (point 1.d.vi) |
||||||||||
|
Organic fertiliser: PFC 1 (A) (point e) |
||||||||||
|
Organo-mineral fertiliser: PFC 1 (B) (points 2-3-4-5) Inorganic fertiliser:
|
||||||||||
|
PFC 1 (point 3.b.c.d) |
||||||||||
|
Inorganic fertiliser: Coated fertiliser PFC 1 (C)(I) (a) (point 4) |
||||||||||
|
Annex III – Part II – PFC 2 |
||||||||||
|
|
||||||||||
|
Expressed as % by mass of product passing through a sieve of 1,0 mm |
||||||||||
|
Expressed as % by mass |
||||||||||
|
Expressed as % by mass |
||||||||||
|
Except for oxide and hydroxide limes |
||||||||||
|
Annex III – Part II – PFC 3 |
||||||||||
|
PFC 3 (Point 1) |
||||||||||
|
If exceeding 0,5 % by mass: N, P2O5 and K2O PFC 3 (Point 2) |
||||||||||
|
Organic soil improver PFC 3(A) |
||||||||||
|
Organic soil improver PFC 3(A) given as mS/m |
||||||||||
|
Organic soil improver PFC 3(A) expressed as % by mass organic carbon (C org) = organic matter × 0,56 |
||||||||||
|
Organic soil improver PFC 3(A) expressed as % by mass followed by a description of the origin of the organic matter used |
||||||||||
|
Organic soil improver PFC 3(A) |
||||||||||
|
Annex III – Part II – PFC 4 |
||||||||||
|
given as mS/m except for mineral wool; |
||||||||||
|
|
||||||||||
|
|
||||||||||
|
(calcium chloride/ diethylenetriaminepentaacetic acid; ‘CAT-soluble’) if above 150 mg/l |
||||||||||
|
(calcium chloride/ diethylenetriaminepentaacetic acid; ‘CAT-soluble’) if above 20 mg/l |
||||||||||
|
(calcium chloride/ diethylenetriaminepentaacetic acid; ‘CAT-soluble’) if above 150 mg/l |
||||||||||
|
Physical data (for Fertiliser) |
Annex III – Part II |
||||||||||
|
Organic fertiliser: PFC 1 A point g, if applicable Inorganic fertiliser: PFC 1 (C)(I) solid:‘granules’, ‘pellets’, ‘powder’ (powder, where at least 90 % by mass of the product can pass through a sieve with a mesh of 1 mm), ‘prills’ (PFC 1 (C)(I) (a) point 3) liquid: PFC (1)(C)(I)(b): ‘in suspension’ or ‘in solution’ (PFC 1 C.I b point 1) |
||||||||||
|
Inorganic fertiliser: PFC 1 (C)(I)(a) (point 2): expressed as % by mass of the product passing through a determined sieve. |
||||||||||
|
Plant Biostimulants |
Annex III – Part II – PFC 6 |
||||||||||
|
PFC 6 (a) |
||||||||||
|
PFC 6 (c) |
||||||||||
|
PFC 6 (d) |
||||||||||
|
Related to the efficacy of the product, including soil management practices, chemical fertilisation, incompatibility with plant protection products, recommended spraying nozzles size, sprayer pressure and other anti-drift measures. PFC 6 (e) |
||||||||||
|
Microbial Plant Biostimulant PFC 6 (A) Intentionally added strains when micro-organism has several strains |
||||||||||
|
+ quantity (concentration) |
Microbial Plant Biostimulant PFC 6 (A) Expressed as the number of active units per volume or weight, or in any other manner that is relevant to the micro-organism, e.g. colony forming units per gram (cfu/g). |
||||||||||
|
+ phrase: ‘Micro-organisms may have the potential to provoke sensitising reactions’ |
Microbial Plant Biostimulant PFC 6 A |
||||||||||
|
Complementary statements |
If applicable |
||||||||||
|
Voluntary statement, under conditions: Annex III – Part I: General Requirements (point 9) |
||||||||||
|
If applicable |
||||||||||
|
If intentionally added micronutrients: Annex III – Part II Organo-mineral fertiliser: PFC (1) (B) (point 5b) Inorganic fertiliser
|
||||||||||
|
Annex III – Part II Inorganic fertiliser: Coated fertiliser: PFC 1 (C)(I) (a) (point 4) here or in ‘Instructions for intended use, including application rates, timing and frequency, and target plants or mushrooms’ section |
||||||||||
|
List of ingredients |
Annex III – Part I: General Requirements (point 1.h) |
||||||||||
|
Ingredients above 5 % by product weight |
||||||||||
|
Annex III – Part II – PFC 5 All ingredients in decreasing order |
||||||||||
|
Nitrogen (N) or phosphorus pentoxide (P2O5) above 0,5 % by mass |
Fertiliser: For PFC 1 (point 2) and when N and P2O5 are above 0,5 % by mass and not declared in ‘Content’ section Indication separate from the nutrient declaration |
||||||||||
|
Instructions for use |
|
||||||||||
|
Annex III – Part I: General Requirements (point 1.d) |
||||||||||
|
Annex III – Part I: General Requirements (point 3) If fertilising product contains a substance for which maximum residue limits for food and feed have been established |
||||||||||
|
Annex III – Part I: General Requirements (point 1.f) For products containing a polymer belonging to CMC 9. |
||||||||||
|
Recommended storage conditions |
Annex III – Part I: General Requirements (point 1.e) |
||||||||||
|
Safety/Environment |
|
||||||||||
|
Annex III – Part I: General Requirements (point 1.g) |
||||||||||
|
Annex III –Part I: General Requirements (points 4-5-6-7) |
||||||||||
|
Annex III – Part II – Inorganic fertiliser PFC 1 (C) (I) (point 1.e on urea & air quality) |
||||||||||
|
Additional information (optional information, under conditions) |
Annex III – Part I: General Requirements (point 8) under conditions |
||||||||||
|
Voluntary statement, under conditions, Annex III – Part II:
|
||||||||||
|
Requirements with no specific position on the label: |
|
||||||||||
|
Annex III – Part II PFC 1 (A) (f) & PFC 4 & PFC 6 (b) PFC 6 (b) |
||||||||||
|
Article 6 (point 6.5) |
||||||||||
|
Annex III – Part I: General Requirements (point 1.c) |
||||||||||
|
Chapter II Article 6 (point 6.6) |
||||||||||
|
Chapter II Article 8 (point 3) |
||||||||||
|
Chapter II Article 11 a Packaging and repackaging by importers and distributors |
||||||||||
|
CE Marking |
Article 18 (point 1) |
||||||||||
|
+ identification number of the notified body, if applicable |
Article 18 (point 3) – following CE marking where applicable under Annex IV module A1 and module D1 |

