ISSN 1977-0677
Official Journal
of the European Union
L 379

English edition
Legislation
Volume 63
13 November 2020
|
ISSN 1977-0677 |
||
|
Official Journal of the European Union |
L 379 |
|

|
||
|
English edition |
Legislation |
Volume 63 |
|
|
|
|
|
(1) Text with EEA relevance. |
|
EN |
Acts whose titles are printed in light type are those relating to day-to-day management of agricultural matters, and are generally valid for a limited period. The titles of all other Acts are printed in bold type and preceded by an asterisk. |
II Non-legislative acts
REGULATIONS
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/1 |
COMMISSION DELEGATED REGULATION (EU) 2020/1676
of 31 August 2020
amending Article 25 of Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures as regards bespoke paints
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 (1), and in particular Article 53(1) thereof,
Whereas:
|
(1) |
Regulation (EC) No 1272/2008 was amended by Commission Regulation (EU) 2017/542 (2) to add certain requirements for the submission of information relating to emergency health response and for the inclusion of a ‘unique formula identifier’ (UFI) in the supplemental information provided on the label of a hazardous mixture. Importers and downstream users are required to start complying with the requirements in stages, according to a series of compliance dates depending on the use for which a mixture is placed on the market. |
|
(2) |
The paints sector has raised a specific concern regarding the workability of the emergency health response requirements in the case of paints formulated in limited amounts on a tailor-made basis for an individual consumer or professional user at the point of sale. In order to satisfy customer demand for very specific paint shades, formulators can be asked to formulate and supply paints with an almost unlimited number of different compositions. Compliance with the emergency health response requirements would therefore require formulators either to submit information and create UFIs in advance for an extremely large number of paints of all possible colour combinations, many of which may never be supplied in reality, or else to postpone each supply at the point of sale until the information had been submitted and the UFI had been created. Either approach would place a disproportionate burden on the bespoke paints industry. |
|
(3) |
In order to avoid disproportionate administrative burden, in particular for small and medium-sized enterprises, for bespoke paints, the requirements are to be amended by Commission Delegated Regulation (EU) 2020/1677 (3) to provide for the possibility to exempt bespoke paints from the notification obligations in Annex VIII to Regulation (EC) No 1272/2008 and from the obligation in that Annex to create a UFI. However, in that case, in order to allow poison centres to formulate a suitable emergency health response, the individual mixtures contained in bespoke paints are to remain subject to all the requirements of Annex VIII. |
|
(4) |
In the light of that, it is appropriate to amend Article 25 of Regulation (EC) No 1272/2008 in order to lay down a rule for bespoke paints for which no information is notified and no corresponding UFI created requiring the UFIs of all the mixtures contained in the bespoke paint to be indicated on the label of the bespoke paint. In addition, if the concentration of a mixture with a UFI contained in the bespoke paint exceeds 5 %, the concentration should be included in the supplemental information indicated on the label of the bespoke paint, since mixtures in bespoke paints of such concentration are more likely to be relevant for emergency health responses. |
|
(5) |
Considering that the compliance date for mixtures for consumer and professional use of 1 January 2021 laid down in Annex VIII to Regulation (EC) No 1272/2008 is approaching, and that this Regulation enables all sectors to comply with that Annex, this Regulation should enter into force as early as possible. |
|
(6) |
Regulation (EC) No 1272/2008 should therefore be amended accordingly, |
HAS ADOPTED THIS REGULATION:
Article 1
In Article 25 of Regulation (EC) No 1272/2008, the following paragraph is added:
‘8. In the case of a bespoke paint for which no submission in accordance with Annex VIII has been made and no corresponding unique formula identifier has been created, the unique formula identifiers of all the mixtures contained in the bespoke paint in a concentration exceeding 0,1 % which themselves are subject to notification under Article 45 shall be included in the supplemental information on the label of the bespoke paint, located together and listed in descending order of the mixtures’ concentration in the bespoke paint, in accordance with the provisions of Section 5 of Part A of Annex VIII.
In a case falling within the first subparagraph, where the concentration of a mixture with a unique formula identifier in the bespoke paint exceeds 5 %, the concentration of that mixture shall also be included in the supplemental information on the label of the bespoke paint next to its unique formula identifier, in accordance with Section 3.4 of Part B of Annex VIII.
For the purposes of this paragraph, “bespoke paint” means a paint that is formulated in limited amounts on a tailor-made basis for an individual consumer or professional user at the point of sale by tinting or colour mixing.’
Article 2
This Regulation shall enter into force on the day after its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in the Member States.
Done at Brussels, 31 August 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 353, 31.12.2008, p. 1.
(2) Commission Regulation (EU) 2017/542 of 22 March 2017 amending Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures by adding an Annex on harmonised information relating to emergency health response (OJ L 78, 23.3.2017, p. 1).
(3) Commission Delegated Regulation (EU) 2020/1677 of 31 August 2020 amending Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures in order to improve the workability of information requirements related to emergency health response (See page 3 of this Official Journal).
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/3 |
COMMISSION DELEGATED REGULATION (EU) 2020/1677
of 31 August 2020
amending Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures in order to improve the workability of information requirements related to emergency health response
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 (1), and in particular Article 45(4) thereof,
Whereas:
|
(1) |
Regulation (EC) No 1272/2008 was amended by Commission Regulation (EU) 2017/542 (2) to add certain requirements for the submission of information relating to emergency health response and for the inclusion of a ‘unique formula identifier’ in the supplemental information provided on the label of a hazardous mixture. The requirements were amended by Commission Delegated Regulation (EU) 2020/11 (3). Importers and downstream users are required to start complying with the requirements in stages, according to a series of compliance dates depending on the use for which a mixture is placed on the market |
|
(2) |
Concerns have been raised by various industry sectors regarding the workability of the emergency health response information requirements in certain cases, notably with regard to the difficulty of knowing the exact composition of mixtures in cases where raw materials with a highly variable or unknown composition are used in the manufacture of the mixture, in cases where toxicologically very similar components supplied by multiple, different suppliers are used together in the same production line, or in cases involving complex supply chains. Concerns have also been raised, in the case of bespoke mixtures, about the impossibility of knowing in advance which exact bespoke mixtures are to be placed on the market. |
|
(3) |
It is necessary to address the situation where different but toxicologically very similar components are used in a mixture, and where it is unknown which component is present in a particular mixture placed on the market at a given time. To ensure that the emergency health response requirements can be complied with properly in practice, importers and downstream users should be allowed to group toxicologically similar components of a mixture together in an interchangeable component group and provide information on the total concentration of those components present in the mixture, without having to specify their separate concentrations. In order to allow poison centres to formulate a suitable emergency health response, components should only be grouped in an interchangeable component group if their classification for health and physical effects is identical and if the hazards identification and the additional hazard information are identical for all possible combinations of the resulting final mixture incorporating those components. For components classified for certain hazard classes, it should also be necessary for them to have the same technical function and the same toxicological properties in order to be grouped. |
|
(4) |
In order to address particular difficulties encountered by the gypsum, ready-mixed concrete and cement sectors and to allow them to comply with the emergency health response requirements without reducing the level of safety, it should be possible for emergency health response information relating to certain standardised mixtures within those three sectors to be submitted by reference to a standard composition. However, in order to allow poison centres to formulate a suitable emergency health response, this option should only be available in cases where the mixture classification does not change according to the mixture’s composition within the concentration ranges specified in the standard formula, and where the information on composition is at least as detailed as the information contained in the mixture’s safety data sheet, drawn up in accordance with Article 31 of Regulation (EC) No 1907/2006 of the European Parliament and of the Council (‘safety data sheet’) (4). In the event that the information contained in the safety data sheet is more detailed than the information on the composition in the standard formula, importers and downstream users should be required to notify the information in the safety data sheet instead. |
|
(5) |
In order to address particular difficulties anticipated for certain fuels, and taking into account the facts that fuels placed on the market normally conform to a technical standard and that poison centres have communicated a low number of poisoning incidents with fuels, it should be possible, until a more suitable solution is found, to submit emergency health response information by reference to the information contained in the safety data sheet, as well as any other known information on the products’ chemical composition. |
|
(6) |
In order to satisfy customer demand for very specific paint shades, formulators are sometimes asked to formulate and supply paints on a bespoke basis at the point of sale. These bespoke paints could have an almost unlimited number of different compositions. Therefore, without any mitigating measures, compliance with the emergency health response requirements in Annex VIII to Regulation (EC) No 1272/2008 would require formulators of bespoke paints either to submit information and create unique formula identifiers (UFIs) in advance for an extremely large number of paints of all possible colour combinations, many of which might never be supplied in reality, or to postpone each supply until the information had been submitted and the UFI had been created. Either approach would place a disproportionate burden on the bespoke paints industry, in particular small and medium-sized enterprises, without improving the level of safety significantly. |
|
(7) |
Poison centres have not communicated a significant number of accidents related to paints. In light of the apparently lower risks compared to other mixtures, it is justified to allow a more flexible approach, as this would not be reducing the current level of safety. |
|
(8) |
It is therefore appropriate to provide for the possibility to exempt bespoke paints from the notification obligations in Annex VIII and from the requirement to create a UFI. However, in that case, in order to allow poison centres to formulate a suitable emergency health response, the individual mixtures contained in bespoke paints should remain subject to all the requirements of that Annex. Alongside this Regulation, Commission Delegated Regulation (EU) 2020/1676 (5) amends Article 25 of Regulation (EC) No 1272/2008 to add a new rule, in the case of bespoke paints for which no submission in accordance with Annex VIII has been made and no corresponding UFI has been created, requiring the UFIs of all the individual mixtures contained in the bespoke paint to be indicated on the label of the bespoke paint, together with the specific concentration of each such mixture with a UFI that is present in a concentration exceeding 5 %. |
|
(9) |
Given the number of changes to Annex VIII to Regulation (EC) No 1272/2008, it is appropriate to replace the whole Annex for the sake of legal clarity. |
|
(10) |
Considering that the compliance date for mixtures for consumer and professional use of 1 January 2021 laid down in Annex VIII to Regulation (EC) No 1272/2008 is approaching, and that this Regulation enables all sectors to comply with that Annex, this Regulation should enter into force as early as possible. |
|
(11) |
Regulation (EC) No 1272/2008 should therefore be amended accordingly, |
HAS ADOPTED THIS REGULATION:
Article 1
Annex VIII to Regulation (EC) No 1272/2008 is replaced by the text in the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the day after its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 31 August 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 353, 31.12.2008, p. 1.
(2) Commission Regulation (EU) 2017/542 of 22 March 2017 amending Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures by adding an Annex on harmonised information relating to emergency health response (OJ L 78, 23.3.2017, p. 1).
(3) Commission Delegated Regulation (EU) 2020/11 of 29 October 2019 amending Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures as regards information relating to emergency health response (OJ L 6, 10.1.2020, p. 8).
(4) Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC (OJ L 396, 30.12.2006, p. 1).
(5) Commission Delegated Regulation (EU) 2020/1676 of 31 August 2020 amending Article 25 of Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures as regards bespoke paints (See page 1 of this Official Journal).
ANNEX
‘ANNEX VIII
HARMONISED INFORMATION RELATING TO EMERGENCY HEALTH RESPONSE AND PREVENTATIVE MEASURES
PART A
GENERAL REQUIREMENTS
1. APPLICATION
|
1.1. |
Importers and downstream users placing on the market mixtures for consumer use, within the meaning of Section 2.4 of Part A of this Annex, shall comply with this Annex from 1 January 2021. |
|
1.2. |
Importers and downstream users placing on the market mixtures for professional use, within the meaning of Section 2.4 of Part A of this Annex, shall comply with this Annex from 1 January 2021. |
|
1.3. |
Importers and downstream users placing on the market mixtures for industrial use or mixtures with an end use not subject to notification within the meaning of Section 2.4 of Part A of this Annex, shall comply with this Annex from 1 January 2024. |
|
1.4. |
Importers and downstream users having submitted information relating to hazardous mixtures to a body appointed in accordance with Article 45(1) before the dates of applicability mentioned in Sections 1.1, 1.2 and 1.3 and which are not in accordance with this Annex, shall for those mixtures not be required to comply with this Annex until 1 January 2025. |
|
1.5. |
By way of derogation from Section 1.4, if one of the changes described in Section 4.1 of Part B of this Annex occurs before 1 January 2025, importers and downstream users shall comply with this Annex before placing that mixture, as changed, on the market. |
2. PURPOSE, SCOPE AND DEFINITIONS
|
2.1. |
This Annex sets out the requirements that importers and downstream users placing mixtures on the market, hereinafter “submitters” shall fulfil in respect of the submission of information so that appointed bodies shall have at their disposal the information to carry out the tasks for which they are responsible under Article 45. |
|
2.2. |
This Annex shall not apply to mixtures for scientific research and development and to mixtures for product and process oriented research and development as defined in Article 3(22) of Regulation (EC) No 1907/2006.
This Annex shall not apply to mixtures classified only for one or more of the following hazards:
|
|
2.2a. |
In the case of bespoke paints, submitters may, without prejudice to Article 25(8), opt not to submit information and not to create a Unique Formula Identifier in accordance with this Annex. |
|
2.3. |
In the case of mixtures with an end use not subject to notification or mixtures placed on the market for industrial use only, submitters may opt for a limited submission, as an alternative to general submission requirements, in accordance with the second subparagraph of Section 3.1 of Part B, provided that a rapid access to additional detailed product information is available in accordance with Section 1.3 of that Part. |
|
2.4. |
For the purposes of this Annex, the following definitions shall apply:
|
3. SUBMISSION REQUIREMENTS
|
3.1. |
Before placing mixtures on the market, submitters shall provide information relating to mixtures classified as hazardous on the basis of their health or physical effects to the bodies appointed under Article 45(1) (“appointed bodies”), in the Member State or Member States where the mixture is placed on the market.
The submission shall contain the information laid down in Part B. It shall be submitted by electronic means in an XML format provided by the Agency and made available free of charge. |
|
3.2. |
Where following receipt of a submission under Section 3.1 an appointed body makes a reasoned request to the submitter that additional information or clarification is necessary for that appointed body to carry out the tasks for which it is responsible under Article 45, the submitter shall provide the necessary information or clarification requested without undue delay. |
|
3.3. |
The submission shall be in the official language(s) of the Member State(s) where the mixture is placed on the market, unless the Member State(s) concerned provide(s) otherwise. |
|
3.4. |
The intended use of the mixture shall be described in accordance with a harmonised product categorisation system provided by the Agency. |
|
3.5. |
A submission update shall be made without undue delay when the conditions laid down in Section 4.1 of Part B are met. |
4. GROUP SUBMISSION
|
4.1. |
A single submission may be provided for more than one mixture where all the mixtures in a group have the same classification for health and physical hazards. Such a submission shall be referred to as a “group submission”. |
|
4.2. |
A group submission shall only be permitted when all mixtures in the group contain the same components (as identified in Section 3.2 of Part B), and for each of the components, the reported concentration range is the same for all mixtures (as provided in Section 3.4 of Part B). |
|
4.3. |
By way of derogation from Section 4.2, a group submission shall also be allowed where the difference in the composition between different mixtures in the group only concerns perfumes, provided that the total concentration of the differing perfumes contained in each mixture does not exceed 5 %. |
|
4.4. |
In the case of a group submission, the information required in Part B shall be provided for each of the mixtures contained in the group where applicable. |
5. UNIQUE FORMULA IDENTIFIER (UFI)
|
5.1. |
The submitter shall create a Unique Formula Identifier (“UFI”) by electronic means made available by the Agency. The UFI is a unique alphanumeric code that unambiguously links the submitted information on the composition of a mixture or a group of mixtures to a specific mixture or group of mixtures. The assignment of a UFI is free of charge.
A new UFI shall be created when a change in the composition of the mixture or group of mixtures fulfils one or more of the conditions laid down in points (a), (b) and (c) of the fourth indent of the first subparagraph of Section 4.1 of Part B or, as the case may be, one or other of the conditions laid down in the second subparagraph of that Section. By way of derogation from the second subparagraph of this Section, a new UFI shall not be required for mixtures in a group submission containing perfumes provided that the change in the composition only concerns those perfumes or the addition of new perfumes. By way of derogation from the second subparagraph of this Section, a new UFI shall not be required where a change fulfilling the condition foreseen in point (a) of the fourth indent of the first subparagraph of Section 4.1 of Part B solely concerns one or more components grouped in an interchangeable component group already included in the submission in accordance with Section 3.5 of Part B. |
|
5.2. |
The UFI shall be preceded by the acronym “UFI” in capital letters followed by a colon (“UFI:”) and it shall be clearly visible, legible and indelibly marked. |
|
5.3. |
Instead of including the UFI in the supplemental information on the label, the submitter may opt to print or affix it on the inner packaging located with the other label elements.
Where the inner packaging is either in such a shape or so small that it is impossible to affix the UFI on it, the submitter may print or affix the UFI located with the other label elements on an outer packaging. In the case of mixtures which are not packaged, the UFI shall be indicated in the Safety Data Sheet or be included in the copy of the label elements referred to in Article 29(3), as applicable. In the case of packaged mixtures supplied for use at an industrial site, instead of including the UFI on the label or packaging, the submitter may opt to indicate it in the Safety Data Sheet. |
6. FORMATS AND TECHNICAL SUPPORT FOR SUBMISSION OF INFORMATION
|
6.1. |
The Agency shall specify, maintain and update the UFI generator, the XML formats for submissions and a harmonised product categorisation system and make them available free of charge on its website. |
|
6.2. |
The Agency shall provide technical and scientific guidance, technical support and tools facilitating the submission of information. |
PART B
INFORMATION CONTAINED IN A SUBMISSION
1. IDENTIFICATION OF THE MIXTURE AND OF THE SUBMITTER
1.1. Product identifier of the mixture
The product identifier shall be provided in accordance with Article 18(3)(a).
The complete trade name(s) of the mixture shall be provided, including, where relevant, brand name(s), name of the product and variant names as they appear on the label, without abbreviations and enabling its specific identification.
In addition, the UFI(s) shall be included in the submission.
1.2. Details of the submitter and contact point
The name, full address, telephone number and email address of the submitter shall be provided, and, if different, the name, full address, telephone number and email address of the point of contact to be used for obtaining further information relevant for emergency health response purposes.
1.3. Name, telephone number and email address for rapid access to additional product information
In the case of a limited submission as laid down in Section 2.3 of Part A, a name, a telephone number and an email address shall be provided at which rapid access to detailed additional product information relevant for emergency health response purposes is available in the language provided in Section 3.3 of Part A. The telephone number shall be accessible 24 hours per day, 7 days per week.
2. HAZARDS IDENTIFICATION AND ADDITIONAL INFORMATION
This Section sets out the information requirements related to the health and physical hazards of the mixture and the appropriate warning information associated with those hazards, as well as the additional information to be included in a submission.
2.1. Classification of the mixture
The classification of the mixture for health and physical hazards (hazard class, category and statement) shall be provided in accordance with the classification rules in Annex I.
2.2. Label elements
The following label elements required in accordance with Article 17 shall be provided, if applicable:
|
— |
hazard pictogram codes (Annex V), |
|
— |
signal word, |
|
— |
hazard statement codes (Annex III, including supplemental hazard information), |
|
— |
precautionary statement codes (Annex IV). |
2.3. Toxicological information
The submission shall include the information on the toxicological effects of the mixture or its components that is required in Section 11 of the Safety Data Sheet of the mixture, in accordance with Annex II to Regulation (EC) No 1907/2006.
2.4. Additional information
The following additional information shall be provided:
|
— |
the type(s) and size(s) of the packaging used to place the mixture on the market for consumer or professional use, |
|
— |
the colour(s) and the physical state(s) of the mixture, as supplied, |
|
— |
the pH, if available, of the mixture as supplied, or where the product is a solid, the pH of an aqueous liquid or solution at a given concentration. The concentration of the test mixture in water shall be indicated. If the pH is not available, the reasons shall be given, |
|
— |
product category (see Section 3.4 of Part A), |
|
— |
use (consumer, professional, industrial, or a combination of any of the three). |
3. INFORMATION ON MIXTURE COMPONENTS
3.1. General requirements
The chemical identity and the concentrations of the components contained in the mixture shall be indicated in the submission in accordance with Sections 3.2, 3.3 and 3.4.
By way of derogation from the first subparagraph, in the case of a limited submission as laid down in Section 2.3 of Part A, the information to be submitted on the composition of a mixture for industrial use or a mixture with an end use not subject to notification may be limited to the information contained in the Safety Data Sheet in accordance with Annex II to Regulation (EC) No 1907/2006, provided that additional information on the composition is rapidly available on request in emergencies in accordance with Section 1.3.
Components which are not present in a mixture shall not be notified. However, if they are notified as part of an interchangeable component group in accordance with Section 3.5 or their concentration has been submitted as a range of percentages in accordance with Sections 3.6 or 3.7, they may be notified if they will certainly be present in the mixture at some point in time.
By way of derogation from the third subparagraph, in a group submission, perfume components in mixtures shall be present in at least one of the mixtures
For group submissions where the perfumes vary between the mixtures contained in the group, a list shall be provided of the mixtures and the perfumes they contain, including their classification.
3.2. Identification of mixture components
A mixture component is either a substance or a mixture in mixture.
3.2.1. Substances
The product identifier for the substances identified according to Section 3.3 shall be provided in accordance with Article 18(2). However, an INCI name, a colour index name or another international chemical name may be used, provided the chemical name is well known and unambiguously defines the substance identity. The chemical name of substances for which an alternative chemical name has been allowed in accordance with Article 24 shall be provided as well.
3.2.2. Mixture in mixture
When a mixture is used in the composition of a second mixture placed on the market, the first mixture is referred to as a mixture in mixture (“MIM”).
Information on the substances contained in a MIM shall be provided in accordance with the criteria of Section 3.2.1, unless the submitter does not have access to information on the full composition of the MIM. In the latter case,
|
(a) |
if a UFI has been created for the MIM and the appointed body has received the information on the MIM in a prior submission, the MIM shall be identified by means of its product identifier in accordance with Article 18(3)(a), together with its concentration and UFI; |
|
(b) |
if a UFI has been created for the MIM, but the appointed body has not received the information on the MIM in a prior submission, the MIM shall be identified by means of its product identifier in accordance with Article 18(3)(a), together with its concentration and UFI and the compositional information contained in the Safety Data Sheet in accordance with Annex II to Regulation (EC) No 1907/2006 of the MIM and any other known components, as well as the name, email address and telephone number of the MIM supplier; |
|
(c) |
in absence of a UFI, the MIM shall be identified by means of its product identifier in accordance with Article 18(3)(a), together with its concentration and the compositional information contained in the Safety Data Sheet in accordance with Annex II to Regulation (EC) No 1907/2006 of the MIM and any other known components, as well as the name, email address and telephone number of the MIM supplier. |
3.2.3. Identification by generic component identifiers
By way of derogation from Sections 3.2.1 and 3.2.2, the generic component identifiers “perfumes”, or “colouring agents” may be used for mixture components used exclusively to add perfume or colour, where the following conditions are met:
|
— |
the mixture components are not classified for any health hazard, |
|
— |
the concentration of mixture components identified with a given generic component identifier does not exceed in total:
|
3.3. Mixture components subject to submission requirements
The following mixture components shall be indicated:
|
(1) |
mixture components classified as hazardous on the basis of their health or physical effects which:
|
|
(2) |
mixture components not classified as hazardous on the basis of their health or physical effects which are identified and present in concentrations equal to or greater than 1 %. |
3.4. Concentration and concentration ranges of the mixture components
Submitters shall provide the information laid down in Sections 3.4.1 and 3.4.2 with regard to the concentration of the mixture components, identified in accordance with Section 3.3.
3.4.1. Hazardous components of major concern for emergency health response and preventative measures
When mixture components are classified in accordance with this Regulation for at least one of the hazard categories listed below, their concentration in the mixture shall be expressed as exact percentages, in descending order by mass or volume.
|
— |
Acute toxicity, Category 1, 2 or 3, |
|
— |
Specific target organ toxicity – Single exposure, Category 1 or 2, |
|
— |
Specific target organ toxicity – Repeated exposure, Category 1 or 2, |
|
— |
Skin corrosion, category 1, 1A, 1B or 1C, |
|
— |
Serious eye damage, Category 1. |
As an alternative to providing concentrations as exact percentages, a range of percentages may be submitted in accordance with Table 1.
Table 1
Concentration ranges applicable to hazardous components of major concern for emergency health response
|
Concentration range of the hazardous component contained in the mixture (%) |
Maximum width of the concentration range to be used in the submission |
|
≥ 25 – < 100 |
5 % units |
|
≥ 10 – < 25 |
3 % units |
|
≥ 1 – < 10 |
1 % units |
|
≥ 0,1 – < 1 |
0,3 % units |
|
> 0 – < 0,1 |
0,1 % units |
3.4.2. Other hazardous components and components not classified as hazardous
The concentration of the hazardous components in the mixture that are not classified for any of the hazard categories listed in Section 3.4.1 and of the identified components not classified as hazardous shall be expressed, in accordance with Table 2, as ranges of percentages in descending order by mass or volume. As an alternative, exact percentages may be provided.
Table 2
Concentration ranges applicable to other hazardous components and components not classified as hazardous
|
Concentration range of the component contained in the mixture (%) |
Maximum width of the concentration range to be used in the submission |
|
≥ 25 – < 100 |
20 % units |
|
≥ 10 – < 25 |
10 % units |
|
≥ 1 – < 10 |
3 % units |
|
> 0 – < 1 |
1 % units |
By way of derogation from the first subparagraph, for perfume components in a group submission that are not classified or only classified for skin sensitisation Category 1, 1A or 1B or aspiration toxicity, submitters shall not be required to provide information on their concentration.
3.5. Grouping of components in an interchangeable component group
Components may be grouped in a submission in an interchangeable component group provided that:
|
(a) |
for all components in the interchangeable component group:
|
|
(b) |
for all possible combinations of the resulting final mixture based on the components in the interchangeable component group, the hazards identification and additional information referred to in Section 2 of Part B are identical. |
Alternatively, components that are classified only for skin corrosion, skin irritation, eye damage, eye irritation, aspiration toxicity, or respiratory or skin sensitisation, or a combination thereof, may be grouped in an interchangeable component group provided that:
|
(a) |
the classification for health and physical hazards (hazard class and category) is identical for all components; and |
|
(b) |
the pH, where applicable, of all components classified for skin corrosion, skin irritation, eye damage, or eye irritation is either acidic, neutral or alkaline; and |
|
(c) |
the interchangeable component group does not contain more than five components; and |
|
(d) |
for all possible combinations of the resulting final mixture based on the components grouped in the interchangeable component group, the hazards identification and additional information referred to in Section 2 of Part B are identical. |
3.5.1. Name of interchangeable component group and identification of grouped components
An interchangeable component group shall be given a name which corresponds to the technical function(s) of the grouped components for which they were incorporated in the mixture.
Each component in an interchangeable component group shall be identified in accordance with Section 3.2.1 or 3.2.2, as applicable.
3.5.2. Concentration and concentration ranges of grouped components
By way of derogation from the first subparagraph of Section 3.4, for components grouped in an interchangeable component group, submitters shall provide the information laid down in Sections 3.4.1 and 3.4.2 with regard to the total concentration of all components present in the mixture and grouped in the interchangeable component group.
When mixture components grouped in an interchangeable component group are classified in accordance with this Regulation for at least one of the hazard categories listed in Section 3.4.1, the total concentration of the components present in the mixture and grouped in the interchangeable component group shall be expressed as exact percentages, in descending order by mass or volume. As an alternative, a range of percentages may be submitted in accordance with Table 1 of that Section.
The total concentration of the hazardous components present in the mixture and grouped in an interchangeable component group that are not classified for any of the hazard categories listed in Section 3.4.1, and the total concentration of the identified components present in the mixture and grouped in an interchangeable component group not classified as hazardous, shall be expressed, in accordance with Table 2 of Section 3.4.2, as ranges of percentages in descending order by mass or volume. As an alternative, exact percentages may be provided.
3.6. Mixtures complying with standard formulas
By way of derogation from Sections 3.2, 3.3 and 3.4, for a mixture with a composition conforming with a standard formula specified in Part D, where the mixture classification does not change depending on the components’ concentration within the ranges of percentages specified in the corresponding standard formula:
|
— |
if the information on composition in the standard formula, together with information as specified in Sections 3.2 to 3.4 on the identity and concentration of the components not specified in the standard formula, is not less detailed than that contained in the Safety Data Sheet in accordance with Annex II to Regulation (EC) No 1907/2006, the identity and concentration of one or more of the mixture’s components may be submitted as specified in the standard formula for the components mentioned in that formula and as specified in Sections 3.2 to 3.4 for the other components, |
|
— |
if the information referred to in the previous indent is less detailed than that contained in the Safety Data Sheet in accordance with Annex II to Regulation (EC) No 1907/2006, the information on the identity and concentration of all the mixture’s components contained in the Safety Data Sheet in accordance with Annex II to Regulation (EC) No 1907/2006 shall be given. |
3.7. Fuels
By way of derogation from Sections 3.2, 3.3 and 3.4, for those fuels listed in Table 3, the identity and concentration of the mixture’s components listed in the Safety Data Sheet in accordance with Annex II to Regulation (EC) No 1907/2006 may be submitted. The identity and concentration of any other known component shall also be submitted.
Table 3
List of fuels
|
Fuel |
Product description |
|
Gasoline EN228 |
Automotive fuels – Unleaded petrol |
|
Gasoline E85 |
Automotive fuels – Ethanol (E85) automotive fuel |
|
Gasoline alkylate |
Motor fuels – special petrol for powered implements |
|
LPG |
Liquefied Petroleum Gas used as fuel |
|
LNG |
Liquefied Natural Gas used as fuel |
|
Diesel fuel |
Automotive fuels – diesel engine fuels with/without biofuel |
|
Paraffinic diesel fuels (e.g GTL, BTL or HVO) |
Automotive fuels – Paraffinic diesel fuel from synthesis or hydrotreatment |
|
Heating oil |
Liquid mineral fuels with the characteristics of domestic fuel oil |
|
MK 1 diesel |
Automotive fuels – Diesel fuel oil of environmental class 1 and 2 for high-speed diesel engines |
|
Aviation fuels |
Aviation turbine engine and piston engine fuels |
|
Kerosene – Illuminating paraffin |
Illuminating paraffin lampoil Type B and C |
|
Heavy fuel oil |
All grades of heavy fuel oil |
|
Marine fuel |
Marine fuels, containing or not biodiesel |
|
Fatty acid methyl esters (FAME) – Diesel B100 |
Fatty acid methyl esters (FAME) for use in diesel engines and heating applications |
3.8. Classification of mixture components
The classification for health and physical effects (hazard classes, hazard categories and hazard statements) of substances identified in accordance with Section 3.3 and contained in the mixture shall be provided. This includes the classification for at least all substances, indicated pursuant to Point 3.2.1 of Annex II to Regulation (EC) No 1907/2006 in the Safety Data Sheet of the mixture and in the Safety Data Sheet of any MIM contained in the mixture. For MIMs identified in accordance with Section 3.3 where the submitter does not have access to the full composition of the MIM, the classification for health and physical effects of the MIM shall be provided in addition.
4. SUBMISSION UPDATE
4.1. Conditions for submission update
Where one of the following changes applies to a mixture in an individual or group submission, submitters shall provide a submission update before placing that mixture, as changed, on the market:
|
— |
when the mixture product identifier or the UFI has changed, |
|
— |
when the mixture classification for health or physical hazards has changed, |
|
— |
when relevant new toxicological information that is required in Section 11 of the Safety Data Sheet becomes available on the hazardous properties of the mixture or its components, |
|
— |
if a change in the composition of the mixture fulfils one of the following conditions:
|
By way of derogation from the fourth indent of the first subparagraph, the following shall apply:
|
(a) |
a submission update for mixtures with a composition conforming with any of the standard formulas specified in Part D is required only when the composition of the mixture changes in such a manner that the mixture’s composition no longer conforms with the standard formula; |
|
(b) |
for mixtures where the information on composition is provided based on the Safety Data sheet in accordance with Section 3.6 or 3.7 a submission update is required when Section 3 of the Safety Data Sheet is updated. Table 4 Variations of the concentration of components requiring a submission update
|
When perfumes in a group submission change, the list of mixtures and the perfumes they contain as required in Section 3.1 shall be updated.
4.2. Content of the submission update
The submission update shall comprise a revised version of the previous submission containing the new information available as described in Section 4.1.
PART C
SUBMISSION FORMAT
1. SUBMISSION FORMAT
1.1. Submission Format
The submission of information to appointed bodies in accordance with Article 45 shall be in a format to be provided by the Agency. The submission format shall address the following elements:
1.2. Identification of the mixture, submitter and contact point
Product identifier
|
— |
Complete trade name(s) of the product (in case of group submission, all product identifiers shall be listed) |
|
— |
Other names, synonyms |
|
— |
Unique Formula Identifier(s) (UFI) |
|
— |
Other identifiers (authorisation number, company product codes) |
Contact details of the submitter and contact point
|
— |
Name |
|
— |
Full address |
|
— |
Telephone number |
|
— |
Email address |
Contact details for rapid access to additional product information (24 hours/7 days). Only for limited submission.
|
— |
Name |
|
— |
Telephone number (accessible 24 hours per day, 7 days per week) |
|
— |
Email address |
1.3. Classification of the mixture, label elements and toxicology
Classification of the mixture and label elements
|
— |
Hazard class and category |
|
— |
Hazard pictogram codes (Annex V) |
|
— |
Signal word |
|
— |
Hazard statement codes, including supplemental hazard information codes (Annex III) |
|
— |
Precautionary statement codes (Annex IV) |
Toxicological information
|
— |
Description of the toxicity of the mixture or its components (as required in Section 11 of the Safety Data Sheet in accordance with Annex II to Regulation No 1907/2006) |
Additional information on the mixture
|
— |
Colour(s) |
|
— |
The pH, if available, of the mixture as supplied, or where the mixture is a solid, the pH of an aqueous liquid or solution at a given concentration. The concentration of the test mixture in water shall be indicated. If the pH is not available, the reasons shall be given. |
|
— |
Physical state(s) |
|
— |
Packaging (type(s) and size(s)) |
|
— |
Intended use (product category) |
|
— |
Uses (consumer, professional, industrial) |
1.4. Information on the mixture components and interchangeable component groups
Identification of the mixture components
|
— |
Chemical/trade name of the components |
|
— |
CAS number (where applicable) |
|
— |
EC number (where applicable) |
|
— |
UFI (where applicable) |
Name of interchangeable component groups (where applicable)
Concentration and concentration ranges of the mixture components
|
— |
Exact concentration or concentration range |
Classification of mixture components
|
— |
Hazard classification (where applicable) |
|
— |
Additional identifiers (where applicable and relevant for health response) |
List according to Part B, Section 3.1, fifth subparagraph (where applicable)
PART D
STANDARD FORMULAS
For standard formulas 1-17 the following conditions apply:
|
— |
Heavy metal, trace elements: As, Ba, Cd, Cr, Co, Cu, Hg, Mo, Ni, Pb, Sb, Sn, Te, Tl, V are below 0,1 w/w % and Mn, Sr, Zn are below 1 w/w % |
|
— |
PAHs are not present |
Note applying to standard formulas 1-17:
|
— |
(1) UVCB substance consists of variable amounts of calcite, tricalcium silicate, dicalcium silicate, calcium oxide, quartz, potassium chloride, potassium sulfate, calcium sulfate, sodium aluminium silicate, magnesium aluminium silicate, muscovite, … |
1. CEMENT
|
Cement Standard Formula – 1 |
||
|
Product description |
Portland cement with one main constituent: clinker |
|
|
Component name |
EC No |
Concentration (w/w%) |
|
Portland cement clinker |
266-043-4 |
86,5 – 100 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 2 |
||
|
Product description |
Portland-slag cement and Blast furnace cement with two main constituents: clinker and slag |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
4,6 – 94 |
|
Granulated blast furnace slag |
266-002-0 |
5,5 – 95 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 3 |
||
|
Product description |
Portland-silica fume cement Portland cements with two main constituents: clinker and silica fume |
|
|
Component name |
EC No |
Concentration (w/w%) |
|
Portland cement clinker |
266-043-4 |
82 – 94 |
|
Silica fume |
273-761-1 |
5,5 – 10 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 4 |
||
|
Product description |
Portland-pozzolana cement, Pozzolanic cement Portland cements with two main constituents: clinker and pozzolan (natural or natural calcined pozzolan) |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
41 – 94 |
|
Natural (calcined) pozzolana |
310-127-6 |
5,5 – 55 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-303-2 |
0 – 0,1 |
|
Cement Standard Formula – 5 |
||
|
Product description |
Portland-fly ash cement, Pozzolanic cement Portland cements with two main constituents: clinker and fly ash (siliceous and calcareous fly ash) |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
41 – 94 |
|
Fly ash |
931-322-8 |
5,5 – 55 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 6 |
||
|
Product description |
Portland-burnt shale cement Portland cements with two main constituents: clinker and burnt shale |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
59 – 94 |
|
Burnt shale |
297-648-1 |
5,5 – 35 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 7 |
||
|
Product description |
Portland-limestone cement Portland cements with two main constituents: clinker and limestone |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
59 – 94 |
|
Limestone |
215-279-6 |
5,5 – 35 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 8 |
||
|
Product description |
Portland-composite cement, Composite cement (slag – limestone) Portland cements with three main constituents: clinker, slag and limestone |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
31,9 – 88 |
|
Granulated blast furnace slag |
266-002-0 |
5,5 – 59 |
|
Limestone |
215-279-6 |
5,5 – 29 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 9 |
||
|
Product description |
Portland-composite cement, Composite Cement (slag – fly ash) Portland cements with three main constituents: clinker, blast-furnace slag, siliceous and calcareous fly ash |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
18,2 – 88 |
|
Granulated blast furnace slag |
266-002-0 |
5,5 – 59 |
|
Fly ash |
931-322-8 |
5,5 – 49 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 10 |
||
|
Product description |
Portland-composite cement, Composite cement (slag – pozzolana) Portland cements with three main constituents: clinker, blast-furnace slag, natural or natural calcined pozzolan |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
18,2 – 88 |
|
Granulated blast furnace slag |
266-002-0 |
5,5 – 49 |
|
Natural (calcined) pozzolana |
310-127-6 |
5,5 – 49 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 11 |
||
|
Product description |
Portland-composite cement (slag – burnt shale) Portland cements with three main constituents: clinker, blast-furnace slag, burnt shale |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
59 – 94 |
|
Granulated blast furnace slag |
266-002-0 |
5,5 – 29 |
|
Burnt shale |
297-648-1 |
5,5 – 29 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 12 |
||
|
Product description |
Portland-composite cement (limestone – fly ash) Portland cements with three main constituents: clinker, limestone, siliceous and calcareous fly ash |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
46 – 94 |
|
Limestone |
215-279-6 |
5,5 – 29 |
|
Fly ash |
931-322-8 |
5,5 – 44 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 13 |
||
|
Product description |
Portland-composite cement (limestone – pozzolana) Portland cements with three main constituents: clinker, limestone, natural or natural calcined pozzolan |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
46 – 94 |
|
Limestone |
215-279-6 |
5,5 – 29 |
|
Natural (calcined) pozzolana |
310-127-6 |
5,5 – 44 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 14 |
||
|
Product description |
Portland-composite cement (limestone – burnt shale) Portland cements with three main constituents: clinker, limestone and burnt shale |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
59 – 94 |
|
Limestone |
215-279-6 |
5,5 – 29 |
|
Burnt shale |
297-648-1 |
5,5 – 29 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 15 |
||
|
Product description |
Portland-composite cement, Pozzolanic cement (fly ash – pozzolana) Portland cements with three main constituents: clinker, siliceous and calcareous fly ash, natural or natural calcined pozzolan |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
41 – 94 |
|
Natural (calcined) pozzolana |
310-127-6 |
5,5 – 55 |
|
Fly ash |
931-322-8 |
5,5 – 55 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 16 |
||
|
Product description |
Portland-composite Portland cements with four main constituents: clinker and three of these constituents: blast-furnace slag, silica fume, fly ash, pozzolan, burnt shale, limestone |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
59 – 94 |
|
Granulated blast furnace slag Natural (calcined) pozzolana Fly ashes Burnt shale Limestone Silica fume |
266-002-0 310-127-6 931-322-8 297-648-1 215-279-6 273-761-1 |
5,5 – 23 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 17 |
||
|
Product description |
Composite cement Portland cements with four main constituents: clinker, slag, siliceous fly ash and natural or natural calcined pozzolan |
|
|
Constituent |
EC No |
Concentration (w/w%) |
|
Portland cement clinker |
266-043-4 |
18,3 – 64 |
|
Granulated blast furnace slag |
266-002-0 |
16,5 – 49 |
|
Natural (calcined) pozzolana |
310-127-6 |
5,5 – 43 |
|
Fly ash |
931-322-8 |
5,5 – 43 |
|
Calcium sulfate |
231-900-3 |
0 – 8 |
|
Flue dust (1) |
270-659-9 |
0 – 5 |
|
Inorganic natural mineral materials |
310-127-6 |
|
|
Iron(II) sulfate |
231-753-5 |
0 – 1 |
|
Tin(II) sulfate |
231-302-2 |
0 – 0,1 |
|
Cement Standard Formula – 18 |
||
|
Product description |
Calcium aluminate cement |
|
|
Constituent |
EC No |
Concentration (w/w %) |
|
Calcium aluminate cement clinker |
266-045-5 |
86,5 – 100 |
|
Grinding aid |
- |
0 – 0,2 |
|
Cement Standard Formula – 19 |
||
|
Product description |
Masonry cements – with clinker and lime – MC 5, MC 12,5, MC 22,5 |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
25 – 60 |
|
Building lime acc. to EN 459 |
215-138-9, |
1 – 75 |
|
Hydrated lime acc. to EN 459 |
215-137-3 |
|
|
Other, non-hazardous inorganic constituent |
310-127-6 |
0 – 74 |
|
Inorganic pigments acc. to EN 12878 |
- |
0 – 1 |
|
Cement Standard Formula – 20 |
||
|
Product description |
Masonry cements – with clinker and without lime – MC 5, MC 12,5, MC 22,5 |
|
|
Component name |
EC No |
Concentration (w/w %) |
|
Portland cement clinker |
266-043-4 |
25 – 60 |
|
Other, non-hazardous inorganic constituent |
310-127-6 |
40 – 75 |
|
Inorganic pigments acc. to EN 12878 |
|
0 – 1 |
2. GYPSUM BINDER
|
Gypsum binder Standard Formula |
||
|
Component name |
EC No |
Concentration (w/w %) |
|
Calcium sulphate |
231-900-3 |
≥ 50 and < 100 |
|
Calcium dihydroxide |
215-137-3 |
> 0 and ≤ 5 |
3. READY MIXED CONCRETE
|
Ready mixed concrete Standard Formula 1 Concrete strength classes C8/10, C12/15, C16/20, C20/25, C25/30, C28/35, C32/40, C35/45, C40/50, C45/55, C50/60 LC8/9, LC12/13, LC16/18, LC20/22, LC25/28, LC30/33, LC35/38, LC40/44, LC45/50, LC50/55, LC55/60 |
||
|
Component name |
EC No |
Concentration (w/w %) |
|
Cement |
270-659-9 |
3 – 18 |
|
Water |
231-791-2 |
5 – 8 |
|
Aggregates |
273-727-6 |
70 – 80 |
|
Air entrainers (admixture) |
- |
0 – 0,08 |
|
Plasticisers/superplasticisers (admixture) |
- |
0 – 0,15 |
|
Retarders (admixture) |
- |
0 – 0,4 |
|
Accelerators (admixture) |
- |
0 – 0,2 |
|
Water resisting (admixture) |
- |
0 – 0,25 |
|
Fly ash |
931-322-8 |
0 – 8 |
|
Silica fume |
273-761-1 |
0 – 3 |
|
GGBS |
266-002-0 |
0 – 6 |
|
Ready mixed concrete Standard Formula 2 Concrete strength classes C55/67, C60/75, C70/85, C80/95, C90/105, C100/105, LC 60/66, LC70/77, LC80/88 |
||
|
Component name |
EC No |
Concentration (w/w %) |
|
Cement |
270-659-9 |
12 – 25 |
|
Water |
231-791-2 |
5 – 8 |
|
Aggregates |
273-727-6 |
70 – 80 |
|
Air entrainers (admixture) |
- |
0,04 – 0,08 |
|
Plasticisers/superplasticisers (admixture) |
- |
0 – 0,15 |
|
Retarders (admixture) |
- |
0 – 0,4 |
|
Accelerators (admixture) |
- |
0 – 0,2 |
|
Water resisting (admixture) |
- |
0 – 0,25 |
|
Fly ash |
931-322-8 |
0 – 8 |
|
Silica fume |
273-761-1 |
0 – 3 |
|
GGBS |
266-002-0- |
0 – 6 |
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/24 |
COMMISSION IMPLEMENTING REGULATION (EU) 2020/1678
of 6 November 2020
approving amendments to the specification for a Protected Designation of Origin or a Protected Geographical Indication (‘Rioja’ (PDO))
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007 (1), and in particular Article 99 thereof,
Whereas:
|
(1) |
The Commission has examined the application for the approval of amendments to the specification for the Protected Designation of Origin ‘Rioja’, forwarded by Spain in accordance with Article 105 of Regulation (EU) No 1308/2013. |
|
(2) |
The Commission has published the application for the approval of the amendments to the specification in the Official Journal of the European Union, as required by Article 97(3) of Regulation (EU) No 1308/2013 (2). |
|
(3) |
No statement of objection has been received by the Commission under Article 98 of Regulation (EU) No 1308/2013. |
|
(4) |
The amendments to the specification should therefore be approved in accordance with Article 99 of Regulation (EU) No 1308/2013. |
|
(5) |
The measures provided for in this Regulation are in accordance with the opinion of the Committee for the Common Organisation of the Agricultural Markets, |
HAS ADOPTED THIS REGULATION:
Article 1
The amendments to the specification published in the Official Journal of the European Union regarding the name ‘Rioja’ (PDO) are hereby approved.
Article 2
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 6 November 2020.
For the Commission,
On behalf of the President,
Janusz WOJCIECHOWSKI
Member of the Commission
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/25 |
COMMISSION IMPLEMENTING REGULATION (EU) 2020/1679
of 6 November 2020
conferring protection under Article 99 of Regulation (EU) No 1308/2013 of the European Parliament and of the Council on the name ‘Soltvadkerti’ (PDO)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007 (1), and in particular Article 99 thereof,
Whereas:
|
(1) |
In accordance with Article 97(2) and (3) of Regulation (EU) No 1308/2013, the Commission has examined the application to register the name ‘Soltvadkerti’ forwarded by Hungary and has published it in the Official Journal of the European Union (2). |
|
(2) |
No statement of objection has been received by the Commission under Article 98 of Regulation (EU) No 1308/2013. |
|
(3) |
In accordance with Article 99 of Regulation (EU) No 1308/2013, the name ‘Soltvadkerti’ should be protected and entered in the register referred to in Article 104 of that Regulation. |
|
(4) |
The measures provided for in this Regulation are in accordance with the opinion of the Committee for the Common Organisation of the Agricultural Markets, |
HAS ADOPTED THIS REGULATION:
Article 1
The name ‘Soltvadkerti’ (PDO) is hereby protected.
Article 2
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 6 November 2020.
For the Commission,
On behalf of the President,
Janusz WOJCIECHOWSKI
Member of the Commission
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/26 |
COMMISSION IMPLEMENTING REGULATION (EU) 2020/1680
of 6 November 2020
conferring protection under Article 99 of Regulation (EU) No 1308/2013 of the European Parliament and of the Council on the name ‘Friuli’/‘Friuli Venezia Giulia’/‘Furlanija’/‘Furlanija Julijska krajina’ (PDO)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007 (1), and in particular Article 99 thereof,
Whereas:
|
(1) |
In accordance with Article 97(2) and (3) of Regulation (EU) No 1308/2013, the Commission has examined the application to register the name ‘Friuli’/‘Friuli Venezia Giulia’/‘Furlanija’/‘Furlanija Julijska krajina’ forwarded by Italy and has published it in the Official Journal of the European Union (2). |
|
(2) |
No statement of objection has been received by the Commission under Article 98 of Regulation (EU) No 1308/2013. |
|
(3) |
In accordance with Article 99 of Regulation (EU) No 1308/2013, the name ‘Friuli’/‘Friuli Venezia Giulia’/‘Furlanija’/‘Furlanija Julijska krajina’ should be protected and entered in the register referred to in Article 104 of that Regulation. |
|
(4) |
The measures provided for in this Regulation are in accordance with the opinion of the Committee for the Common Organisation of the Agricultural Markets, |
HAS ADOPTED THIS REGULATION:
Article 1
The name ‘Friuli’/‘Friuli Venezia Giulia’/‘Furlanija’/‘Furlanija Julijska krajina’ (PDO) is hereby protected.
Article 2
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 6 November 2020.
For the Commission,
On behalf of the President,
Janusz WOJCIECHOWSKI
Member of the Commission
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/27 |
COMMISSION REGULATION (EU) 2020/1681
of 12 November 2020
amending Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council as regards removal from the Union list of certain flavouring substances
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC (1), and in particular Article 11(3),
Having regard to Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings (2), and in particular Article 7(4) thereof,
Whereas:
|
(1) |
Annex I to Regulation (EC) No 1334/2008 lays down a Union list of flavourings and source materials approved for use in and on foods and their conditions of use. |
|
(2) |
Commission Implementing Regulation (EU) No 872/2012 (3) adopted the list of flavouring substances and introduced that list in Part A of Annex I to Regulation (EC) No 1334/2008. |
|
(3) |
Annex I to Regulation (EC) No 1334/2008 may be updated in accordance with the common procedure referred to in Article 3(1) of Regulation (EC) No 1331/2008, either on the initiative of the Commission or following an application submitted by a Member State or by an interested party. |
|
(4) |
The Union list of flavourings and source materials laid down in Annex I to Regulation (EC) No 1334/2008 contains, among others, a number of flavouring substances for which, at the time of adoption of the list by Regulation (EU) No 872/2012, the European Food Safety Authority (‘the Authority’) had not been able to rule out a safety risk to the health of the consumer on the basis of the data available and had, therefore, considered that additional data was necessary to complete their evaluation. Those substances were included in the Union list of flavouring substances but on the condition that safety data addressing the concerns expressed by the Authority was submitted before the expiry of specific deadlines established in Part A of Annex I to Regulation (EC) No 1334/2008. These substances and their deadlines were identified by footnotes numbered from 1 to 4. |
|
(5) |
Among the substances included in the Union list of flavourings and source materials but identified by way of a footnote reference requiring additional scientific data to be submitted by 31 December 2012, there were the following five substances alpha-Damascone (Fl No 07.134) (representative substance of the group), delta-Damascone (Fl No 07.130), cis-1-(2,6,6-trimethyl-2-cyclohexen-1-yl)but-2-en-1-one (Fl No 07.225), trans-1-(2,6,6-trimethyl-2-cyclohexen-1-yl)but-2-en-1-one (Fl No 07.226) and alpha-Damascenone (Fl No 07.231) (‘the concerned substances’). These substances are part of the subgroup 2.4 of substances from Flavouring Group FGE.19, and were included in the Flavouring Group FGE 210. As regards these substances, the Authority had indicated in its opinion on the Flavouring Group Evaluation 210 of 2009 (4) that they contain a structural alert for genotoxicity in their molecular structure as they are alpha, beta-unsaturated ketones, and that additional genotoxicity data was needed to rule out the concern on their genotoxicity in accordance with the Authority’s document on the ‘Genotoxicity test strategy for substances belonging to subgroups of the Flavouring Group FGE.19’ (5). |
|
(6) |
On 28 December 2012, data was submitted concerning subgroup 2.4 of substances from Flavouring Group FGE.19. |
|
(7) |
The Authority evaluated the submitted data in revision 1 of the Opinion on the genotoxic potential of the substances of Flavouring Group FGE 210 from chemical group 2.4 of Flavouring Group FGE 19 published on 19 February 2014 (6). However, the Authority considered the submitted data was still insufficient to rule out the genotoxic potential of the concerned substances and it requested further additional data on genotoxicity on the substances representative for this subgroup. |
|
(8) |
New data was submitted in 2014. The Authority evaluated the new data in revision 2 of its Opinion, published on 10 July 2015 (7). However, the Authority considered that the new data was insufficient to rule out the genotoxic potential of the concerned substances and requested once more further scientific data to be submitted on the genotoxicity of the concerned substances. |
|
(9) |
Further data was submitted in 2016 on the concerned substances. Following this submission, the Authority requested further information and specific studies to be performed by letters of 8 November 2016, 9 February 2017, 29 June 2017 and 8 February 2019. However, the new data provided did not always correspond to the studies requested by the Authority and were not suitable to answer properly the Authority’s concerns. Taking into account all that additional data submitted, the Authority evaluated again the genotoxic potential of the concerned substances in revision 3 of the Opinion on FGE.210 (8) published on 22 May 2019. The Authority concluded that the concern for genotoxicity cannot be ruled out for the five concerned substances. |
|
(10) |
In view of the fact that neither the data submitted within the initial deadline nor the data submitted following the Authority’s successive requests after that deadline had allowed the Authority in 2019 to rule out the concerns expressed in its Opinion of 2009, the Commission considers that it is not established that the concerned substances do not pose a safety risk to the health of the consumer. Therefore, on the basis of the scientific evidence submitted within the framework set out in Part A of Annex I to Regulation (EC) No 1334/2008 for the substances pending the completion of their evaluation, the use of the concerned substances does not comply with the general conditions of use for flavourings set out in Article 4 of Regulation (EC) No 1334/2008. |
|
(11) |
Consequently, the concerned substances should be removed from the Union list in order to protect human health. |
|
(12) |
Part A of Annex I to Regulation (EC) No 1334/2008 should therefore be amended accordingly. |
|
(13) |
Due to technical reasons, transitional periods should be provided for concerning food to which any of the five flavouring substances have been added and which have been placed on the market or dispatched from third countries to the Union and were en route prior to the entry into force of this Regulation. The transitional period should not apply to preparations to which any of these five flavouring substances have been added and which are not intended to be consumed as such as the composition of these preparations are known by their manufacturers when they prepare them. |
|
(14) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Plants, Animals, Food and Feed, |
HAS ADOPTED THIS REGULATION:
Article 1
Part A of Annex I to Regulation (EC) No 1334/2008 is amended in accordance with the Annex to this Regulation.
Article 2
1. Foods to which any of the flavouring substances listed in the Annex to this Regulation have been added and which were lawfully placed on the market prior to the date of entry into force of this Regulation may continue to be marketed until their date of minimum durability or ‘use by’ date.
2. Foods imported into the Union, to which one of the flavouring substances listed in the Annex to this Regulation have been added may be marketed until their date of minimum durability or ‘use by’ date where the importer of such food can demonstrate that they were dispatched from the third country concerned and were en route to the Union before the date of entry into force of this Regulation.
3. The transitional periods provided in paragraphs 1 and 2 shall not apply to preparations not intended to be consumed as such to which any of these five flavouring substances have been added.
4. For the purposes of this Regulation, preparations shall be understood as mixtures of one or more flavourings to which other food ingredients such as food additives, enzymes or carriers may be also incorporated to facilitate their storage, sale, standardisation, dilution or dissolution.
Article 3
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 12 November 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 354, 31.12.2008, p. 34.
(2) OJ L 354, 31.12.2008, p. 1.
(3) Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC (OJ L 267, 2.10.2012, p. 1).
(4) Scientific Opinion on Flavouring Group Evaluation 210: alpha, beta-unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19; EFSA Journal (2009) ON-1030, 1-18. https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2009.1030
(5) Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19 – Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF); EFSA Journal (2008) 854, 1-5. https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2008.854
(6) Scientific Opinion on Flavouring Group Evaluation 210, Revision 1 (FGE.210Rev1): Consideration of genotoxic potential for alpha,beta-unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. EFSA Journal 2014;12(2):3587, 35 pp. doi:10.2903/j.efsa.2014.3587
(7) Scientific Opinion on Flavouring Group Evaluation 210 Revision 2 (FGE.210Rev2): Consideration of genotoxic potential for alpha, beta-unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. 10 July 2015. EFSA Journal 2015;13(7):4172.doi:10.293/j.efsa.2015.4172
(8) Scientific Opinion on Flavouring Group Evaluation 210 Revision 3 (FGE.210Rev3): Consideration of genotoxic potential for alpha, beta-unsaturated alicyclic ketones and precursors from chemical subgroup 2.4 of FGE.19. EFSA Journal 2019; 17(5):5676.
ANNEX
In Section 2 of Part A of Annex I to Regulation (EC) No 1334/2008, the following entries are deleted:
|
‘07.130 |
delta-Damascone |
57378-68-4 |
386 |
|
|
|
2 |
JECFA/EFSA |
|
07.134 |
alpha-Damascone |
43052-87-5 |
385 |
11053 |
|
|
2 |
JECFA/EFSA |
|
07.225 |
cis-1-(2,6,6-Trimethyl-2-cyclohexen-1-yl)but-2-en-1-one |
23726-94-5 |
|
|
At least 92 %; secondary component 4 % trans-isomer |
|
2 |
EFSA |
|
07.226 |
trans-1-(2,6,6-Trimethyl-2-cyclohexen-1-yl)but-2-en-1-one |
24720-09-0 |
|
|
|
|
2 |
EFSA |
|
07.231 |
alpha-Damascenone |
35044-63-4 |
|
|
|
|
2 |
EFSA’ |
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/31 |
COMMISSION REGULATION (EU) 2020/1682
of 12 November 2020
amending Annex III to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (1), and in particular Article 31(1) thereof,
Whereas:
|
(1) |
The substances 2-hydroxyethyl methacrylate (HEMA) and 11,14-Dioxa-2,9-diazaheptadec-16-enoic Acid, 4,4,6,16-tetramethyl-10,15-dioxo, 2-[(2-methyl-1-oxo-2-propenyl)oxy]ethyl ester (Di-HEMA Trimethylhexyl Dicarbamate or Di-HEMA TMHDC) are currently not subject to prohibition or restriction pursuant to Regulation (EC) No 1223/2009. |
|
(2) |
On 2 July 2014, the Swedish Medical Products Agency, which is the Swedish competent authority for the purposes of Regulation (EC) No 1223/2009, adopted and communicated a decision under Article 27 of Regulation (EC) No 1223/2009 introducing provisional restrictive measures on a nail cosmetic product that had caused a high number of undesirable effects. The substances identified as likely to cause those undesirable effects were HEMA and Di-HEMA TMHDC. |
|
(3) |
According to Article 27(2) of Regulation (EC) No 1223/2009, the Swedish Medical Products Agency communicated immediately to the Commission and the competent authorities of the other Member States the measures taken and any supporting data. |
|
(4) |
The Scientific Committee on Consumer Safety (SCCS) concluded in its opinion of 21-22 June 2018 (2) that ‘HEMA and di-HEMA-TMHDC, when applied appropriately to the nail plate (…) as part of an artificial nail modelling system, are not likely to pose a risk of sensitisation, provided that their use is restricted to the nail plate only and contact with the adjacent skin is avoided’. The SCCS further concluded that ‘[b]oth HEMA and di-HEMA-TMHDC are weak to moderate sensitisers and pose a risk of sensitisation from misuse of the products or from inappropriately carried out application or from unintentional contamination of the skin adjacent to the nails under normal and reasonably foreseeable conditions of use’. |
|
(5) |
According to Article 3 of Regulation (EC) No 1223/2009, cosmetic products made available on the market must be safe for human health when used under normal or reasonably foreseeable conditions of use. |
|
(6) |
When assessing ‘normal or reasonably foreseeable conditions of use’, account has to be taken of possible misuse, inappropriate or unintentional use. In the case of products requiring high level of precision, it is necessary to take into account situations of insufficient precision in their application. |
|
(7) |
Cases of sensitisation to nail products containing HEMA and Di-HEMA TMHDC reported in some Member States lead the Commission to the conclusion that there is a risk that such products may be applied with insufficient precision, causing contact with the skin adjacent to the nail plate. |
|
(8) |
A distinction should be made between professional and consumer use of cosmetic products. Higher safety standards are expected from professionals. In particular, a professional is expected to have more skills, experience and knowledge on the use of a cosmetic product compared to the consumer. |
|
(9) |
Possible health and safety risks for professionals are regulated by certain Union Directives laying down minimum requirements, in particular Council Directives 89/391/EEC (3) and 98/24/EC (4). Additional professional safety rules may apply. |
|
(10) |
As regards consumers, since the SCCS opinion considers the substances HEMA and Di-HEMA TMHDC safe in nail products only when applied on the nail plate and since ‘normal or reasonably foreseeable conditions of use’ should take into account the possibility of application on the skin adjacent to the nail plate, there is a potential risk to human health from the use of HEMA and Di-HEMA TMHDC in nail products. |
|
(11) |
As the use of nail products containing HEMA and Di-HEMA TMHDC by professionals is expected to be safer for the consumer, such products should be used only by professionals and therefore the warning ‘for professional use only’ should be added on the package of such products. |
|
(12) |
To draw attention of both professionals and consumers to the potential health risk, the warning ‘can cause an allergic reaction’ should be added on the package of nail products containing HEMA and Di-HEMA TMHDC. |
|
(13) |
Therefore, the safeguard measure taken by Sweden should be considered as justified. Consequently, it is necessary to impose a restriction on the use of HEMA and Di-HEMA TMHDC in nail products. |
|
(14) |
Annex III to Regulation (EC) No 1223/2009 should therefore be amended accordingly. |
|
(15) |
It is appropriate to provide for a reasonable period of time in order for the industry to adapt to the new requirements. |
|
(16) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Cosmetic Products, |
HAS ADOPTED THIS REGULATION:
Article 1
Annex III to Regulation (EC) No 1223/2009 is amended in accordance with the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 12 November 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 342, 22.12.2009, p. 59.
(2) SCCS/1592/17;
https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_o_214.pdf
(3) Council Directive 89/391/EEC of 12 June 1989 on the introduction of measures to encourage improvements in the safety and health of workers at work (OJ L 183, 29.6.1989, p. 1).
(4) Council Directive 98/24/EC of 7 April 1998 on the protection of the health and safety of workers from the risks related to chemical agents at work (fourteenth individual Directive within the meaning of Article 16(1) of Directive 89/391/EEC) (OJ L 131, 5.5.1998, p. 11).
ANNEX
In Annex III to Regulation (EC) No 1223/2009, in the table, the following entries are added:
|
Ref No |
Substance identification |
Restrictions |
Wording of conditions of use and warnings |
|||||
|
Chemical name/ INN |
Name of Common Ingredients Glossary |
CAS number |
EC number |
Product type, body parts |
Maximum concentration in ready for use preparation |
Other |
|
|
|
a |
b |
c |
d |
e |
f |
g |
h |
i |
|
‘313 |
2-Hydroxyethyl Methacrylate (*1) |
HEMA |
868-77-9 |
212-782-2 |
Nail products |
|
Professional use only |
For professional use only Can cause an allergic reaction |
|
314 |
11,14-Dioxa-2,9-diazaheptadec-16-enoic Acid, 4,4,6,16-tetramethyl-10,15-dioxo, 2-[(2-methyl-1-oxo-2-propenyl)oxy]ethyl ester (*2) |
DI-HEMA TRIMETHYLHEXYL DICARBAMATE |
41137-60-4/72869-86-4 |
255-239-5/276-957-5 |
Nail products |
|
Professional use only |
For professional use only Can cause an allergic reaction |
(*1) From 3 June 2021 products containing that substance and not complying with those conditions shall not be placed on the Union market. From 3 September 2021 products containing that substance and not complying with those conditions shall not be made available on the Union market.
(*2) From 3 June 2021 products containing that substance and not complying with those conditions shall not be placed on the Union market. From 3 September 2021 products containing that substance and not complying with those conditions shall not be made available on the Union market.’
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/34 |
COMMISSION REGULATION (EU) 2020/1683
of 12 November 2020
amending Annexes II and III to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (1), and in particular Article 31(1) thereof,
Whereas:
|
(1) |
Following the publication of a scientific study in 2001, entitled ‘Use of permanent hair dyes and bladder cancer risk’, the Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers, subsequently replaced by the Scientific Committee on Consumer Products (SCCP) pursuant to Commission Decision 2004/210/EC (2), concluded that the potential health risks of the use of hair dyes were of concern. |
|
(2) |
The SCCP further recommended an overall safety assessment strategy for hair dye substances including the requirements for testing substances used in hair dye products for their potential genotoxicity or carcinogenicity. |
|
(3) |
Following the opinions of the SCCP, the Commission agreed with Member States and stakeholders on an overall strategy to regulate substances used in hair dye products according to which the industry was required to submit files, containing updated scientific data on the safety of hair dye substances, for a risk assessment by the SCCP. |
|
(4) |
Having succeeded the SCCP pursuant to Commission Decision 2008/721/EC (3), the Scientific Committee on Consumer Safety (SCCS) assessed the safety of individual hair dye substances for which updated files had been submitted by the industry. |
|
(5) |
As a result of the assessement by the SCCS, in order to ensure the safety of hair dye products for human health it is necessary to prohibit the use of three hair dye substances, 1,2,4-Trihydroxybenzene (4), 2-[(4-Amino-2-nitrophenyl)-amino]-benzoic acid (5) and 4-Amino-3-hydroxytoluene (6), based on the final opinions given by the SCCS on their safety. In addition, in light of the final opinions by the SCCS on other six hair dye substances, Dimethylpiperazinium Aminopyrazolopyridine HCl (7), Methylimidazoliumpropyl p-phenylenediamine HCl (8), HC Orange No 6 (9), Acid Orange 7 (10), Tetrabromophenol Blue (11) and Indigofera Tinctoria (12), it is appropriate to limit their maximum concentrations for use in hair dye products. |
|
(6) |
The definition of a hair product laid down in point (1)(c) of the Preamble to Annexes II to VI to Regulation (EC) No 1223/2009 excludes the application of hair dye substances on eyelashes, based on a different level of risk of applying a cosmetic product on the hair on the head as compared to applying the same product on eyelashes. A specific safety assessment was therefore needed for the application of hair dye substances on eyelashes. |
|
(7) |
The substance 2-Methoxymethyl-p-Phenylenediamine and its sulfate are listed under entry 292 in Annex III to Regulation (EC) No 1223/2009. Taking into account the conclusions of the latest SCCS opinion (13) on the use of those substances on eyelashes, the field of application of the restriction to which they are subject should be extended to products intended for colouring eyelashes. |
|
(8) |
In order to avoid any risk related to self-application by consumers of products intended for colouring eyelashes that contain 2-Methoxymethyl-p-Phenylenediamine and its sulfate, those products should be allowed for professional use only. |
|
(9) |
In order to inform consumers and professionals about possible adverse effects of the use of hair dyes and products intended for colouring eyelashes with the aim to lower the risk of skin sensitisation to those products, appropriate warnings should be printed on their labels. |
|
(10) |
Regulation (EC) No 1223/2009 should therefore be amended accordingly. |
|
(11) |
It is appropriate to provide for reasonable periods of time in order for the industry to adapt to the new requirements and to phase out cosmetic products which do not comply with those requirements. |
|
(12) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Cosmetic Products, |
HAS ADOPTED THIS REGULATION:
Article 1
Regulation (EC) No 1223/2009 is amended in accordance with the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 12 November 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 342, 22.12.2009, p. 59.
(2) Commission Decision 2004/210/EC of 3 March 2004 setting up Scientific Committees in the field of consumer safety, public health and the environment (OJ L 66, 4.3.2004, p. 45).
(3) Commission Decision 2008/721/EC of 5 September 2008 setting up an advisory structure of Scientific Committees and experts in the field of consumer safety, public health and the environment and repealing Decision 2004/210/EC (OJ L 241, 10.9.2008, p. 21).
(4) SCCS/1598/18.
(5) SCCS/1497/12.
(6) SCCS/1400/11.
(7) SCCS/1584/17.
(8) SCCS/1609/19.
(9) SCCS/1579/16.
(10) SCCS/1536/14.
(11) SCCS/1610/19.
(12) SCCS/1615/20.
(13) SCCS/1603/18.
ANNEX
Regulation (EC) No 1223/2009 is amended as follows:
|
(1) |
in Annex II, in the table, the following entries are added:
|
|||||||||||||||||||
|
(2) |
in Annex III, the table is amended as follows:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(*1) From 3 September 2021 hair and eyelash dye products containing those substances shall not be placed on the Union market.
From 3 June 2022 hair and eyelash dye products containing those substances shall not be made available on the Union market.’;
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/42 |
COMMISSION REGULATION (EU) 2020/1684
of 12 November 2020
amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (1), and in particular Article 31(2) thereof,
Whereas:
|
(1) |
The Scientific Committee on Consumer Safety (SCCS) concluded in its opinion of 13 December 2019 (2) (‘the SCCS opinion’) that the use of Methoxypropylamino Cyclohexenylidene Ethoxyethylcyanoacetate as a UV filter in cosmetic products at a maximum concentration of 3 % is safe. Inhalation toxicity was not assessed in the SCCS opinion because no data were provided. Hence, the SCCS opinion is not applicable to any cosmetic product in form of spray that could lead to exposure of the end-user’s lungs by inhalation. |
|
(2) |
The SCCS also concluded in its opinion that Methoxypropylamino Cyclohexenylidene Ethoxyethylcyanoacetate is a secondary amine, and thus is prone to nitrosation and formation of nitrosamine. It should not be used in combination with nitrosating substances. The nitrosamine content should be less than 50 ppb. |
|
(3) |
In light of the SCCS opinion and in order to take into account technical and scientific progress, the use of Methoxypropylamino Cyclohexenylidene Ethoxyethylcyanoacetate as a UV filter in cosmetic products should be authorised at a maximum concentration of 3 %, except in applications that may lead to exposure of the end-user’s lungs by inhalation. |
|
(4) |
Annex VI to Regulation (EC) No 1223/2009 should therefore be amended accordingly. |
|
(5) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Cosmetic Products, |
HAS ADOPTED THIS REGULATION:
Article 1
Annex VI to Regulation (EC) No 1223/2009 is amended in accordance with the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 12 November 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 342, 22.12.2009, p. 59.
(2) SCCS/1605/19.
ANNEX
In Annex VI to Regulation (EC) No 1223/2009, the following entry is added:
|
Reference number |
Substance identification |
Conditions |
Wording of conditions of use and warnings |
|||||||||||
|
Chemical name/INN/XAN |
Name of Common Ingredients Glossary |
CAS number |
EC number |
Product type, Body parts |
Maximum concentration in ready for use preparation |
Other |
||||||||
|
a |
b |
c |
d |
e |
f |
g |
h |
i |
||||||
|
‘32 |
2-ethoxyethyl (2Z)-2-cyano-2-[3-(3-methoxypropylamino) cyclohex-2-en-1-ylidene]acetate |
Methoxypropylamino Cyclohexenylidene Ethoxyethylcyanoacetate |
1419401-88-9 |
700-860-3 |
|
3 % |
|
|
||||||
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/44 |
COMMISSION IMPLEMENTING REGULATION (EU) 2020/1685
of 12 November 2020
amending Regulation (EU) No 37/2010 to classify the substance bupivacaine as regards its maximum residue limit
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and the Council (1), and in particular Article 14, in conjunction with Article 17 thereof,
Having regard to the opinions of the European Medicines Agency formulated on 20 February 2020 and 18 June 2020 by the Committee for Medicinal Products for Veterinary Use,
Whereas:
|
(1) |
Article 17 of Regulation (EC) No 470/2009 requires that the maximum residue limit (‘MRL’) for pharmacologically active substances intended for use in the Union in veterinary medicinal products for food-producing animals or in biocidal products used in animal husbandry is established in a Regulation. |
|
(2) |
Table 1 of the Annex to Commission Regulation (EU) No 37/2010 (2) sets out the pharmacologically active substances and their classification regarding MRLs in foodstuffs of animal origin. |
|
(3) |
The substance bupivacaine is not included in that table. |
|
(4) |
An application for the establishment of MRLs for bupivacaine for cutaneous and epilesional use only in porcine, for piglets up to 7 days of age, and in bovine, for calves up to 2 months of age, has been submitted to the European Medicines Agency (‘Agency’). |
|
(5) |
The Agency, based on the opinion of the Committee for Medicinal Products for Veterinary Use, has concluded that the establishment of an MRL for bupivacaine in porcine and bovine, within those age limitations, is not necessary for the protection of human health and recommended a ‘no MRL required’ classification. |
|
(6) |
According to Article 5 of Regulation (EC) No 470/2009, the Agency is to consider using MRLs established for a pharmacologically active substance in a particular foodstuff for another foodstuff derived from the same species, or MRLs established for a pharmacologically active substance in one or more species for other species. |
|
(7) |
The Agency has considered that the extrapolation of the ‘no MRL required’ classification for bupivacaine in porcine and bovine to other food-producing species is not appropriate at this time due to insufficient data. |
|
(8) |
Regulation (EU) No 37/2010 should therefore be amended accordingly. |
|
(9) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Veterinary Medicinal Products, |
HAS ADOPTED THIS REGULATION:
Article 1
The Annex to Regulation (EU) No 37/2010 is amended as set out in the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 12 November 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 152, 16.6.2009, p. 11.
(2) Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin (OJ L 15, 20.1.2010, p. 1).
ANNEX
In Table 1 of the Annex to Regulation (EU) No 37/2010, an entry for the following substance is inserted in alphabetical order:
|
Pharmacologically active Substance |
Marker residue |
Animal Species |
MRLs |
Target Tissues |
Other Provisions (according to Article 14(7) of Regulation (EC) No 470/2009) |
Therapeutic Classification |
|
‘Bupivacaine |
NOT APPLICABLE |
Porcine |
No MRL required |
NOT APPLICABLE |
For use in piglets up to 7 days of age only. For cutaneous and epilesional use only. |
Local anaesthetic’ |
|
Bovine |
For use in calves up to 2 months of age only. For cutaneous and epilesional use only. |
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/47 |
COMMISSION IMPLEMENTING REGULATION (EU) 2020/1686
of 12 November 2020
making imports of certain hot-rolled flat products of iron, non-alloy or other alloy steel originating in Turkey subject to registration
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) 2016/1036 of the European Parliament and of the Council of 8 June 2016 on protection against dumped imports from countries not members of the European Union (1) (‘the basic Regulation’), and in particular Article 14(5) thereof,
After informing the Member States,
Whereas:
|
(1) |
On 14 May 2020, the European Commission (‘the Commission’) announced, by a notice published in the Official Journal of the European Union (2) (‘the Notice of Initiation’), the initiation of an anti-dumping proceeding (‘the anti-dumping proceeding’) with regard to imports into the Union of certain hot-rolled flat products of iron, non-alloy or other alloy steel originating in Turkey following a complaint lodged on 31 March 2020 by Eurofer (‘the complainant’) on behalf of producers representing more than 25 % of the total Union production of certain hot-rolled flat products of iron, non-alloy or other alloy steel. |
1. PRODUCT SUBJECT TO REGISTRATION
|
(2) |
The products subject to registration (‘the product concerned’) are flat-rolled products of iron, non-alloy steel or other alloy steel, whether or not in coils (including ‘cut-to-length’ and ‘narrow strip’ products), not further worked than hot-rolled, not clad, plated or coated. These products are currently falling under CN codes 7208 10 00, 7208 25 00, 7208 26 00, 7208 27 00, 7208 36 00, 7208 37 00, 7208 38 00, 7208 39 00, 7208 40 00, 7208 52 10, 7208 52 99, 7208 53 10, 7208 53 90, 7208 54 00, 7211 13 00, 7211 14 00, 7211 19 00, ex 7225 19 10 (TARIC code 7225191090), 7225 30 90, ex 7225 40 60 (TARIC code 7225406090), 7225 40 90, ex 7226 19 10 (TARIC code 7226191090), 7226 91 91 and 7226 91 99. The CN and TARIC codes are given for information only. |
|
(3) |
Not subject to registration are: (i) products of stainless steel and grain-oriented silicon electrical steel; (ii) products of tool steel and high-speed steel; (iii) products, not in coils, without patterns in relief, of a thickness exceeding 10 mm and of a width of 600 mm or more; and (iv) products, not in coils, without patterns in relief, of a thickness of 4,75 mm or more but not exceeding 10 mm and of a width of 2 050 mm or more. |
2. REQUEST
|
(4) |
On 17 September 2020, the complainant submitted a registration request pursuant to Article 14(5) of the basic Regulation. The complainant requested that imports of the product concerned be made subject to registration so that measures may be applied against those imports retroactively from the date of such registration. |
|
(5) |
The following interested parties submitted comments in reaction to the request: the Government of Turkey, the Consortium of Users (users), Colakoglu and Erdemir Groups and Habas (exporting producers), the Turkish Steel Exporters’ Association (‘ÇİB’) and the Turkish Steel Producers Association (‘TCUD’). |
3. GROUNDS FOR REGISTRATION
|
(6) |
According to Article 14(5) of the basic Regulation, the Commission may direct the customs authorities to take the appropriate steps to register imports, so that measures may subsequently be applied against those imports from the date of such registration, provided all conditions set out in the basic Regulation are met. Imports may be made subject to registration following a request from the Union industry, which contains sufficient evidence to justify such action. |
|
(7) |
The complainant alleged that, on the basis of the most recent available statistics, there had been a substantial rise in imports following the initiation of the investigation which was likely to seriously undermine the remedial effect of definitive duties. Moreover the complainant argued that there was a history of dumping from Turkey over an extended period and that importers were, or should have been, aware of the dumping practices from Turkey. |
|
(8) |
The Commission examined the request in the light of Article 10(4) of the basic Regulation. The Commission verified whether the importers were aware, or should have been aware, of the dumping as regards the extent of the dumping and the injury alleged or found. It also analysed whether there was a further substantial rise in imports which, in the light of its timing as well as volume and other circumstances, was likely to seriously undermine the remedial effect of an eventual definitive anti-dumping duty to be applied. |
3.1. Awareness of the importers of the dumping, the extent thereof and the alleged injury
|
(9) |
At this stage, the Commission has at its disposal sufficient evidence that imports of the product concerned from Turkey are being dumped. The complaint provided sufficient evidence of dumping based on a comparison of the normal value thus established with the export price (at ex-works level) of the product concerned when sold for export to the Union. As a whole, and given the extent of the alleged dumping margins ranging from 4 % to 8 %, this evidence provided sufficient support that the exporting producers practice dumping. |
|
(10) |
The complaint also provided sufficient evidence of alleged injury to the Union industry, including a negative development of key performance indicators of the Union industry. |
|
(11) |
That information was contained both in the non-confidential version of the complaint and in the Notice of Initiation published on 14 May 2020. By its publication in the Official Journal of the European Union, the Notice of Initiation is a public document accessible to all importers. Furthermore, as interested parties in the investigation, importers have access to the non-confidential version of the complaint and the non-confidential file. Therefore, the Commission considered that, on this basis, importers, were aware, or should have been aware, of the dumping, the extent thereof and the alleged injury (3). |
|
(12) |
As stated in recital 5 several interested parties submitted comments to the request for registration. With regard to the first criterion, the Government of Turkey, Habas, CIB and the Colakoglu and Erdemir Groups highlighted that the mere initiation of an anti-dumping investigation does not automatically mean that dumping is actually taking place, as certain investigations (some concerning Turkey) are terminated without the imposition of duties. Rather it would be only a ‘one-sided allegation’. Thus, an importer cannot be aware of something (‘dumping practices’) that the investigation has not yet established. The Government of Turkey, Habas and CIB also contested certain additional evidence provided by Eurofer in the request, including the claim that the CEO of a Turkish exporting producer was aware of the upcoming anti-dumping investigation and the information that imports of the product concerned are subject to trade defence measures in third countries. Lastly, TCUD noted that some of the claims made by Eurofer regarding anti-dumping measures against Turkish imports of the product concerned were not truthfully described in the submission. In this respect, TCUD argued that one company was exempted from the US measures following a challenge before the relevant courts and that another third country (Morocco) removed the anti-dumping duties against Turkey following the ruling of a WTO panel. |
|
(13) |
Article 14(5) of the basic Regulation stipulates that, as of the initiation of the investigation and having informed the Member States in due time, the Commission may direct the customs authorities to take the appropriate steps to register imports, so that measures may subsequently be applied against those imports from the date of such registration. The main objective of registration in this case is to allow the possibility to retroactively impose provisional measures up to 90 days preceding their application in accordance with Article 10(4) of the basic Regulation. In light of this provision, the Commission cannot register imports after a provisional duty has already been imposed. |
|
(14) |
If, as suggested by some parties, registration is only possible after a finding of dumping has been made by an investigation, then by definition it could never take place prior to the imposition of provisional duties. Such approach would completely deprive the registration tool of its effet utile (4). Therefore, the Commission needs to take a decision on whether to register imports or not before any provisional duty is in place. In this respect the Commission noted that at the stage when the complainant filed the request for registration the best information available was that of the complaint, on the basis of which, as provided in the Notice of Initiation, the Commission initiated the anti-dumping investigation, and which indicated the existence of evidence tending to show injurious dumping. Therefore, the Commission rejected these claims as unfounded. Moreover, in view of this finding, the Commission did not need to assess the additional evidence referred to above regarding imports of the Turkish exporting producers to third countries and the related arguments. |
|
(15) |
The Commission thus concluded that the first criterion for registration was met. |
3.2. Further substantial rise in imports
|
(16) |
The Commission analysed this criterion based on the statistical data for the product concerned available in the Surveillance 2 database. For assessing whether a further substantial increase had taken place since the initiation of the investigation, the Commission first defined the periods of time to be compared. On the one hand, it assessed the import data from Turkey following the initiation of the anti-dumping investigation (i.e. the point in time as of when importers were aware, or should have been aware, of dumping practices) until the most recent period, i.e. the period June-mid-October 2020. On the other hand, the Commission calculated Turkish imports for the same period during the investigation period (5) (i.e. June-mid-October 2019) and the monthly average import volumes in the full investigation period. |
|
(17) |
The Commission considered that, in this case, a comparison between the post-initiation average monthly import volumes with the average monthly Turkish import volumes in the entire investigation period would not be sufficient to assess whether there was a substantial increase in imports in view of the market developments and stockpiling effect derived from the ongoing safeguard measures affecting the product concerned. Thus, the Commission considered it appropriate to base its determination on the comparison between the post-initiation average monthly import volumes with the same period in the investigation period in order to better reflect the potential influence of the ongoing safeguard measures on the behaviour of import flows from Turkey in the period assessed (6). Comparing the post-initiation import volumes with import volumes in the same period of the previous year would, in addition, take due account of possible seasonality effects, if any (7). |
|
(18) |
Regarding the influence of the safeguard measures on the Turkish import flows, the Commission noted that throughout the whole year 2019 (the investigation period), Turkey’s imports of the product concerned were affected by numerous adjustments to the functioning of the safeguard measures that impacted the export behaviour from Turkey. |
|
(19) |
First, January 2019, by far the best-performing month of Turkish imports of the product concerned, coincided with the last month of provisional safeguard measures. In this respect, there was uncertainty in the market as to the shape definitive measures would take. The Commission noted that any volume of free-of-duty Tariff-Rate Quota (‘TRQ’) not sold by the end of that month would not have been carried over to the next period, i.e. volumes of free-of-duty TRQ would have been ‘lost’. A similar situation, i.e. last month(s) prior to a new period of the measures, occurred in the quarter February-March 2019. Second, in February, April, July and October of the year 2019 there were openings of new free-of-duty TRQ batches. Under the safeguard measures on certain steel products, every opening of TRQ has generally lead to large volumes being imported in the early stages of each of the quarters. Last, the Commission announced in August 2019, in the framework of the first review investigation of the steel safeguard measures, that it would make Turkish imports of the product concerned subject to a 30 % cap over the TRQ available under the relevant product category, thus also leading to an increase in imports ahead of such change in the TRQ administration. |
|
(20) |
The Commission further highlighted that the above events impacted the flow of import volumes as shown in the statistics. Notably, the months of January, March and September rank first, second and fourth in terms of volumes exported monthly by Turkey in 2019 (in some cases being substantially higher than any other month in that year) (8). Hence the Commission concluded that all these regulatory actions in the framework of the steel safeguard measures influenced significantly the behaviour of market operators and the trend of import flows across the year. |
|
(21) |
The situation in a substantially shorter period (between June and mid-October) was less affected by regulatory changes in the management of the TRQs. Thus, the Commission compared the monthly average Turkish import volume in the period June-mid-October 2020 with the monthly average volume of imports in the same period of the preceding year, 2019. |
|
(22) |
The comparison shows that the monthly average import volume from Turkey in this period increased by 6 %.
Source: Surveillance 2 database. |
||||||||||||||
|
(23) |
The Commission further examined the import trends in the selected period and the respective period within the investigation period in order to determine whether that 6 % increase constituted a substantial increase in imports in this case. In particular, the Commission observed that negligible import volumes from Turkey took place in the month of September 2020. Such exceptionally low volume of imports was largely due to the fact that under the steel safeguard measures, Turkey had virtually exhausted its country-specific TRQ in that month in product category 1 (where the product concerned belongs) and therefore it would have been only able to export any meaningful amounts of the product concerned subject to a 25 % duty (10). This exceptionally low import volume in September 2020 reduced to a large extent the monthly average import volumes in the relevant period chosen by the Commission to carry out the comparison (June-mid-October 2020). At the same time, the Commission noted that precisely in the same month of the preceding year, in September 2019, there was a very high volume of imports due to the fact that in October 2019 the adjustments following the first review investigation of the safeguard measures would take effect. Therefore, in anticipation of this event, Turkish producers exported very large amounts during the month of September 2019. |
|
(24) |
In light of the considerations in the previous recital, the Commission considered that the imports of the months of September of, respectively 2019 and 2020 were not representative and assessed also the import levels without including that month in both years. Such comparison confirmed that the increases of import volumes in comparison with the same months during the investigation period had been very significant. For example, when comparing the period June-August in 2020 and the same period in 2019 there was an increase by 44 %. A significant increase by 49 % would also take place when comparing the period June-mid-October 2020 with the same period in 2019, in the absence of the month of September. In light of these considerations, the Commission concluded that the increase of imports in this case is substantial. |
|
(25) |
Regarding this criterion, the Government of Turkey, Habas, CIB, the Colakoglu and Erdemir Groups and the Consortium of HRFS users contested the period proposed by the complainant to assess whether a substantial rise in imports took place. Instead, these parties proposed different periods to carry out such comparison that in their view would show that this criterion was not met. Habas and the Consortium of HRFS users suggested that comparing on a quarterly basis the same periods of 2019 and the first half of 2020, i.e. the first two quarters of each year, would show a decrease in imports. CIB and the Colakoglu and Erdemir Groups argued that the period chosen by Eurofer, namely June-July 2020, was too short, and thus not representative. Moreover, CIB and the Consortium of HRFS users alleged that the reasons for any increase in imports would have nevertheless been the consequence of the relaxation of the lockdown measures in the Union related to the COVID-19 pandemic, which coincided with the early stages of the post-initiation period. CIB argued that the Commission must compare the volume of imports that occurred after initiation of the investigation with the volume of imports that occurred during the investigation period. This party further argued that Turkey’s imports would have shown a steady decline in January-May 2020 and that in the months of June and July 2020, they would have just increased to return to ‘their normal level’. Moreover, CIB concluded that the Commission should disregard the post-initiation data of June 2020 because of the impact of the alleged particular ‘post-quarantine’ situation. In such case the comparison with the monthly average in the investigation period would show an increase of 12 %, which could not qualify as ‘further substantial increase’, but rather as a ‘very modest’ increase. Other parties proposed to assess the import trends in the first seven months (January-July) of the periods 2017-2020, the import trends throughout the years 2017 to 2020 (annualised) (11), the monthly average in the years 2018 and 2019 with the monthly average in the period January-July 2020, the monthly average of the period January-July 2018 and 2019 with the same period in the year 2020, and the monthly average in the investigation period with the import volume in the month of July 2020 (12), to show that there would have been a decline in imports. The Colakoglu and Erdemir Groups also took issue with the fact that the request did not include any import data between the end of the investigation period and the initiation of the investigation, despite the time lapsed between the two. |
|
(26) |
Furthermore, the Consortium of HRFS users additionally claimed that registration would reduce the level of imports and cause a shortage of supply which would be threatening the operations of certain users’ production facilities established in the Union. This would allegedly disrupt the market and cause irreparable damage to independent users of the product concerned. |
|
(27) |
All interested parties that reacted to the registration request from Eurofer concurred in arguing that because of the impact of the steel safeguard measures on Turkey, it would not be able to rise further its exports in the coming months (i.e. those prior to the imposition of provisional anti-dumping measures), and that therefore the Commission should disregard the request for registration. |
|
(28) |
With regard to the different periods proposed by the parties, the Commission refers to the reasoning and explanations it laid out in section 3.2 above. Nevertheless, there are a number of claims in this respect that warrant a specific rebuttal. In the first place, the Commission notes that while it usually also compares the monthly average imports of a post-initiation period with the average import levels in the investigation period, such comparison may not always be appropriate. In this case, the Commission has found and explained in recitals 16 to 20 above that, in view of the particular market circumstances with regard to the imports under investigation, using the average imports of the year 2019 is not the most appropriate basis for comparison. |
|
(29) |
Second, the Commission notes that one of the proposals referred to trends from a period prior to the initiation of the anti-dumping investigation. This would be inconsistent with the finding that the awareness of dumping practices from Turkey stems from the publication of the Notice of Initiation. Thus, the trend in imports from a period prior to the point where an importer could have been aware of the existence of dumping, is not to be taken into consideration for assessing the merits of a registration request. |
|
(30) |
Third, the Commission further notes that, while Eurofer’s request constitutes the starting point for the analysis, the Commission nevertheless also has to carry out a comprehensive analysis of its own. In this regard, the Commission has at its disposal a set of data for a more recent period than that provided by Eurofer in the request (namely, until mid-October 2020) (13). Moreover, the Commission also deemed it more appropriate to compare this set of data with the same period in the investigation period, for the reasons developed above. |
|
(31) |
Thus, the Commission does not consider that the different proposals have shown any compelling reasons that would render them more apt to assess the import trends than that developed in section 3.2. Therefore, the Commission rejected these claims. |
|
(32) |
Regarding the claims that because of the safeguard measures in place, there would in any event be no risk of a further substantial rise in imports, the Commission disagreed for the following reasons. |
|
(33) |
First, the registration does not prejudge a decision on retroactive collection of the provisional anti-dumping duties. Whether safeguards measures will prevent an increase of imports until the imposition of provisional measures should be assessed in the context of a possible retroactive collection of duties (14). |
|
(34) |
In any event, the Commission acknowledges that, as a result of the changes introduced by the safeguard measures as of 1 July 2020, Turkey has become subject to a country-specific TRQ whose quarterly volumes are set out in Annex II of Commission Implementing Regulation (EU) 2020/894 (15). If these volumes are exhausted, Turkey is nevertheless still allowed to continue exporting into the Union (16). |
|
(35) |
However, the Commission does not agree that this fact alone would automatically prevent Turkish imports from rising substantially before the imposition of provisional anti-dumping duties, if any. This is so because by the time provisional duties may be imposed at the latest, this is by mid-January 2021, two batches of free-of-duty country-specific TRQ would have been released for Turkey (17). Moreover, any eventual comparison between import volumes to assess whether retroactive collection of duties is warranted could also cover a period prior to that when Turkey became subject to a country-specific TRQ as explained in recitals 18 and 19 above. Therefore, at this stage, there is no evidence that the overall volumes that Turkey could export free-of-duty (and under the 25 % duty) until an eventual imposition of provisional anti-dumping measures could not qualify as a further substantial rise. The Commission will any event only be able to conclude on this matter in case definitive anti-dumping duties were imposed at the end of the ongoing investigation. |
|
(36) |
Therefore the Commission rejects the claims that because of the safeguard measures in place a further substantial rise in imports should be ruled out. |
|
(37) |
Lastly, the Commission notes that the comment made by the Consortium of users regarding the impact of registration on the supply of the product concerned and on independent users does not fall under the legal requirements that are to be assessed when deciding on the registration of imports. Therefore the Commission does not address it at this stage of the proceeding. |
|
(38) |
In view of the above considerations, the Commission found that the second criterion for registration was also met. |
3.3. Undermining of the remedial effect of the duty
|
(39) |
The Commission has at its disposal sufficient evidence that additional injury would be caused by a continued rise in imports from Turkey at further decreasing prices. |
|
(40) |
As established in section 3.2 there is sufficient evidence of a substantial rise in imports of the product concerned. |
|
(41) |
In addition, there is evidence of a decreasing trend in the import prices of the product concerned. In this regard import prices from Turkey into the Union in the period June-mid-October 2020 have decreased, on average, by 13 % when compared to the same period in 2019 and by 14 % when compared to the monthly average in the investigation period.
Source: Surveillance 2 database. |
||||||||||||||
|
(42) |
The further rise in imports following the initiation of the case is thus likely, in light of its timing, volume and other circumstances, such as the pricing behaviour of exporting producers, to seriously undermine the remedial effect of any definitive duty, unless such duty would be applied retroactively. |
|
(43) |
The Commission therefore concluded that the third criterion for registration was also met. |
4. PROCEDURE
|
(44) |
The Commission has concluded that there is sufficient evidence to justify making imports of the product concerned subject to registration in accordance with Article 14(5) of the basic anti-dumping Regulation. |
|
(45) |
All interested parties are invited to make their views known in writing and to provide supporting evidence. The Commission may hear interested parties, provided that they make a request in writing and show that there are particular reasons why they should be heard. |
5. REGISTRATION
|
(46) |
Under Article 14(5) of the basic Regulation imports of the product concerned should be made subject to registration for the purpose of ensuring that, should the investigation result in findings leading to the imposition of anti-dumping duties, those duties can, if the necessary conditions are fulfilled, be levied retroactively on the registered imports in accordance with the applicable legal provisions. |
|
(47) |
Any future liability would emanate from the findings of the anti-dumping investigation. |
|
(48) |
The allegations in the complaint resulting in the initiation of an anti-dumping investigation estimate dumping margins from 4 % to 8 % and injury elimination levels ranging from 10 % to 25 % for the product concerned. The amount of possible future liability can be estimated at the level of the highest dumping margin estimated on the basis of the complaint, namely 8 % as a proportion of the CIF import value of the product concerned. |
6. PROCESSING OF PERSONAL DATA
|
(49) |
Any personal data collected in the context of this registration will be treated in accordance with Regulation (EU) 2018/1725 of the European Parliament and of the Council (18), |
HAS ADOPTED THIS REGULATION:
Article 1
1. The customs authorities are hereby directed, pursuant to Article 14(5) of Regulation (EU) 2016/1036, to take the appropriate steps to register imports into the Union of flat-rolled products of iron, non-alloy steel or other alloy steel, whether or not in coils (including ‘cut-to-length’ and ‘narrow strip’ products), not further worked than hot-rolled, not clad, plated or coated. These products are currently falling under CN codes 7208 10 00, 7208 25 00, 7208 26 00, 7208 27 00, 7208 36 00, 7208 37 00, 7208 38 00, 7208 39 00, 7208 40 00, 7208 52 10, 7208 52 99, 7208 53 10, 7208 53 90, 7208 54 00, 7211 13 00, 7211 14 00, 7211 19 00, ex 7225 19 10 (TARIC code 7225191090), 7225 30 90, ex 7225 40 60 (TARIC code 7225406090), 7225 40 90, ex 7226 19 10 (TARIC code 7226191090), 7226 91 91 and 7226 91 99 and originating in Turkey.
2. Registration shall expire nine months following the date of entry into force of this Regulation.
3. All interested parties are invited to make their views known in writing, to provide supporting evidence or to request to be heard within 21 days from the date of publication of this Regulation.
Article 2
This Regulation shall enter into force on the day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 12 November 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 176, 30.6.2016, p. 21.
(2) OJ C 166, 14.5.2020, p. 9.
(3) See judgment of the General Court (Second Chamber) of 8 May 2019 in T-749/16, Stemcor vs European Commission, paragraph 56.
(4) See judgment of the General Court (Second Chamber) of 8 May 2019 in T-749/16, Stemcor vs European Commission, paragraph 33.
(5) The investigation period (IP) covered the period from 1 January 2019 to 31 December 2019.
(6) Imports from Turkey of the product concerned are subject to safeguard measures since mid-July 2018 (under product category 1). Tariff-Rate Quota’s (‘TRQs’) under the safeguard measures are administered quarterly. Therefore, by comparing the same periods, the trend will be less impacted by the numerous changes to the TRQ administration that took place throughout the investigation period which is a full year (2019), as Turkey was subject to three different regimes under the TRQ administration system in the course of the year 2019.
(7) The Commission noted that for the product concerned, the summer period shows consistently a decline in consumption as compared to the period prior to it. The periods used by the Commission in this case would thus remove any distortion as far as possible seasonality effects are concerned.
(8) In fact, the average import volumes in these months is more than twice the average import volumes of the remaining nine months in the year 2019.
(9) This comparison is considered more appropriate, see recitals 17 to 21 above.
(10) Until the next country-specific TRQ batch was available.
(11) Consortium of HRFS users.
(12) Colakoglu and Erdemir Groups.
(13) Eurofer’s request for registration only considered data for the months of June and July 2020.
(14) This is, when the import data for the relevant period to be assessed for determining whether a retroactive collection of duties is warranted or not, is available.
(15) Commission Implementing Regulation (EU) 2020/894 of 29 June 2020 amending Implementing Regulation (EU) 2019/159 imposing definitive safeguard measures against imports of certain steel products (OJ L 206, 30.6.2020, p. 27).
(16) Subject to a 25 % duty until the opening of the next batch of TRQs in the next quarter.
(17) Those corresponding to the quarters October-December 2020 and January-March 2021 respectively.
(18) Regulation (EU) 2018/1725 of the European Parliament and of the Council of 23 October 2018 on the protection of natural persons with regard to the processing of personal data by the Union institutions, bodies, offices and agencies and on the free movement of such data, and repealing Regulation (EC) No 45/2001 and Decision No 1247/2002/EC (OJ L 295, 21.11.2018, p. 39).
DIRECTIVES
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/55 |
COMMISSION DELEGATED DIRECTIVE (EU) 2020/1687
of 2 September 2020
amending the Annex to Council Framework Decision 2004/757/JHA as regards the inclusion of the new psychoactive substance N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H-benzimidazole-1-ethanamine (isotonitazene) in the definition of ‘drug’
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Council Framework Decision 2004/757/JHA of 25 October 2004 laying down minimum provisions on the constituent elements of criminal acts and penalties in the field of illicit drug trafficking (1), and in particular Articles 1a and 8a thereof,
Whereas:
|
(1) |
A risk assessment report on the new psychoactive substance N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H-benzimidazole-1-ethanamine (isotonitazene) was drawn up in compliance with Article 5c of Regulation (EC) No 1920/2006 of the European Parliament and of the Council (2) by the Scientific Committee of the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA; the Centre), extended following the procedure laid down in Article 5c(4) of the same Regulation, on 26 May 2020. The Centre submitted the risk assessment report to the Commission and to the Member States on 29 May 2020. |
|
(2) |
Isotonitazene is a synthetic opioid analgesic and is closely related to etonitazene and clonitazene, both of which are under international control under the 1961 United Nations Single Convention on Narcotic Drugs, as amended by the 1972 Protocol. |
|
(3) |
Isotonitazene has been available in the Union since at least April 2019 and has been detected in five Member States as well as in the United Kingdom. 24 seizures in total were reported by four Member States; in addition, one Member State reported a collected sample and the United Kingdom reported post-mortem biological samples. Isotonitazene in general is likely to be under-detected since the substance is not routinely screened for due to its novelty on the market. In most cases, the substance was seized as powder, but it was also identified in liquid form. The detected quantities are relatively small. However, they should be seen within the context of the high potency of isotonitazene. |
|
(4) |
Two deaths have been reported so far by Germany and the United Kingdom where isotonitazene was involved. The deaths occurred in 2019. No detailed information is available for the death case in Germany. In the case reported by the United Kingdom, several other substances were identified in the postmortem biological samples (3). No acute intoxications with confirmed exposure to isotonitazene were reported so far. It is likely that naloxone works as an antidote to poisoning caused by isotonitazene as for other synthetic opioids. Both intoxications and deaths are likely to be under-detected and under-reported as they are not routinely screened for and as the substance appeared very recently on the Union market. |
|
(5) |
There is no direct evidence showing the involvement of organised crime in the manufacture, distribution (trafficking) and supply of isotonitazene within the Union. The available information suggests that isotonitazene is produced by chemical companies based outside the Union. |
|
(6) |
Isotonitazene appears to be sold online in small and wholesale amounts, mainly as a powder; it is also sold as ready to-use nasal sprays. Information from seizures suggests that isotonitazene may have also been sold on the illicit opioid market. Due to this, users may not be aware that they are using isotonitazene. |
|
(7) |
Isotonitazene has no recognised human or veterinary medical use in the Union nor, it appears, elsewhere. There are no indications that the substance may be used for any other purpose aside from as an analytical reference standard and in scientific research. |
|
(8) |
The risk assessment report reveals that many of the questions related to isotonitazene that are posed by the lack of data on the risks to individual health, risks to public health and social risks could be answered through further research. There is no specific information on the social risks posed by isotonitazene. However, the available evidence and information on the health risks that the substance poses, given also that the substance is relatively unknown, provides sufficient ground for including isotonitazene in the definition of ‘drug’. |
|
(9) |
Isotonitazene is not listed for control under the 1961 United Nations Single Convention on Narcotic Drugs, as amended by the 1972 Protocol, or under the 1971 United Nations Convention on Psychotropic Substances. Isotonitazene is not currently under assessment by the United Nations system. |
|
(10) |
Given that four Member States control isotonitazene under national drug control legislation and one Member State as well as the United Kingdom and Norway control isotonitazene under other legislation, including this substance in the definition of ‘drug’ and thereby covering it by provisions on the criminal offences and sanctions as defined in Framework Decision 2004/757 would help avoid the emergence of obstacles in cross-border law enforcement and judicial cooperation, and would help protect from the risks that its availability and use can pose. |
|
(11) |
Article 1a of Framework Decision 2004/757/JHA confers the power to adopt delegated acts upon the Commission with a view to giving a quick and expertise-based response at Union level to the emergence of new psychoactive substances detected and reported by the Member States, by amending the Annex to that Framework Decision to include those substances in the definition of ‘drug’. |
|
(12) |
The available information would suggest that the consumption of isotonitazene causes harm to health associated with its acute toxicity and abuse liability or dependence producing potential. This harm to health is considered life-threatening. In addition, there is a potential for severe physical and mental impairment and a significant spread of diseases, including the transmission of blood-borne viruses. These effects, including dependence, are comparable to other opioid analgesics that are under international control. |
|
(13) |
As the conditions and procedure for triggering the exercise of the powers to adopt a delegated act have been met, a delegated directive should be adopted in order to include isotonitazene in the Annex to Framework Decision 2004/757/JHA and, as a consequence thereof, subject that substance to the Union criminal law provisions on illicit drug trafficking. |
|
(14) |
Ireland is bound by Framework Decision 2004/757/JHA, as amended by Directive (EU) 2017/2103 of the European Parliament and of the Council (4), and is therefore taking part in the adoption and application of this Decision. |
|
(15) |
Denmark is bound by Framework Decision 2004/757/JHA as applicable until 21 November 2018, but is not bound by Directive (EU) 2017/2103. It is therefore not taking part in the adoption and application of this Directive and is not bound by it or subject to its application. |
|
(16) |
In accordance with the Joint Political Declaration of 28 September 2011 of Member States and the Commission on explanatory documents (5), Member States have undertaken to accompany, in justified cases, the notification of their transposition measures with one or more documents explaining the relationship between the components of a directive and the corresponding parts of national transposition instruments. |
|
(17) |
Framework Decision 2004/757/JHA should therefore be amended accordingly, |
HAS ADOPTED THIS DIRECTIVE:
Article 1
Amendment to Framework Decision 2004/757/JHA
In the Annex to Framework Decision 2004/757/JHA, the following point 17 is added:
|
‘17. |
N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H-benzimidazole-1-ethanamine (isotonitazene). (*1) |
Article 2
Transposition
1. Member States shall bring into force the laws, regulations and administrative provisions necessary to comply with this Directive by 3 June 2021 at the latest. They shall forthwith communicate to the Commission the text of those provisions.
When Member States adopt those provisions, they shall contain a reference to this Directive or be accompanied by such a reference on the occasion of their official publication. Member States shall determine how such reference is to be made.
2. Member States shall communicate to the Commission the text of the main provisions of national law which they adopt in the field covered by this Directive.
Article 3
Entry into force
This Directive shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
Article 4
This Directive is addressed to the Member States in accordance with the Treaties.
Done at Brussels, 2 September 2020.
For the Commission
The President
Ursula VON DER LEYEN
(1) OJ L 335, 11.11.2004, p. 8.
(2) Regulation (EC) No 1920/2006 of the European Parliament and of the Council of 12 December 2006 on the European Monitoring Centre for Drugs and Drug Addiction (OJ L 376, 27.12.2006, p. 1).
(3) Deaths have also been reported by Canada (three cases) and the United States (18 cases).
(4) Directive (EU) 2017/2103 of the European Parliament and of the Council of 15 November 2017 amending Council Framework Decision 2004/757/JHA in order to include new psychoactive substances in the definition of ‘drug’ and repealing Council Decision 2005/387/JHA (OJ L 305, 21.11.2017, p. 12).
DECISIONS
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/58 |
DECISION (EU) 2020/1688 OF THE EUROPEAN CENTRAL BANK
of 25 September 2020
amending Decision (EU) 2020/187 on the implementation of the third covered bonds purchase programme (ECB/2020/48)
THE GOVERNING COUNCIL OF THE EUROPEAN CENTRAL BANK,
Having regard to the Treaty on the Functioning of the European Union and in particular the first indent of Article 127(2) thereof,
Having regard to the Statute of the European System of Central Banks and of the European Central Bank, and in particular the second subparagraph of Article 12.1 in conjunction with the first indent of Article 3.1, and Article 18.1 thereof,
Whereas:
|
(1) |
Decision ECB/2014/40 of the European Central Bank (1) established the third covered bond purchase programme (CBPP3) and has been recast in Decision (EU) 2020/187 of the European Central Bank (ECB/2020/8) (2). Alongside the asset-backed securities purchase programme, the secondary markets public sector asset purchase programme and the corporate sector purchase programme, the CBPP3 is part of the expanded asset purchase programme (APP) of the European Central Bank (ECB). The APP aims to enhance the transmission of monetary policy, facilitate the provision of credit to the euro area economy, ease borrowing conditions for households and firms and contribute to returning inflation rates to levels below but close to 2 % over the medium term, consistent with the ECB’s primary objective of maintaining price stability. |
|
(2) |
On 5 March 2020, the Governing Council decided, in principle, to automatically limit an eligible counterparty’s access to Eurosystem monetary policy operations in certain pre-defined circumstances. Consequently, the Governing Council also decided that where and for as long as such an automatic limitation or a suspension or exclusion applies pursuant to Guideline (EU) 2015/510 of the European Central Bank (ECB/2014/60) (3), a corresponding automatic exclusion of purchases of covered bonds under the CBPP3 issued by that counterparty should also apply. The Governing Council further decided that amendments to reflect these decisions should apply from the date from which the annual amendment of Guideline (EU) 2015/510 (ECB/2014/60) applies. |
|
(3) |
Therefore, Decision (EU) 2020/187 (ECB/2020/8) should be amended accordingly, |
HAS ADOPTED THIS DECISION:
Article 1
Amendment
Decision (EU) 2020/187 (ECB/2020/8) is amended as follows:
In Article 3, point (d) of paragraph 3 is replaced by the following:
|
‘(d) |
Covered bonds issued by credit institutions whose access to Eurosystem monetary policy operations has been limited, suspended or excluded pursuant to Guideline (EU) 2015/510 (ECB/2014/60) shall be automatically excluded from purchases under the CBPP3 for the duration of the limitation, suspension or exclusion. By derogation from the first sentence of this point (d), the Governing Council shall retain its power, following a case-by-case assessment, to reassess the exclusion of covered bonds issued by a credit institution whose access to Eurosystem monetary policy operations has been limited, suspended or excluded pursuant to Guideline (EU) 2015/510 (ECB/2014/60) and to revoke the exclusion, if deemed appropriate’. |
Article 2
Entry into force
This Decision shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
It shall apply from 1 January 2021.
Done at Frankfurt am Main, 25 September 2020.
For the Governing Council of the ECB
The President of the ECB
Christine LAGARDE
(1) Decision ECB/2014/40 of the European Central Bank of 15 October 2014 on the implementation of the third covered bond purchase programme (OJ L 335, 22.11.2014, p. 22).
(2) Decision (EU) 2020/187 of the European Central Bank of 3 February 2020 on the implementation of the third covered bond purchase programme (ECB/2020/8) (OJ L 39, 12.2.2020, p. 6).
(3) Guideline (EU) 2015/510 of the European Central Bank of 19 December 2014 on the implementation of the Eurosystem monetary policy framework (General Documentation Guideline) (ECB/2014/60) (OJ L 91, 2.4.2015, p. 3).
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/60 |
EUROPEAN SECURITIES AND MARKETS AUTHORITY DECISION (EU) 2020/1689
of 16 September 2020
renewing the temporary requirement to natural or legal persons who have net short positions to lower the notification thresholds of net short positions in relation to the issued share capital of companies whose shares are admitted to trading on a regulated market to notify the competent authorities above a certain threshold in accordance with point (a) of Article 28(1) of Regulation (EU) No 236/2012 of the European Parliament and of the Council
THE EUROPEAN SECURITIES AND MARKETS AUTHORITY BOARD OF SUPERVISORS,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to the Agreement on the European Economic Area, in particular Annex IX thereof,
Having regard to Regulation (EU) No 1095/2010 of the European Parliament and of the Council of 24 November 2010 establishing a European Supervisory Authority (European Securities and Markets Authority), amending Decision No 716/2009/EC and repealing Commission Decision 2009/77/EC (1), and in particular Article 9(5), 43(1) and 44(1) thereof,
Having regard to Regulation (EU) No 236/2012 of the European Parliament and of the Council of 14 March 2012 on short selling and certain aspects of credit default swaps (2), and in particular Article 28(1) thereof,
Having regard to Commission Delegated Regulation (EU) No 918/2012 of 5 July 2012 supplementing Regulation (EU) No 236/2012 of the European Parliament and of the Council on short selling and certain aspects of credit default swaps with regards to definitions, the calculation of net short positions, covered sovereign credit default swaps, notification thresholds, liquidity thresholds for suspending restrictions, significant falls in the value of financial instruments and adverse events (3), and in particular Article 24 thereof,
Having regard to the European Securities and Markets Authority Decision (EU) 2020/525 of 16 March 2020 to require natural or legal persons who have net short positions to temporarily lower the notification thresholds of net short positions in relation to the issued shares capital of companies whose shares are admitted to trading on a regulated market above a certain threshold to notify the competent authorities in accordance with point (a) of Article 28(1) of Regulation (EU) No 236/2012 of the European Parliament and of the Council (4),
Having regard to the European Securities and Markets Authority Decision (EU) 2020/1123 of 10 June 2020 renewing the temporary requirement to natural or legal persons who have net short positions to temporarily lower the notification thresholds of net short positions in relation to the issued share capital of companies whose shares are admitted to trading on a regulated market to notify the competent authorities above a certain threshold in accordance with point (a) of Article 28(1) of Regulation (EU) No 236/2012 of the European Parliament and of the Council (5),
Whereas:
1. INTRODUCTION
|
(1) |
With Decision (EU) 2020/525, ESMA required natural or legal persons with net short positions in relation to the issued share capital of companies whose shares are admitted to trading on a regulated market to notify the competent authorities details of any such position reaching, exceeding or falling below 0,1 % of the issued share capital in accordance with point (a) of Article 28(1) of Regulation (EU) No 236/2012. |
|
(2) |
The measure imposed by Decision (EU) 2020/525 addressed the necessity for national competent authorities and ESMA to be able to monitor the net short positions that market participants have entered into in relation to shares admitted to trading on a regulated market, on account of exceptional circumstances present in financial markets. |
|
(3) |
With Decision (EU) 2020/1123, ESMA renewed the temporary requirement since, notwithstanding a partial recovery of the EU financial markets from the losses registered since the outbreak of the pandemic, the outlook for a future recovery remained uncertain and threats to the orderly functioning and integrity of financial markets and the stability of the financial system were still present. |
|
(4) |
In accordance with Article 28(10) of Regulation (EU) No 236/2012, ESMA has to review this measure at appropriate intervals and at least every three months. |
|
(5) |
ESMA performed this review based on an analysis of performance indicators, including prices, volatility, credit default swaps spread indices, as well as the evolution of net short positions, especially those between 0,1 and 0,2 %. Pursuant to the conducted analysis, ESMA has decided that it should renew the measure for an additional three months. |
2. ABILITY OF THE MEASURE TO ADDRESS RELEVANT THREATS AND CROSS-BORDER IMPLICATIONS (ARTICLE 28(2)(a) OF REGULATION (EU) No 236/2012)
(a) Threat to the orderly functioning and integrity of the financial markets
|
(6) |
The COVID-19 pandemic continues to have an adverse impact on the real economy with the overall outlook for a future recovery remaining uncertain, particularly in light of recent developments in the EU and beyond. ESMA notes that the number of COVID-19 cases has significantly increased in several jurisdictions over the last few weeks, raising concerns about the possibility of a second wave of COVID-19 infections exacerbating the uncertainty of any future outlook. |
|
(7) |
Equity markets in the EU, as demonstrated by the Eurostoxx 50 Index, lost 14 % in the period between 20 February and 3 September 2020, compared to a loss of value of 13 % between 20 February and 4 June (Figure 1). There was a significant improvement of the Eurostoxx 50 Index with respect to the levels reached in March (where the drop was of approximately 30 %, compared to February 2020, as indicated in Decision (EU) 2020/525) but without recovering to pre-COVID-19 levels. |
|
(8) |
The volatility measured by the VSTOXX (6) also remains relatively elevated compared to February 2020. The level measured in September (+ 15 %) is slightly higher than the one in June (+ 13 %). The same is also true, and even to a larger extent, for the VIX (7) (+ 18 % in September against + 9 % in June) (Figure 1 and Figure 2). |
|
(9) |
The level of credit default swaps (CDS) has decreased between 17 % and 28 % from 4 June to 3 September (Figure 1). However, to better understand the informative power of CDSs at this moment, the data has to be assessed in the context of the European Central Bank’s (ECB) decision on the Pandemic Emergency Purchase Programme (PEPP). From the beginning of March, the CDS spreads started to be highly volatile and indicative of a high financial risk. The renewal by ESMA of Decision (EU) 2020/525, based on data up to 4 June, was also based on the fact that CDS spreads were high and volatile at that time and were considered as an indication of the perception of risk in the market. |
|
(10) |
However, the increase of the PEPP by a further EUR 600 billion decided on 4 June brought the ECB’s total announced monetary stimulus to EUR 1,35 trillion, reducing the informative value of CDS spreads in relation to the risk perception of the market. |
|
(11) |
Similar considerations can be made in relation to the informative value of the sovereign bond markets, which, as in the case of CDS spreads, are affected by the monetary policy of the central banks. As it appears in Figure 1, the 10Y government bonds yields of DE, FR, GB and IT show a decrease with respect to June levels on average by 22 basis points. |
|
(12) |
As a consequence, ESMA considers that the evolution of stock markets, which is significantly less impacted by the central banks’ monetary policy, should provide better insights to the current level of risk in EU financial markets. |
|
(13) |
Similar to the Eurostoxx 50, the STOXX EUROPE 800 excluding Switzerland Index lost approximately 17 % since 20 February 2020, compared to a drop of 16 % in June. The STOXX Europe Total Market Banks (ref. European banks) decreased by 37 % since February, while in June the loss was of approximately 30 %. |
|
(14) |
As mentioned in ESMA’s Report on Trends, Risks and Vulnerabilities No 2 of September 2020 (TRV), in Q2 2020 there were signs of differentiation across sectors, affecting in particular credit institutions. As of end-June, EU airlines and banking sector indices were still 36 % and 30 %, respectively, below their early January levels. Furthermore, Figure 3 shows that the banking and the insurance sectors have been rather stable since June, but the performance between 20 February and 3 September compared to the one between 20 February and 4 June shows a deterioration: – 38 % v – 33 % for the banking sector and – 21 % v – 20 % for the insurance sector. On the contrary, the performance of the non-financial (8) sectors has improved to a small extent: – 9 % v – 11 %. |
|
(15) |
The price recovery of certain sectors took place in the context of a further deteriorating macroeconomic environment and a deep and globally synchronized recession. As indicated in the TRV, the current apparent decoupling of financial market performance from underlying economic activity raises a question about the sustainability of the market rebound going forward. |
|
(16) |
Considering the aggregated national or EU level, the price recovery does not seem to have materialised: Figure 5 shows that price decreases are widespread across the Union, from June to September there were no significant improvements and the stock indices performance for 22 EU markets indicates that the drop from 20 February to 3 September was worse than the drop from 20 February to 4 June. Furthermore, the stock markets of 25 jurisdictions, compared to 24 in June, lost at least 10 % of their value when comparing the prices of 3 September to those of 20 February 2020. Over the same period, the share prices of European credit institutions have lost between 10 % and 59 %, compared to losses in value between 9 and 48 % recorded in the period from February to June. |
|
(17) |
The percentage of shares with a net short position between 0,1 and 0,2 % steadily increased over the period from 16 March until 11 June 2020 and then remained stable until 4 September 2020 (9) at an average of 13 % over the total net short positions (Figure 6). Furthermore, lower reporting threshold had demonstrated that, in some countries, net short positions between 0,1 % and 0,2 % represented up to approximately 50 % of the total positions reported. In conclusion, the percentage of net short positions between 0,1 and 0,2 %, which had to be reported due to the temporary lower notification threshold, remains a relevant portion of the total net short positions and has strong informative value for regulators in the current context. |
|
(18) |
Overall, significant price decreases in key sectors, relatively high volatility, the potential decoupling of financial market performance and underlying economic activity and a steadily high level of net short positions, coupled with the uncertainty about the evolution of the COVID-19 pandemic and its impact on the real economy, signal that the EU financial markets remain in a fragile state. Such state makes it more likely that short selling pressures could initiate or exacerbate negative developments in the coming months which, in turn, can negatively affect market confidence or the integrity of the price determination mechanism. |
|
(19) |
ESMA therefore considers that the combination of the circumstances described above constitutes a serious threat to the orderly functioning and integrity of the financial markets. |
(b) Threat to the stability of the whole or part of the financial system in the Union
|
(20) |
As explained by the ECB in its Financial Stability Review (10), financial stability is a condition in which the financial system – which comprises financial intermediaries, markets and market infrastructures – is capable of withstanding shocks and the unravelling of financial imbalances. |
|
(21) |
The COVID-19 pandemic continues to have a severe impact on the real economy in the Union. As reported in ESMA’s TRV, notwithstanding the market rebound, the market environment remains fragile and ESMA sees, going forward, a ‘prolonged period of risk to institutional and retail investors of further – possibly significant – market corrections and (…) very high risks across the whole of the ESMA remit’ (11). In that respect, ESMA alerted the public to a potential decoupling of financial market performance and underlying economic activity. |
|
(22) |
Section 2(a) above contains further information on the performance of banking, insurance sectors and financial markets. |
|
(23) |
These widespread price decreases have put the majority of EU shares across all sectors into a situation of fragility in which further price declines not triggered by additional fundamental information could have highly detrimental consequences. |
|
(24) |
In the still uncertain situation, ESMA considers that substantial selling pressure and unusual volatility in the price of shares could be triggered by different factors, including by an increasing number of market participants engaging in short selling and building up significant net short positions. |
|
(25) |
In particular, ESMA notes that the widespread price losses for credit institutions, which constituted one of the parameters for the renewal decision taken in June, have not improved. This indicates that credit institutions, which in certain cases are systemically important, remain potentially vulnerable to short selling strategies and to the building up of significant net short positions, regardless of whether these strategies and positions are supported by fundamental information. |
|
(26) |
The risk remains that the accumulation of short selling strategies and the building up of significant net short positions could lead to disorderly downward price spirals for certain issuers, with potential spill over effects within the same Member State or across the EU, that in turn, could eventually put the financial system of one or several Member States at risk. |
|
(27) |
Notwithstanding the partial recovery observed in certain sectors of European financial markets, ESMA considers that the current market circumstances continue to seriously threaten the stability of the financial system in the Union. |
|
(28) |
Within the limit of ESMA’s mandate, the intended renewal of the measure obliges natural or legal persons who have a net short position in shares admitted to trading on a regulated market to report to national competent authorities at a lower threshold than the one established in Article 5 of Regulation (EU) No 236/2012. |
|
(29) |
The renewed measure should maintain the improved capacity of national competent authorities and ESMA to assess the evolving situation adequately, differentiate between market movements led by fundamental information from those that might be initiated or exacerbated by short selling and react if the integrity, orderly functioning and stability of the markets require more stringent actions. |
(c) Cross-border implications
|
(30) |
Another condition for ESMA to be able to take this measure is that the identified threats have cross-border implications. |
|
(31) |
As described above, equity markets across the EU, considering both national and pan-European indices, have not fully recovered from the severe price decreases observed in March. |
|
(32) |
Given the fact that the financial markets of most EU Member States are affected by these threats, albeit to different degrees, the cross border implications remain particularly serious as the interconnectedness of EU financial markets raise the likelihood of potential spill over or contagion effects across markets in case of short selling pressure. |
|
(33) |
ESMA therefore considers that the threats to market integrity, orderly functioning and financial stability described above have cross-border implications. Due to the nature of the COVID-19 crisis, they actually have a pan-EU and global character. |
3. NO COMPETENT AUTHORITY HAS TAKEN MEASURES TO ADDRESS THE THREAT OR ONE OR MORE OF THE COMPETENT AUTHORITIES HAVE TAKEN MEASURES THAT DO NOT ADEQUATELY ADDRESS THE THREAT (ARTICLE 28(2)(b) OF REGULATION (EU) No 236/2012)
|
(34) |
Another condition for ESMA to adopt the measure in this Decision is that a competent authority or competent authorities have not taken action to address the threat or the actions that have been taken do not adequately address the threat. |
|
(35) |
The market integrity, orderly functioning and financial stability concerns described in Decision (EU) 2020/525, which remain valid for this Decision, have led some national competent authorities to take national actions aimed at restricting the short selling of shares in Spain, France, Austria, Belgium, Greece and Italy (12) that expired on 18 May. |
|
(36) |
Following the expiration or lifting of those temporary measures, no further measures based on Regulation (EU) No 236/2012 have been taken in the EU, and as of the date of this Decision there are no such measures in force. |
|
(37) |
At the time of adoption of this Decision, no competent authorities have adopted measures to increase their visibility of the evolution of net short positions through the establishment of lower reporting thresholds, as they can rely on Decision (EU) 2020/1123. |
|
(38) |
The necessity of having increased visibility of net short positions is even more acute in a context where the above mentioned restrictions imposed under Article 20 of Regulation (EU) No 236/2012 are no longer in place and the uncertainty in relation to the prolonged COVID-19 impact remains. As short selling and transactions with equivalent effect are no longer subject to other external constraints, national competent authorities across the EU need to be able to identify in advance whether net short positions are being built up to an extent which may lead to the threats to financial markets and financial stability described above manifesting themselves and being exacerbated by short selling pressure. |
|
(39) |
In light of the abovementioned pan-EU threats, it remains evident that the information received by national competent authorities under the ordinary reporting threshold set out in Article 5(2) of Regulation (EU) No 236/2012 is not sufficient under the current stressed market conditions. ESMA considers that maintaining the lower reporting threshold should ensure that all national competent authorities across the EU and ESMA have the best possible data set available to monitor market trends and prepare themselves and ESMA to take further measures, if necessary. |
4. EFFICIENCY OF THE MEASURE (ARTICLE 28(3)(a) OF REGULATION (EU) No 236/2012)
|
(40) |
ESMA also has to take into account to what extent the renewed measure significantly addresses the threats identified. |
|
(41) |
ESMA considers that despite the extraordinary losses that were incurred in the trading of shares on regulated markets since 20 February 2020, markets have functioned orderly and that the integrity of markets has been largely preserved. |
|
(42) |
ESMA has therefore analysed the current circumstances, in particular with reference to how far they constitute threats to the integrity of markets and to financial stability in the Union and whether the renewed ESMA measure would be efficient in addressing such threats by taking a forward-looking approach. |
(a) The measure significantly addresses the threat to the orderly functioning and integrity of financial markets
|
(43) |
Under the above described circumstances, any sudden increase in selling pressure and market volatility due to short selling and building up of short positions can amplify downward trends in financial markets. While short selling at other times may serve positive functions in terms of determining the correct valuation of issuers, in current market circumstances it may pose an additional threat to the orderly functioning and integrity of markets. |
|
(44) |
In particular, given the horizontal impact of the continued emergency situation that affects a broad set of shares across the Union, any sudden fall in share prices may be exacerbated by additional selling pressure resulting from short selling and increased net short positions that, if below the normal thresholds for notification to the national competent authorities under Article 5 of Regulation (EU) No 236/2012, would therefore go undetected without the renewed measure. |
|
(45) |
For the above reasons, national competent authorities and ESMA need to be aware as soon as possible of market participants engaging in short sales and building up significant net short positions to prevent, if necessary, that those positions become signals leading to a cascade of selling orders and a consequent significant fall in prices. |
|
(46) |
ESMA considers that, without this measure being renewed for an additional three months, national competent authorities and ESMA would not have the capacity to adequately monitor the market in the current uncertain and fragile environment. This is accentuated by the apparent decoupling of financial market performance and the underlying economic activity, coupled with the evolving nature of the COVID-19 pandemic. Such factors could trigger a sudden and significant selling pressure and an unusual additional volatility in the price of Union shares that, in turn, could be further amplified by the accumulation of short positions. |
|
(47) |
At the same time, ESMA considers it appropriate to maintain the publication threshold laid down in Article 6 of Regulation (EU) No 236/2012, which equals 0,5 % of the issued share capital of the company, as the lowering of this threshold does not appear to be necessary from the perspectives of maintaining orderly markets and addressing risks to financial stability. ESMA continues monitoring on an ongoing basis the market conditions and will take further measures, where needed. |
(b) The measure significantly addresses the threat to the stability of the whole or part of the financial system in the Union
|
(48) |
As described above, in the great majority of EU equity markets performance has worsened between June and September 2020, when compared to 20 February. Overall, trading in shares since 20 February 2020 was and still is characterised by selling pressure and a relatively high level of volatility. As evidenced above, various risk factors continue to have an impact on many sectors of the real economy and on EU financial markets. In this environment engaging in short selling and building up significant net short positions can amplify selling pressure and downward trends which in turn may exacerbate a threat which can have highly detrimental effects on the financial stability of financial institutions and companies from other sectors. |
|
(49) |
In that context, data limitations for national competent authorities and ESMA would restrict their capacity to address any potential negative effects on the economy and ultimately the financial stability of the Union as a whole. |
|
(50) |
Therefore, ESMA’s renewed measure to temporarily lower the reporting thresholds of net short positions to national competent authorities efficiently addresses this threat to the stability of parts or ultimately the whole of the Union financial system by reducing data limitations and enhancing the national competent authorities capacity to address upcoming threats at an early stage. |
(c) Improvement of the ability of the competent authorities to monitor the threat
|
(51) |
In ordinary market conditions national competent authorities monitor any threat that may derive from short selling and the building up of net short positions with the supervisory tools established in Union legislation, in particular the reporting obligations concerning net short positions established in Regulation (EU) No 236/2012 (13). |
|
(52) |
However, the existing market conditions render it necessary to intensify the monitoring activity of national competent authorities and ESMA of the aggregated net short positions in shares admitted to trading on regulated markets. To that end and given the continued uncertainty related to the COVID-19 pandemic, it remains important that national competent authorities continue receiving information on the build-up of net short positions at the earliest stage possible, before they reach the level of 0,2 % of the issued share capital laid down in Article 5(2) of Regulation (EU) No 236/2012. |
|
(53) |
This is highlighted by the percentage of shares with a net short position between 0,1 and 0,2 %, which steadily increased over the period 16 March until 11 June 2020 and has remained stable since then, until 4 September 2020 (14), at on average 13 % over the whole observation period. Therefore, it can be concluded that the percentage of net short positions between 0,1 and 0,2 %, which had to be reported due to the lower notification threshold imposed by ESMA, remain a relevant portion of the total net short positions. |
|
(54) |
Therefore, ESMA’s renewed measure will maintain the improved ability of national competent authorities to deal with any identified threats at an earlier stage, allowing them and ESMA to timely manage threats to the orderly functioning of markets and to financial stability, should any sign of market stress manifest itself. |
5. THE MEASURES DO NOT CREATE A RISK OF REGULATORY ARBITRAGE (ARTICLE 28(3)(b) OF REGULATION (EU) No 236/2012)
|
(55) |
In order to adopt or renew a measure under Article 28 of Regulation (EU) No 236/2012, ESMA should take into account whether the measure creates a risk of regulatory arbitrage. |
|
(56) |
Since ESMA’s renewed measure concerns the reporting obligations of market participants with respect to all shares admitted to trading on regulated markets in the Union, it will ensure a single reporting threshold for all national competent authorities, ensuring a level-playing field among market participants within and outside the Union in respect of the trading of shares admitted to trading on regulated markets in the Union. |
6. ESMA’S MEASURE DOES NOT HAVE A DETRIMENTAL EFFECT ON THE EFFICIENCY OF FINANCIAL MARKETS, INCLUDING BY REDUCING LIQUIDITY IN THOSE MARKETS OR CREATING UNCERTAINTY FOR MARKET PARTICIPANTS, THAT IS DISPROPORTIONATE TO ITS BENEFITS (ARTICLE 28(3)(c) OF REGULATION (EU) No 236/2012)
|
(57) |
ESMA has to assess whether the measure has detrimental effects which would be considered disproportionate compared to its benefits. |
|
(58) |
ESMA considers it appropriate that national competent authorities closely monitor the evolution of net short positions before considering adopting any more intrusive measure. ESMA notes that the normal reporting thresholds (0,2 % of the issued share capital) may not be adequate in the continued exceptional market conditions to timely identify trends and materialising threats. |
|
(59) |
Although the introduction of an enhanced reporting obligation may have added an additional burden to reporting entities, currently the latter have already adapted their internal systems upon the application of Decisions (EU) 2020/525 and (EU) 2020/1123 and therefore this renewed measure is not expected to further impact the reporting entities’ compliance costs. Additionally, it will not limit the capacity of market participants to enter into or increase their short positions in shares. As a result, the efficiency of the market will not be affected. |
|
(60) |
Compared to other potential and more intrusive measures, this renewed measure should not affect the liquidity in the market as the increased reporting obligation for a limited set of market participants should not change their trading strategies and therefore, their participation in the market. Additionally, the maintained exception foreseen for market making activities and stabilisation programmes is meant not to increase the burden for entities that offer important services in terms of providing liquidity and reducing volatility, particularly relevant in the current situation. |
|
(61) |
In terms of scope of the renewed measure, ESMA believes that limiting it to one or several sectors or to any subset of issuers may not achieve the desired outcome. The magnitude of the price declines recorded after the outbreak of the COVID-19 pandemic, the wide range of shares (and sectors) affected and the degree of interconnection between the EU economies and trading venues, suggest that an EU-wide measure is likely to be more effective than sectoral measures in providing early market intelligence to national competent authorities. |
|
(62) |
In terms of creating market uncertainty, the measure does not introduce new regulatory obligations, as by lowering the relevant threshold it only modifies the normal reporting obligation that has been in force since 2012. ESMA also highlights that the renewed measure remains limited to the reporting of shares that are admitted to trading on a regulated market in the Union to capture those positions where additional reporting appears most relevant. |
|
(63) |
Therefore, ESMA considers that such an enhanced transparency obligation should not have a detrimental effect on the efficiency of financial markets or on investors that is disproportionate to its benefits and should not create any uncertainty in the financial markets. |
|
(64) |
In terms of duration of the measure, ESMA considers that a renewal of the measure for three months is justified considering the information available at this point in time and the remaining overall uncertain outlook in the context of the COVID-19 pandemic. ESMA intends to revert to the regular reporting obligation as soon as the situation improves, but at the same time cannot discard the possibility of extending the measure should the situation worsen or should markets remain in a fragile state. |
|
(65) |
On that basis and as of this date, ESMA deems this Decision to renew the temporary increased transparency measure on net short positions to be proportionate given the continued adverse circumstances. |
7. CONSULTATION AND NOTICE (ARTICLE 28(4) AND (5) OF REGULATION (EU) No 236/2012)
|
(66) |
ESMA has consulted the ESRB. The ESRB has not raised any objections to the adoption of the proposed Decision. |
|
(67) |
ESMA has notified national competent authorities of the intended Decision. |
|
(68) |
ESMA’s renewed measure will apply as of 18 September 2020, |
HAS ADOPTED THIS DECISION:
Article 1
Definition
For the purposes of this Decision, a ‘regulated market’ means a regulated market as referred to in Article 4(1)(21) of Directive 2014/65/EU of the European Parliament and of the Council (15).
Article 2
Temporary additional transparency obligations
1. A natural or legal person who has a net short position in relation to the issued share capital of a company that has its shares admitted to trading on a regulated market shall notify the relevant competent authority, in accordance with Articles 5 and 9 of Regulation (EU) No 236/2012 where the position reaches or falls below a relevant notification threshold referred to in paragraph 2 of this Article.
2. A relevant notification threshold is a percentage that equals 0,1 % of the issued share capital of the company concerned and each 0,1 % above that threshold.
Article 3
Exemptions
1. In accordance with Article 16 of Regulation (EU) No 236/2012, the temporary additional transparency obligations referred to in Article 2 shall not apply to shares admitted to trading on a regulated market where the principal venue for the trading of the shares is located in a third country.
2. In accordance with Article 17 of Regulation (EU) No 236/2012, the temporary additional transparency obligations referred to in Article 2 shall not apply to transactions performed due to market making activities.
3. The temporary additional transparency obligations referred to in Article 2 shall not apply to a net short position in relation to the carrying out of a stabilisation under Article 5 of Regulation (EU) No 596/2014 of the European Parliament and of the Council (16).
Article 4
Entry into force and application
This Decision enters into force on 18 September 2020. It shall apply from the date of its entry into force for a period of three months.
Done at Paris, 16 September 2020.
For the Board of Supervisors
Steven MAIJOOR
The Chair
(1) OJ L 331, 15.12.2010, p. 84.
(3) OJ L 274, 9.10.2012, p. 1.
(4) OJ L 116, 15.4.2020, p. 5.
(5) OJ L 245, 30.7.2020, p. 17.
(6) The VSTOXX measures the implied volatility based on the Eurostoxx 50 option prices.
(7) The VIX Index is calculated by using the midpoint of real-time S&P 500 Index (SPX) option bid/ask quotes.
(8) The non-financial sectors exclude the following sub-sectors: banks, insurance and financial services.
(9) The reports of the daily net short positions from Denmark are missing over the period 31 August-4 September due to a technical issue.
(10) https://www.ecb.europa.eu/pub/financial-stability/fsr/html/ecb.fsr201911~facad0251f.en.html
(11) TRV, Executive Summary, page 4.
(12) In Italy the measure was lifted on 18 May.
(13) Cf. Article 5 of Regulation (EU) No 236/2012.
(14) The reports of the daily net short positions from Denmark are missing over the period 31 August-4 September due to a technical issue.
(15) Directive 2014/65/EU of the European Parliament and of the Council of 15 May 2014 on markets in financial instruments and amending Directive 2002/92/EC and Directive 2011/61/EU (OJ L 173, 12.6.2014, p. 349).
(16) Regulation (EU) No 596/2014 of the European Parliament and of the Council of 16 April 2014 on market abuse (market abuse regulation) and repealing Directive 2003/6/EC of the European Parliament and of the Council and Commission Directives 2003/124/EC, 2003/125/EC and 2004/72/EC (OJ L 173, 12.6.2014, p. 1).
ANNEX
In this Annex, ‘ESMA decision’ refers to Decision (EU) 2020/525.
Figure 1
Financial indicators
|
Equity market performance |
Changes from 20.2.2020 to 3.9.2020 (in %) |
Index level as of 3.9.2020 |
Changes from 20.2.2020 to 4.6.2020 (in %) |
Index level as of 4.6.2020 |
|
STOXX EUROPE 800 ex. Switzerland |
– 17 |
116 |
– 16 |
117 |
|
EURO STOXX INDEX |
– 13 |
362 |
– 13 |
364 |
|
EURO STOXX 50 |
– 14 |
3 304 |
– 13 |
3 323 |
|
US S&P500 |
2 |
3 451 |
– 8 |
3 112 |
|
JP Nikkei |
0 |
23 466 |
– 3 |
22 864 |
|
Global |
– 1 |
228 |
– 9 |
211 |
|
European banks |
– 37 |
93 |
– 30 |
104 |
|
IT financials |
– 30 |
27 |
– 29 |
28 |
|
ES financials |
– 48 |
34 |
– 35 |
42 |
|
DE financials |
– 13 |
123 |
– 11 |
126 |
|
FR financials |
– 34 |
118 |
– 28 |
130 |
|
Volatility |
Changes from 20.2.2020 to 3.9.2020 (in %) |
Index level as of 3.9.2020 |
Changes from 20.2.2020 to 4.6.2020 (in %) |
Index level as of 4.6.2020 |
|
VSTOXX |
15 |
29 |
13 |
28 |
|
VIX |
18 |
33 |
9 |
25 |
|
Credit Default Swaps |
Changes in bps from 20.2.2020 to 3.9.2020 |
CDS spreads in bps as of 3.9.2020 |
Changes in bps from 20.2.2020 to 4.6.2020 |
CDS spreads in bps as of 4.6.2020 |
|
Europe corporate |
5 |
45 |
22 |
62 |
|
Europe high yield |
92 |
290 |
157 |
355 |
|
Europe financials |
15 |
59 |
29 |
73 |
|
Europe financials subordinate |
38 |
124 |
62 |
149 |
|
10Y Government bonds |
Changes in bps from 20.2.2020 to 3.9.2020 |
Bond yields in % as of 3.9.2020 |
Changes in bps from 20.2.2020 to 4.6.2020 |
Bond yields in % as of 4.6.2020 |
|
DE10Y |
– 5 |
– 0,49 |
15 |
– 0,29 |
|
ES10Y |
9 |
0,33 |
32 |
0,55 |
|
FR10Y |
2 |
– 0,19 |
23 |
0,01 |
|
IT10Y |
15 |
1,06 |
51 |
1,42 |
|
US10Y |
– 90 |
0,62 |
– 67 |
0,86 |
|
GB10Y |
– 34 |
0,24 |
– 24 |
0,34 |
|
JP10Y |
8 |
0,04 |
9 |
0,05 |
|
Note: Equity market changes expressed in relative terms, other changes in absolute terms. Sources: Refinitiv EIKON, ESMA. |
||||

Note: Implied volatilities of EURO STOXX 50 (VSTOXX) and S&P 500 (VIX), in %.
Sources: Refinitiv Datastream, ESMA.
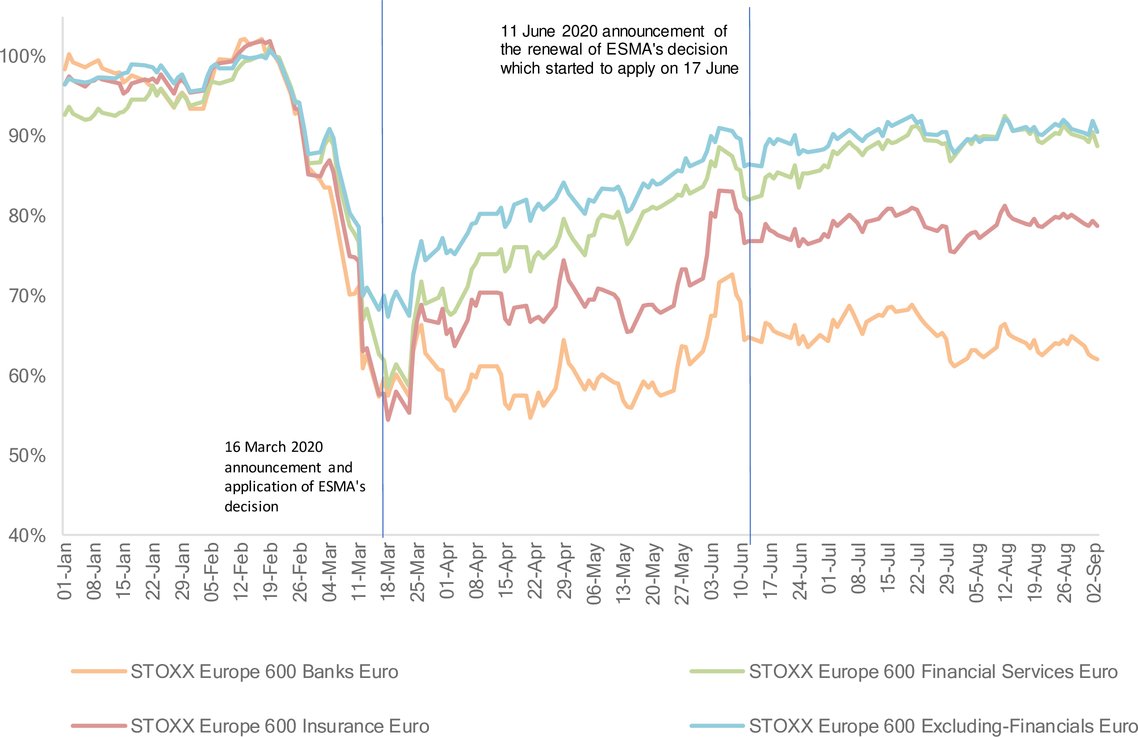
Note: Equity prices. 20.2.2020 = 100.
Sources: Refinitiv Datastream, ESMA.

Note: Equity prices. 20.2.2020 = 100.
Sources: Refinitiv Datastream, ESMA.
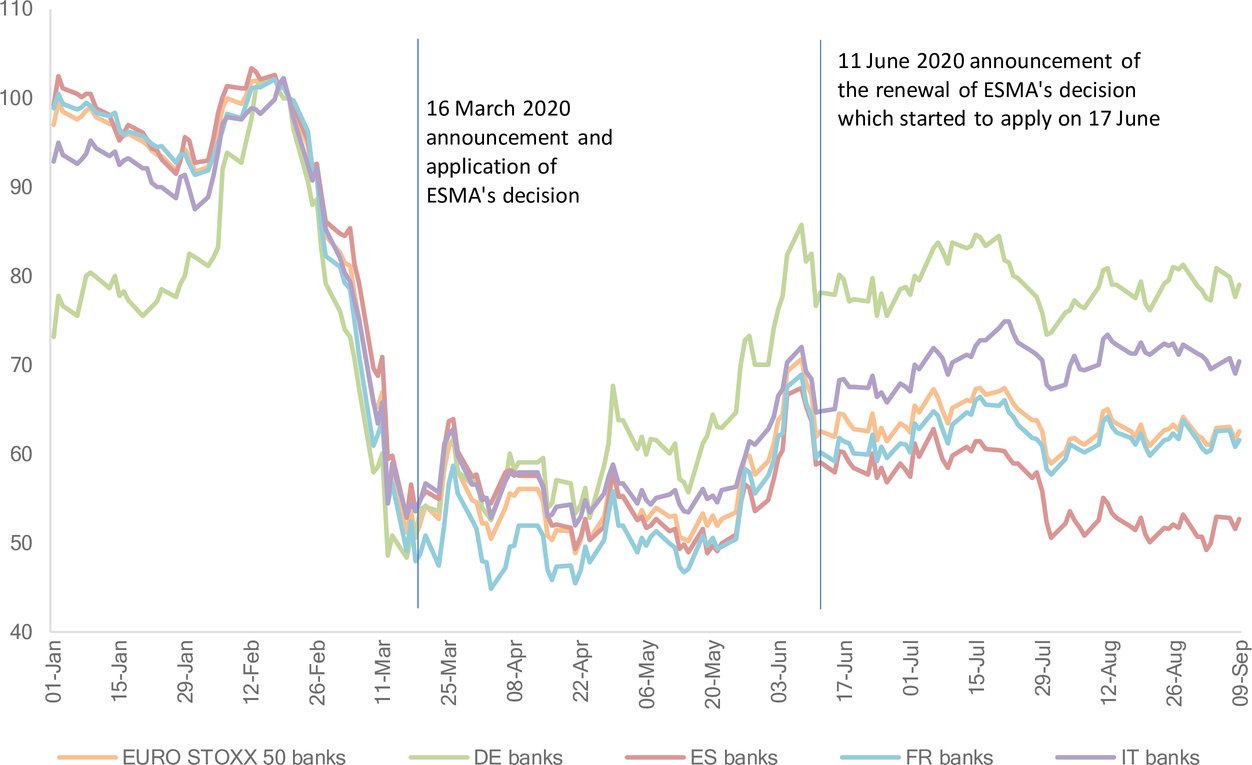
Note: Equity prices. 20.2.2020 = 100.
Sources: Refinitiv Datastream, ESMA.
Figure 4
EU CDS spread indices
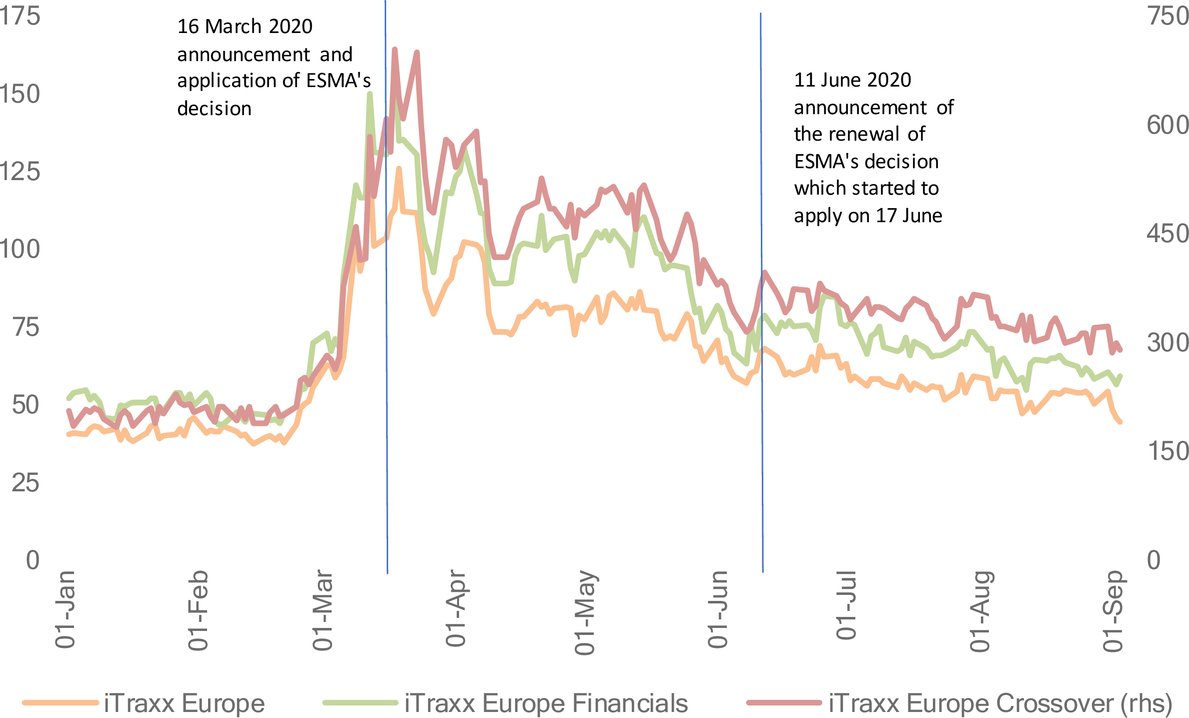
Note: CDS spreads on European IG corporates (iTraxx Europe), European HY corporates (iTraxx Europe Crossover) and European Financials, in bps.
Sources: Refinitiv EIKON, ESMA.
Figure 5
European stock indices performance per country
|
|
Percentage change from 20.2.2020 to 3.9.2020 |
Percentage change from 20.2.2020 to 4.6.2020 |
|
STOXX EUROPE 800 ex. Switzerland |
– 16,67 |
– 15,78 |
|
EURO STOXX INDEX |
– 13,22 |
– 12,72 |
|
EURO STOXX 50 |
– 13,57 |
– 13,07 |
|
AT |
– 30,21 |
– 23,30 |
|
BE |
– 19,22 |
– 14,86 |
|
BG |
– 20,67 |
– 15,69 |
|
CY |
– 39,52 |
– 34,73 |
|
CZ |
– 17,81 |
– 13,94 |
|
DE |
– 4,44 |
– 7,32 |
|
DK |
4,24 |
– 2,48 |
|
EE |
– 13,55 |
– 13,16 |
|
ES |
– 29,45 |
– 21,88 |
|
FI |
– 5,35 |
– 8,66 |
|
FR |
– 17,37 |
– 15,91 |
|
GB |
– 21,32 |
– 13,93 |
|
GR |
– 29,24 |
– 26,64 |
|
HR |
– 19,61 |
– 17,16 |
|
HU |
– 24,05 |
– 17,83 |
|
IE |
– 13,00 |
– 12,90 |
|
IS |
– 1,50 |
– 4,44 |
|
IT |
– 22,04 |
– 20,01 |
|
LT |
5,06 |
– 1,98 |
|
LU |
– 25,92 |
– 21,60 |
|
LV |
5,53 |
– 1,81 |
|
MT |
– 20,80 |
– 12,92 |
|
NL |
– 11,66 |
– 9,17 |
|
NO |
– 11,80 |
– 10,22 |
|
PL |
– 15,75 |
– 13,47 |
|
PT |
– 20,09 |
– 13,64 |
|
RO |
– 10,73 |
– 11,45 |
|
SE |
– 7,28 |
– 9,47 |
|
SI |
– 13,01 |
– 10,83 |
|
SK |
– 7,05 |
0,07 |
|
Sources: Refinitiv EIKON, ESMA. |
||
Figure 6
NSP between 0,1 % and 0,2 % over the period 16 March-4 September 2020 (1)

GUIDELINES
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/77 |
GUIDELINE (EU) 2020/1690 OF THE EUROPEAN CENTRAL BANK
of 25 September 2020
amending Guideline (EU) 2015/510 on the implementation of the Eurosystem monetary policy framework
(ECB/2020/45)
THE GOVERNING COUNCIL OF THE EUROPEAN CENTRAL BANK,
Having regard to the Treaty on the Functioning of the European Union, and in particular the first indent of Article 127(2) thereof,
Having regard to the Statute of the European System of Central Banks and of the European Central Bank, and in particular the first indent of Article 3.1, Articles 9.2, 12.1, 14.3 and 18.2 and the first paragraph of Article 20 thereof,
Whereas:
|
(1) |
Achieving a single monetary policy entails defining the tools, instruments and procedures to be used by the Eurosystem in order to implement such a policy in a uniform manner throughout the Member States whose currency is the euro. |
|
(2) |
Guideline (EU) 2015/510 of the European Central Bank (ECB/2014/60) (1) should be amended to incorporate necessary technical and editorial adjustments relating to certain aspects of monetary policy operations. |
|
(3) |
In order to reduce the overall complexity of the Eurosystem’s collateral framework, the Eurosystem risk exposure and the operational burden on the eligibility assessment, non-legislative covered bonds (i.e. contractual covered bonds) should no longer be accepted as Eurosystem collateral. Therefore, the definitions and provisions relating to covered bonds in the Eurosystem collateral framework should be amended to restrict the type of eligible covered bonds to legislative covered bonds and multi cédulas. |
|
(4) |
To reflect the Eurosystem’s two-tier system for remunerating excess reserve holdings applicable since 30 October 2019 in accordance with Decision (EU) 2019/1743 of the European Central Bank (ECB/2019/31) (2), it should be specified which legal framework applies for the remuneration of minimum reserves and of excess reserve holdings. |
|
(5) |
To further reduce the complexity of the Eurosystem’s collateral framework, and taking into account the limited extent to which they have been used, marketable debt instruments issued or guaranteed by non-financial corporations for which no appropriate credit assessment is available should no longer be accepted as Eurosystem collateral after a transition period. |
|
(6) |
With a view to reflecting recent financial innovations in the area of sustainable finance, the Eurosystem intends to accept certain marketable debt instruments with coupon structures linked to the issuer’s fulfilment of pre-defined sustainability targets. |
|
(7) |
It should be clarified that assets with coupons linked to interpolated reference rates are eligible only under certain conditions, and these conditions should be specified. |
|
(8) |
In order to establish a consistent and transparent approach to the categories of secured marketable assets eligible as collateral for Eurosystem credit operations, secured marketable assets other than ABSs and covered bonds should no longer be accepted as Eurosystem collateral. |
|
(9) |
The loan-level data requirements for asset-backed securities (ABSs) that are eligible as Eurosystem collateral should be adjusted for those ABSs for which loan-level data are reported in accordance with Regulation (EU) 2017/2402 of the European Parliament and of the Council (3). |
|
(10) |
Certain provisions related to the eligibility as Eurosystem collateral of credit claims and to data reporting regarding credit claims should be amended in order to improve the information availability for credit claims under the collateral framework, increase clarity of the rules determining a credit claim’s eligibility as collateral and clarify the verification procedures for such assets. |
|
(11) |
To ensure greater transparency, consistency and legal certainty, the general acceptance criteria for external credit assessment institutions (ECAIs) in the Eurosystem credit assessment framework (ECAF) should be clarified. |
|
(12) |
The rules regarding the use of unsecured debt instruments issued by a counterparty or its closely linked entities should be simplified. |
|
(13) |
In order to increase the transparency of the Eurosystem counterparty framework, the details relating to the length of the grace period applicable to counterparties that do not meet minimum own funds requirements should be clarified. |
|
(14) |
The financial penalty for breaches related to the use of eligible assets as Eurosystem collateral should be adjusted to provide incentives to counterparties to proactively report such breaches. |
|
(15) |
Therefore, Guideline (EU) 2015/510 (ECB/2014/60) should be amended accordingly, |
HAS ADOPTED THIS GUIDELINE:
Article 1
Amendments
Guideline (EU) 2015/510 (ECB/2014/60) is amended as follows:
|
(1) |
Article 2 is amended as follows:
|
|
(2) |
Article 54 is amended as follows:
|
|
(3) |
in Article 61(1) the following sentence is added: ‘Such assets shall only be eligible until the date on which the Eurosystem Collateral Management System starts to operate (“go-live date”).’; |
|
(4) |
Article 63(1) is amended as follows:
|
|
(5) |
the following Article 64a is inserted: ‘Article 64a Marketable assets other than ABSs and covered bonds 1. In order to be eligible, marketable assets other than ABSs, legislative covered bonds and multi cédulas shall be unsecured obligations of both the issuer and guarantor. For marketable assets with more than one issuer or with more than one guarantor, the requirement in this paragraph shall apply to each issuer and each guarantor. 2. Marketable assets which are secured and were eligible before 1 January 2021 but do not comply with the eligibility requirements as set out in this Article shall remain eligible until 1 January 2026, provided that they fulfil all other eligibility criteria for marketable assets. By derogation from the first sentence of this paragraph, covered bonds which are neither legislative covered bonds nor multi cédulas, shall become ineligible from 1 January 2021.’; |
|
(6) |
Article 78 is amended as follows:
|
|
(7) |
Article 80 is replaced by the following: ‘Article 80 Eligibility criteria for covered bonds backed by asset-backed securities 1. Without prejudice to the eligibility of legislative covered bonds pursuant to Article 64a, in order for EEA legislative covered bonds backed by ABSs to be eligible, the cover pool of such bonds (for the purposes of paragraphs 1 to 4, “the cover pool”) shall only contain ABSs that comply with all of the following.
2. Subject to paragraph 4, the NCBs shall use the following measures to verify that the cover pool does not contain ABSs that do not comply with paragraph 1.
3. If the issuer fails to comply with a particular request or if the Eurosystem deems the content of a confirmation incorrect or insufficient to the extent that it is not possible to verify that the cover pool complies with the criteria in paragraph 1, the Eurosystem shall decide not to accept the EEA legislative covered bonds as eligible collateral or to suspend their eligibility. 4. Where the applicable legislation or prospectus exclude the inclusion of ABSs that do not comply with paragraph 1 as cover pool assets, no verification pursuant to paragraph 2 shall be required. 5. For the purposes of paragraph 1(b), the close links shall be determined at the time that the senior units of the ABSs are transferred into the cover pool of the EEA legislative covered bond. 6. The cover pool of non-EEA G10 legislative covered bonds shall not contain ABSs.’; |
|
(8) |
Article 87 is amended as follows:
|
|
(9) |
Article 90 is amended as follows:
|
|
(10) |
Article 100 is replaced by the following: ‘Article 100 Verifications of the procedures and systems used to submit credit claims NCBs, or supervisors or external auditors, shall conduct a verification of the appropriateness of the procedures and systems used by the counterparty to submit the information on credit claims to the Eurosystem prior to the first mobilisation of credit claims by the counterparty. The verification of the procedures and systems shall subsequently be conducted at least once every five years. In the event of significant changes to such procedures or systems, a new verification may be conducted.’; |
|
(11) |
in Article 101, the following new point (aa) is inserted after point (a):
(*5) Regulation (EU) 2016/867 of the European Central Bank of 18 May 2016 on the collection of granular credit and credit risk data (ECB/2016/13) (OJ L 144, 1.6.2016, p. 44).’;" |
|
(12) |
in Article 102, the second sentence is replaced by the following: ‘All the necessary legal formalities to ensure the validity of the agreement and to ensure the mobilisation of a credit claim as collateral shall be fulfilled by the counterparty and/or the transferee, as appropriate.’; |
|
(13) |
in Article 120, paragraphs 2 and 2a are replaced by the following: ‘2. Following the application process outlined in Annex IXc, the Eurosystem reserves the right to decide whether to initiate an ECAF acceptance procedure upon request from a credit rating agency (CRA). In making its decision, the Eurosystem shall take into account, among other things, whether the CRA provides relevant coverage for the efficient implementation of the ECAF in accordance with the requirements set out in Annex IXa. 2a. Following the initiation of an ECAF acceptance procedure, the Eurosystem shall investigate all additional information deemed relevant to ensure the efficient implementation of the ECAF, including the ECAI’s capacity (i) to fulfil the criteria and rules of the ECAF performance monitoring process in accordance with the requirements set out in Annex IX and the specific criteria in Annex IXb (if relevant); and (ii) to comply with the acceptance criteria set out in Annex IXc. The Eurosystem reserves the right to decide whether to accept an ECAI for the purposes of the ECAF on the basis of the information provided and its own due diligence assessment.’; |
|
(14) |
Article 138 is amended as follows:
|
|
(15) |
Article 139 is amended as follows:
|
|
(16) |
in Article 148, paragraph 2 is replaced by the following: ‘2. Counterparties may mobilise eligible assets other than fixed-term deposits, for cross-border use in accordance with the following:
|
|
(17) |
Article 155 is replaced by the following: ‘Article 155 Financial penalties for non-compliance with certain operational rules 1. If a counterparty fails to comply with any of the obligations referred to in Article 154(1), the Eurosystem shall impose a financial penalty for each case of non-compliance. The applicable financial penalty shall be calculated in accordance with Annex VII. 2. Where a counterparty rectifies a failure to comply with an obligation referred to in Article 154(1)(c), and notifies the NCB before the counterparty has been notified of the non-compliance by the NCB, ECB or an external auditor (“self-reported infringement”), the applicable financial penalty as calculated in accordance with Annex VII shall be reduced by 50 %. The reduction of the financial penalty shall also be applicable in cases where the counterparty notifies the NCB of a breach that was not discovered by the ECB or NCB and in relation to assets that have been demobilised. The reduction of the financial penalty shall not be applicable to assets that fall under the scope of an ongoing verification procedure of which the counterparty is aware due to a notification by the NCB, ECB or an external auditor.’; |
|
(18) |
in Article 156(4), point (a) is replaced by the following:
|
|
(19) |
Article 158 is amended as follows:
|
|
(20) |
in Article 159, in paragraph 4, point (b) is replaced by the following:
|
|
(21) |
Annexes I, VIII, IXa and XII are amended in accordance with Annex I to this Guideline; |
|
(22) |
the text set out in Annex II to this Guideline is added as a new Annex IXc. |
Article 2
Taking effect and implementation
1. This Guideline shall take effect on the day of its notification to the national central banks of the Member States whose currency is the euro.
2. The national central banks of the Member States whose currency is the euro shall take the necessary measures to comply with this Guideline and apply them from 1 January 2021. They shall notify the European Central Bank of the texts and means relating to those measures by 6 November 2020 at the latest.
Article 3
Addressees
This Guideline is addressed to all Eurosystem central banks.
Done at Frankfurt am Main, 25 September 2020.
For the Governing Council of the ECB
The President of the ECB
Christine LAGARDE
(1) Guideline (EU) 2015/510 of the European Central Bank of 19 December 2014 on the implementation of the Eurosystem monetary policy framework (General Documentation Guideline) (ECB/2014/60) (OJ L 91, 2.4.2015, p. 3).
(2) Decision (EU) 2019/1743 of the European Central Bank of 15 October 2019 on the remuneration of holdings of excess reserves and of certain deposits (ECB/2019/31) (OJ L 267, 21.10.2019, p. 12).
(3) Regulation (EU) 2017/2402 of the European Parliament and of the Council of 12 December 2017 laying down a general framework for securitisation and creating a specific framework for simple, transparent and standardised securitisation, and amending Directives 2009/65/EC, 2009/138/EC and 2011/61/EU and Regulations (EC) No 1060/2009 and (EU) No 648/2012 (OJ L 347, 28.12.2017, p. 35).
ANNEX I
Annexes I, VIII, IXa and XII to Guideline (EU) 2015/510 (ECB/2014/60) are amended as follows:
|
(1) |
in Annex I, paragraph 5, the second sentence is replaced by the following: ‘Such institutions include, inter alia, institutions subject to reorganisation measures and institutions subject to the freezing of funds and/or other measures imposed by the Union under Article 75 of the Treaty or by a Member State restricting the use of their funds or a decision of the Eurosystem suspending or excluding their access to open market operations or the Eurosystem’s standing facilities.’; |
|
(2) |
Annex VIII is amended as follows:
|
|
(3) |
in Annex IXa, Section 2, paragraph 1 is replaced by the following:
|
|
(4) |
in Annex XII, the term ‘UCITS compliant jumbo covered bond’ is replaced by the term ‘jumbo covered bond’. |
ANNEX II
The following new Annex IXc is added:
‘ANNEX IXc
ECAI ACCEPTANCE CRITERIA AND APPLICATION PROCESS
This Annex sets out in detail the acceptance criteria for external credit assessment institutions (ECAIs) and the process for a credit rating agency (CRA) to apply to become accepted as an ECAI under the Eurosystem credit assessment framework (ECAF), as provided for in Article 120 of this Guideline.
I. APPLICATION PROCESS FOR ACCEPTANCE AS AN ECAI UNDER THE ECAF
|
1. |
An application by a CRA for acceptance as an ECAI under the ECAF must be submitted to the ECB’s Directorate Risk Management (DRMSecretariat@ecb.europa.eu). The application must provide appropriate reasoning and supporting documentation as set out in Section II, demonstrating the applicant’s compliance with the requirements for ECAIs set out in this Guideline. The application, reasoning and supporting documentation must be provided in writing in English, using any applicable templates and in electronic format. |
|
2. |
In the first stage of the application process, the CRA must demonstrate its compliance with the relevant coverage requirements set out in Article 120 of and Annex IXa to this Guideline, as well as in this Annex, and, if the CRA’s application to be accepted under the ECAF was previously rejected by the Eurosystem, how it has addressed its previous non-compliance. The individual steps in this first stage are as follows.
|
|
3. |
If the ECB decides that the CRA complies with the relevant coverage requirements and, where applicable, has remedied its previous non-compliance and the ECB decides to initiate an ECAF acceptance procedure, the CRA may proceed to the second stage of the application process. In the second stage, the CRA must demonstrate its compliance with all other relevant requirements set out in this Guideline. The individual steps in the second stage are as follows.
|
II. INFORMATION REQUIRED FOR AN APPLICATION FOR ECAF ACCEPTANCE TO BE DEEMED COMPLETE
|
1. |
As regards the first stage of the application process, a CRA must provide the following information.
|
|
2. |
The ECB may request supplemental information, for example, to demonstrate the stability of a CRA’s coverage over time, the CRA’s rating issuance practices and the quality of the CRA’s ratings during the relevant coverage period. |
|
3. |
As regards the second stage of the application process, a CRA must provide the following documentation and information:
|
|
4. |
The ECB may request relevant supplemental information from the CRA, such as in relation to the CRA’s ratings of assets, issuers and guarantors which are not eligible under the ECAF, for example, due to geographical restrictions. |
III. ECAF ACCEPTANCE CRITERIA
|
1. |
In order to be accepted in the ECAF, a CRA must comply with the applicable requirements in this Guideline, including relevant coverage so as to ensure the efficient implementation of the ECAF, operational criteria, the availability of information on ECAI credit assessments and for the purposes of the performance monitoring processes and the capacity to fulfil the criteria and rules of the ECAF performance monitoring process. |
|
2. |
In relation to the requirement of relevant coverage:
|
|
3. |
In relation to the availability of information on ECAI credit assessments and for the purposes of the performance monitoring processes:
|
|
4. |
In relation to a CRA’s capacity to fulfil the criteria and rules of the ECAF performance monitoring process, the performance of the CRA’s ratings and its default assignments must be consistent over time to (a) ensure the appropriate mapping of the credit assessment information provided by the credit assessment system to the Eurosystem’s harmonised rating scale; and (b) to maintain the comparability of the results from the CRA’s credit assessments across the ECAF’s systems and sources. The CRA’s observed rating transition tables and default statistics should be in line with the expected values based on the CRA’s own rating scales, because, as set out in Annex IX to this Guideline, deviations between observed default rates and assigned probability of default can call into question the quality of credit assessments, thus hampering the efficient implementation of the ECAF. |
|
5. |
In relation to the operational criteria:
|
|
6. |
All ECAF acceptance criteria must be fulfilled in order for a CRA to be accepted in the ECAF. As the application to be accepted in the ECAF requires a highly technical qualitative and quantitative assessment, the Eurosystem may assess further relevant factors related to the requirements of this Guideline on the ECAF, if necessary. |
IV. ECAF ACCEPTANCE CRITERIA FOR ECAIS AND COMPLIANCE OVER TIME
|
1. |
The acceptance criteria for ECAIs must be fulfilled by CRAs at the time of their application for acceptance and at all times after their acceptance under the ECAF. |
|
2. |
The Eurosystem may apply measures pursuant to Article 126 of this Guideline to a CRA that:
When notifying the CRA of its decision to apply measures pursuant Article 126, the Eurosystem will provide reasons for its decision. |
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/92 |
GUIDELINE (EU) 2020/1691 OF THE EUROPEAN CENTRAL BANK
of 25 September 2020
amending Guideline ECB/2014/31 on additional temporary measures relating to Eurosystem refinancing operations and eligibility of collateral
(ECB/2020/47)
THE GOVERNING COUNCIL OF THE EUROPEAN CENTRAL BANK,
Having regard to the Treaty on the Functioning of the European Union, and in particular the first indent of Article 127(2) thereof,
Having regard to the Statute of the European System of Central Banks and of the European Central Bank, and in particular the first indent of Article 3.1, and Articles 5.1, 12.1, 14.3 and 18.2 thereof,
Whereas:
|
(1) |
Asset-backed securities whose underlying assets include residential mortgages or loans to small and medium-sized enterprises, or both, and which do not fulfil certain requirements specified in Article 3(5) of Guideline ECB/2014/31 (1) should no longer be eligible as Eurosystem collateral, in view of the fact that this asset class has never been used. |
|
(2) |
The method of calculation of financial penalties in cases where credit claims that are not compliant with Article 154(1)(c) of Guideline (EU) 2015/510 of the European Central Bank (ECB/2014/60) (2) are included in a pool of additional credit claims under Article 4 of Guideline ECB/2014/31 should be amended to avoid the imposition of disproportionate financial penalties. |
|
(3) |
Therefore, Guideline ECB/2014/31 should be amended accordingly, |
HAS ADOPTED THIS GUIDELINE:
Article 1
Amendments
Guideline ECB/2014/31 is amended as follows:
|
(1) |
in Article 3, paragraph 5 is deleted; |
|
(2) |
Article 4 is amended as follows:
|
Article 2
Taking effect and implementation
1. This Guideline shall take effect on the date of its notification to the national central banks of the Member States whose currency is the euro.
2. The national central banks of the Member States whose currency is the euro shall take the necessary measures to comply with this Guideline and apply them from 1 January 2021. They shall notify the European Central Bank of the texts and means relating to those measures by 6 November 2020 at the latest.
Article 3
Addressees
This Guideline is addressed to all Eurosystem central banks.
Done at Frankfurt am Main, 25 September 2020.
For the Governing Council of the ECB
The President of the ECB
Christine LAGARDE
(1) Guideline ECB/2014/31 of 9 July 2014 on additional temporary measures relating to Eurosystem refinancing operations and eligibility of collateral and amending Guideline ECB/2007/9 (OJ L 240, 13.8.2014, p. 28).
(2) Guideline (EU) 2015/510 of the European Central Bank of 19 December 2014 on the implementation of the Eurosystem monetary policy framework (General Documentation Guideline) (ECB/2014/60) (OJ L 91, 2.4.2015, p. 3).
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/94 |
GUIDELINE (EU) 2020/1692 OF THE EUROPEAN CENTRAL BANK
of 25 September 2020
amending Guideline (EU) 2016/65 on the valuation haircuts applied in the implementation of the Eurosystem monetary policy framework
(ECB/2020/46)
THE GOVERNING COUNCIL OF THE EUROPEAN CENTRAL BANK,
Having regard to the Treaty on the Functioning of the European Union and in particular the first indent of Article 127(2) thereof,
Having regard to the Statute of the European System of Central Banks and of the European Central Bank, and in particular the first indent of Article 3.1, Articles 9.2, 12.1, 14.3, 18.2 and the first paragraph of Article 20 thereof,
Whereas:
|
(1) |
Adjustments need to be made to the Eurosystem risk control and valuation framework to reflect the fact that non-legislative covered bonds (i.e. contractual covered bonds) should no longer be accepted as Eurosystem collateral. |
|
(2) |
Therefore, Guideline (EU) 2016/65 of the European Central Bank (ECB/2015/35) (1) should be amended accordingly, |
HAS ADOPTED THIS GUIDELINE:
Article 1
Amendments
Guideline (EU) 2016/65 (ECB/2015/35) is amended as follows:
|
(1) |
Article 2 is amended as follows:
|
|
(2) |
in the Annex, Table 1 is replaced by the following: ‘Table 1 Haircut categories for eligible marketable assets based on the type of issuer and/or type of asset
|
Article 2
Taking effect and implementation
1. This Guideline shall take effect on the day of its notification to the national central banks of the Member States whose currency is the euro.
2. The national central banks of the Member States whose currency is the euro shall take the necessary measures to comply with this Guideline and apply them from 1 January 2021.They shall notify the European Central Bank of the texts and means relating to those measures by 6 November 2020 at the latest.
Article 3
Addressees
This Guideline is addressed to the national central banks of the Member States whose currency is the euro.
Done at Frankfurt am Main, 25 September 2020.
For the Governing Council of the ECB
The President of the ECB
Christine LAGARDE
(1) Guideline (EU) 2016/65 of the European Central Bank of 18 November 2015 on the valuation haircuts applied in the implementation of the Eurosystem monetary policy framework (ECB/2015/35) (OJ L 14, 21.1.2016, p. 30).
RULES OF PROCEDURE
|
13.11.2020 |
EN |
Official Journal of the European Union |
L 379/96 |
DECISION OF THE GOVERNING BOARD OF THE BIO-BASED INDUSTRIES JOINT UNDERTAKING
of 26 March 2020
laying down internal rules concerning restrictions of certain rights of data subjects in relation to processing of personal data in the framework of the functioning of the BBI JU
THE GOVERNING BOARD OF THE BIO-BASED INDUSTRIES JOINT UNDERTAKING (hereafter referred to as ‘the BBI JU’),
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) 2018/1725 of the European Parliament and of the Council of 23 October 2018 on the protection of natural persons with regard to the processing of personal data by the Union institutions, bodies, offices and agencies and on the free movement of such data, and repealing Regulation (EC) No 45/2001 and Decision No 1247/2002/EC (1), and in particular Article 25 thereof,
Having regard to Council Regulation (EU) No 560/2014 of 6 May 2014 establishing the Bio-based Industries Joint Undertaking (2), and in particular Article 7(3)(s) of the Annex,
Having regard to the European Data Protection Supervisor Guidance on Article 25 of the new Regulation and internal rules,
After having consulted the EDPS, in accordance with Article 41(2) of Regulation (EU) 2018/1725,
Whereas:
|
(1) |
The BBI JU carries out its activities in accordance with Regulation (EU) No 560/2014. |
|
(2) |
In accordance with Article 25(1) of Regulation (EU) 2018/1725 restrictions of the application of Articles 14 to 22, 35 and 36, as well as Article 4 of that Regulation in so far as its provisions correspond to the rights and obligations provided for in Articles 14 to 22 should be based on internal rules to be adopted by the BBI JU, where these are not based on legal acts adopted on the basis of the Treaties. |
|
(3) |
These internal rules, including its provisions on the assessment of the necessity and proportionality of a restriction, should not apply where a legal act adopted on the basis of the Treaties provides for a restriction of data subject rights. |
|
(4) |
Where the BBI JU performs its duties with respect to data subject’s rights under Regulation (EU) 2018/1725, it shall consider whether any of the exemptions laid down in that Regulation apply. |
|
(5) |
Within the framework of its administrative functioning, the BBI JU may conduct administrative inquiries, disciplinary proceedings, carry out preliminary activities related to cases of potential irregularities reported to OLAF, process whistleblowing cases, process (formal and informal) procedures of harassment, process internal and external complaints, conduct internal audits, carry out investigations by the Data Protection Officer in line with Article 45(2) of Regulation (EU) 2018/1725 and internal (IT) security investigations. |
|
(6) |
The BBI JU processes several categories of personal data, including hard data (‘objective’ data such as identification data, contact data, professional data, administrative details, data received from specific sources, electronic communications and traffic data) and soft data (‘subjective’ data related to the case such as reasoning, behavioural data, appraisals, performance and conduct data and data related to or brought forward in connection with the subject matter of the procedure or activity) (3). |
|
(7) |
The BBI JU, represented by its Executive Director, acts as the data controller irrespective of further delegations of the controller role within the BBI JU to reflect operational responsibilities for specific personal data processing operations. |
|
(8) |
The personal data are stored securely in an electronic environment or on paper preventing unlawful access or transfer of data to persons who do not have a need to know. The personal data processed are retained for no longer than necessary and appropriate for the purposes for which the data are processed for the period specified in the data protection notices, privacy statements or records of the BBI JU. |
|
(9) |
The internal rules should apply to all processing operations carried out by the BBI JU in the performance of administrative inquiries, disciplinary proceedings, preliminary activities related to cases of potential irregularities reported to OLAF, whistleblowing procedures, (formal and informal) procedures for cases of harassment, processing internal and external complaints, internal audits, the investigations carried out by the Data Protection Officer in line with Article 45(2) of Regulation (EU) 2018/1725, (IT) security investigations handled internally or with external involvement (e.g. CERT-EU). |
|
(10) |
They should apply to processing operations carried out prior to the opening of the procedures referred to above, during these procedures and during the monitoring of the follow-up to the outcome of these procedures. It should also include assistance and cooperation provided by the BBI JU to national authorities and international organisations outside of its administrative investigations. |
|
(11) |
In cases where these internal rules apply, the BBI JU must provide justifications explaining why the restrictions are strictly necessary and proportionate in a democratic society and respect the essence of the fundamental rights and freedoms. |
|
(12) |
Within this framework the BBI JU is bound to respect, to the maximum extent possible, the fundamental rights of the data subjects during the above procedures, in particular, those relating to the right of provision of information, access and rectification, right to erasure, restriction of processing, right of communication of a personal data breach to the data subject or confidentiality of communication as enshrined in Regulation (EU) 2018/1725. |
|
(13) |
However, the BBI JU may be obliged to restrict the information to data subject and other data subject’s rights to protect, in particular, its own investigations, the investigations and proceedings of other public authorities, as well as the rights of other persons related to its investigations or other procedures. |
|
(14) |
The BBI JU may thus restrict the information for the purposes of protecting the investigation, and the fundamental rights and freedoms of other data subjects. |
|
(15) |
The BBI JU should periodically monitor that the conditions justifying the restriction apply, and lift the restriction as far as they no longer apply. |
|
(16) |
The Controller should inform the Data Protection Officer at the moment of deferral and during the revisions, |
HAS ADOPTED THIS DECISION:
Article 1
Subject matter and scope
1. This Decision lays down rules relating to the conditions under which the BBI JU in the framework of its procedures set out paragraph 2 may restrict the application of the rights enshrined in Articles 14 to 21, 35 and 36, as well as Article 4 thereof, following Article 25 of Regulation (EU) 2018/1725.
2. Within the framework of the administrative functioning of the BBI JU, this Decision applies to the processing operations on personal data by the Programme Office for the purposes of conducting administrative inquiries, disciplinary proceedings, preliminary activities related to cases of potential irregularities reported to OLAF, processing whistleblowing cases, (formal and informal) procedures of harassment, processing internal and external complaints, conducting internal audits, investigations carried out by the Data Protection Officer in line with Article 45(2) of Regulation (EU) 2018/1725 and (IT) security investigations handled internally or with external involvement (e.g. CERT-EU).
3. The categories of data concerned are hard data (‘objective’ data such as identification data, contact data, professional data, administrative details, data received from specific sources, electronic communications and traffic data) and soft data (‘subjective’ data related to the case such as reasoning, behavioural data, appraisals, performance and conduct data and data related to or brought forward in connection with the subject matter of the procedure or activity).
4. Where the BBI JU performs its duties with respect to data subject’s rights under Regulation (EU) 2018/1725, it shall consider whether any of the exemptions laid down in that Regulation apply.
5. Subject to the conditions set out in this Decision, the restrictions may apply to the following rights: provision of information to data subjects, right of access, rectification, erasure, restriction of processing, communication of a personal data breach to the data subject or confidentiality of communication.
Article 2
Specification of the controller
The controller of the processing operations is the BBI JU, represented by its Executive Director, who may delegate the function of the controller. Data subjects shall be informed of the delegated controller by way of the data protection notices or records published on the website and/or the intranet of the BBI JU.
Article 3
Specification of safeguards
1. The BBI JU shall put in place the following safeguards aimed at preventing abuse or unlawful access or transfer of personal data: (4)
|
(a) |
Paper documents shall be kept in secured cupboards and only accessible to authorized staff; |
|
(b) |
All electronic data shall be stored in a secure IT application according to the BBI JU’s security standards, as well as in specific electronic folders accessible only to authorised staff. Appropriate levels of access shall be granted individually; |
|
(c) |
The database shall be password-protected under a single sign-on system and connected automatically to the user’s ID and password. Replacing users is strictly prohibited. E-records shall be held securely to safeguard the confidentiality and privacy of the data therein; |
|
(d) |
All persons having access to the data are bound by the obligation of confidentiality. |
2. The retention period of the personal data referred to in Article 1(3) shall be no longer than necessary and appropriate for the purposes for which the data are processed. It shall in any event not be longer than the retention period specified in the data protection notices, privacy statements or records referred to in Article 6.
3. Where the BBI JU considers to apply a restriction, the risk to the rights and freedoms of the data subject shall be weighed, in particular, against the risk to the rights and freedoms of other data subjects and the risk of cancelling the effect of the BBI JU’s investigations or procedures for example by destroying evidence. The risks to the rights and freedoms of the data subject concern primarily, but are not limited to, reputational risks and risks to the right of defence and the right to be heard.
Article 4
Restrictions
1. Any restriction shall only be applied by the BBI JU to safeguard:
|
(a) |
the national security, public security or defence of the Member States; |
|
(b) |
the prevention, investigation, detection and prosecution of criminal offences or the execution of criminal penalties, including the safeguarding against and the prevention of threats to public security; |
|
(c) |
other important objectives of general public interest of the Union or of a Member State, in particular the objectives of the common foreign and security policy of the Union or an important economic or financial interest of the Union or of a Member State, including monetary, budgetary and taxation matters, public health and social security; |
|
(d) |
the internal security of Union institutions and bodies, including of their electronic communications networks; |
|
(e) |
the prevention, investigation, detection and prosecution of breaches of ethics for regulated professions; |
|
(f) |
a monitoring, inspection or regulatory function connected, even occasionally, to the exercise of official authority in the cases referred to in points (a) to (c); |
|
(g) |
the protection of the data subject or the rights and freedoms of others; |
|
(h) |
the enforcement of civil law claims. |
2. As a specific application of the purposes described in paragraph 1 above, the BBI JU may apply restrictions in the following circumstances:
|
(a) |
in relation to personal data exchanged with Commission services or other Union institutions, bodies, agencies and offices;
|
|
(b) |
in relation to personal data exchanged with competent authorities of Member States;
|
|
(c) |
in relation to personal data exchanged with third countries or international organisations, where there is clear evidence that the exercise of those rights and obligations is likely to jeopardise the BBI JU’s cooperation with third countries or international organisations in the conduct of its tasks. |
Before applying restrictions in the circumstances referred to in points (a) and (b) of the first subparagraph, the BBI JU shall consult the relevant Commission services, Union institutions, bodies, agencies, offices or the competent authorities of Member States unless it is clear to the BBI JU that the application of a restriction is provided for by one of the acts referred to in those points.
Article 5
Restrictions to the rights of data subjects
1. In duly justified cases and under the conditions stipulated in this decision, the following rights may be restricted by the controller in the context of the processing operations listed in paragraph 2 below where necessary and proportionate:
|
(a) |
The right to information; |
|
(b) |
The right of access; |
|
(c) |
The right of rectification, erasure and restriction of processing; |
|
(d) |
The right to communication of a personal data breach to the data subject; |
|
(e) |
The right to confidentiality of electronic communications; |
2. In accordance with article 25(2)(a) of Regulation (EU) 2018/1725, in duly justified cases and under the conditions stipulated in this decision, restrictions may be applied by the controller in the context of the following processing operations:
|
(a) |
the performance of administrative inquiries and disciplinary proceedings; |
|
(b) |
preliminary activities related to cases of potential irregularities reported to OLAF; |
|
(c) |
whistleblowing procedures; |
|
(d) |
(formal and informal) procedures for cases of harassment; (7) |
|
(e) |
processing internal and external complaints; |
|
(f) |
internal audits; |
|
(g) |
the investigations carried out by the Data Protection Officer in line with Article 45(2) of Regulation (EU) 2018/1725; |
|
(h) |
(IT) security investigations handled internally or with external involvement (e.g. CERT-EU); |
|
(i) |
within the frame of the grant management or procurement procedure, after the closing date of the submission of the calls for proposals or the application of tenders (8). |
The restriction shall continue to apply as long as the reasons justifying it remain applicable.
3. Where the BBI JU restricts, wholly or partly, the application of the rights in paragraph 1 above, it shall take the steps set out in Articles 6 and 7 of this Decision.
4. Where data subjects request access to their personal data processed in the context of one or more specific cases or to a particular processing operation, in accordance with Article 17 of Regulation (EU) 2018/1725, the BBI JU shall limit its assessment of the request to such personal data only.
Article 6
Necessity and proportionality of restrictions
1. Any restriction outlined in Article 5 shall be necessary and proportionate taking into account the risks to the rights and freedoms of data subjects and respect the essence of the fundamental rights and freedoms in a democratic society.
2. If the application of restriction is considered, a necessity and proportionality test shall be carried out based on the present rules. The test shall also be conducted within the framework of the periodic review, following assessment of whether the factual and legal reasons for a restriction still apply. It shall be documented through an internal assessment note for accountability purposes on a case by case basis.
3. Restrictions shall be temporary and lifted as soon as the circumstances that justify them no longer apply. In particular, where it is considered that the exercise of the restricted right would no longer cancel the effect of the restriction imposed or adversely affect the rights or freedoms of other data subjects.
The BBI JU shall review the application of the restriction every six months from its adoption and at the closure of the relevant inquiry, procedure or investigation. Thereafter, the controller shall monitor the need to maintain any restriction every six months.
4. Where the BBI JU applies, wholly or partly, the restrictions outlined in Article 5 of this Decision, it shall record the reasons for the restriction, the legal ground in accordance with paragraph 1 above, including an assessment of the necessity and proportionality of the restriction.
The record and, where applicable, the documents containing underlying factual and legal elements shall be registered. They shall be made available to the European Data Protection Supervisor on request.
Article 7
Obligation to inform
1. The BBI JU shall include in the data protection notices, privacy statements or records in the sense of Article 31 of Regulation (EU) 2018/1725, published on its website and/or on the intranet informing data subjects of their rights in the framework of a given procedure, information relating to the potential restriction of these rights. The information shall cover which rights may be restricted, the reasons and the potential duration.
Without prejudice to the provisions of Article 6(4), the BBI JU, where proportionate, shall also inform individually all data subjects, which are considered persons concerned in the specific processing operation, of their rights concerning present or future restrictions without undue delay and in a written form.
2. Where the BBI JU restricts, wholly or partly, the rights laid out in Article 5, it shall inform the data subject concerned of the restriction applied and of the principal reasons thereof, and of the possibility of lodging a complaint with the European Data Protection Supervisor or of seeking a judicial remedy in the Court of justice of the European Union.
The provision of information referred to in paragraph 2 above may be deferred, omitted or denied if it would cancel the effect of the restriction in accordance with Article 25(8) of Regulation (EU) 2018/1725.
Article 8
Review by the Data Protection Officer
1. The BBI JU shall, without undue delay, inform the Data Protection Officer of the BBI JU (‘the DPO’) whenever the controller restricts the application of data subjects’ rights, or extends the restriction, in accordance with this Decision. The controller shall provide the DPO access to the record containing the assessment of the necessity and proportionality of the restriction and document the date of informing the DPO in the record.
2. The DPO may request the controller in writing to review the application of the restrictions. The controller shall inform the DPO in writing about the outcome of the requested review.
3. The DPO shall be involved throughout the procedure. The controller shall inform the DPO when the restriction has been lifted.
Article 9
Entry into force
This Decision shall enter into force on the twentieth day following its publication in the Official Journal of the European Union.
Done at Brussels, 26 March 2020.
For the Governing Board of the BBI JU
Mat QUAEDVLIEG
The Chairperson
(1) OJ L 295, 21.11.2018, p. 39.
(2) OJ L 169, 7.6.2014, p. 130.
(3) In cases of joint controllership data shall be processed in line with the means and purposes established in the relevant agreement among the joint controllers as defined in Article 28 of Regulation (EU) 2018/1725.
(4) This list is non-exhaustive.
(5) Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) (OJ L 119, 4.5.2016, p. 1).
(6) Directive (EU) 2016/680 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data by competent authorities for the purposes of the prevention, investigation, detection or prosecution of criminal offences or the execution of criminal penalties, and on the free movement of such data, and repealing Council Framework Decision 2008/977/JHA (OJ L 119, 4.5.2016, p. 89).
(7) This processing operation shall not apply to Article 5(1)(d).
(8) This processing operating shall only apply to Article 5(1)(c).