ISSN 1977-0677
Official Journal
of the European Union
L 234

English edition
Legislation
Volume 62
11 September 2019
|
ISSN 1977-0677 |
||
|
Official Journal of the European Union |
L 234 |
|

|
||
|
English edition |
Legislation |
Volume 62 |
|
|
|
Corrigenda |
|
|
|
* |
||
|
|
* |
|
|
|
|
|
(1) Text with EEA relevance. |
|
EN |
Acts whose titles are printed in light type are those relating to day-to-day management of agricultural matters, and are generally valid for a limited period. The titles of all other Acts are printed in bold type and preceded by an asterisk. |
II Non-legislative acts
REGULATIONS
|
11.9.2019 |
EN |
Official Journal of the European Union |
L 234/1 |
COMMISSION IMPLEMENTING REGULATION (EU) 2019/1394
of 10 September 2019
amending and correcting Implementing Regulation (EU) 2015/2447 as regards certain rules on surveillance for release for free circulation and exit from the customs territory of the Union
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) No 952/2013 of the European Parliament and of the Council of 9 October 2013 laying down the Union Customs Code (1), and in particular Articles 8, 58, 100, 132, 157, 161, 184, 193, 217, 232, 268 thereof,
Whereas:
|
(1) |
Council Regulation (EU) No 904/2010 (2) requires Member States to collect and exchange certain information on importations that are exempt from value added tax (VAT) pursuant to Article 143(1)(ca) (special scheme for distance sales) or Article 143(1)(d) and 143(2) of Council Directive 2006/112/EC (3). In addition, pursuant to Article 47(2) of the Code, customs and other competent authorities may, where necessary for the purposes of minimising risk and combating fraud, exchange with each other and with the Commission data received in the context of the entry, exit, transit, movement, storage and end-use of goods. |
|
(2) |
The electronic system that the Commission has put in place to comply with the surveillance obligation in Article 56(5) of the Code, Surveillance, is the most appropriate tool to be used for exchanging that VAT-related information. It is necessary to amend Article 55 of Implementing Regulation (EU) 2015/2447 to clarify who and to what extent may have access to the data stored in the Surveillance system. First, the Commission should be able to disclose the data in Surveillance in an aggregated form. Second, as a general rule, authorised users in the Member States' customs authorities should have access only to the non-aggregated data that that Member State has provided and to data aggregated at Union level. Third, by derogation from the general rule, Article 55 should foresee the possibility that specific Union acts, such as Regulation (EU) No 904/2010, provides that the Commission grants to certain authorities of Member States access to non-aggregated data in a specific manner. |
|
(3) |
In order to be able to collect the information that Regulation (EU) No 904/2010 requires Member States to collect and exchange, Implementing Regulation (EU) 2015/2447 should also be amended to increase the number of data elements that the electronic system collects. In particular, it is necessary that Annexes 21-01 and 21-02 to that Regulation include the data elements that, in Annex B to that Regulation, have the order number 3/40 and 4/4, which concern the additional fiscal references identification numbers and the tax base respectively. |
|
(4) |
Following the amendment to Article 278 of the Code to prolong the deadline for the transitional use of means other than the electronic data-processing techniques provided for in the Code (4), the provision in Implementing Regulation (EU) 2015/2447 establishing a transitional list of data for the purpose of surveillance (Annex 21-02) should be amended. The provision should clarify that the transitional list of data can be used for the purposes of surveillance at release for free circulation until the national import systems are operational, that is, pursuant to Article 278(2) of the Code, until end of 2022 at the latest. By contrast, the transitional list can be used for the purposes of surveillance at export until the national export systems are operational, that is, pursuant to Article 278(3) of the Code, until end of 2025 at the latest. |
|
(5) |
Until the upgrade of the Import Control System referred to in the Annex to Commission Implementing Decision (EU) 2016/578 (5), the risk analysis of goods for which the obligation to lodge an entry summary declaration is waived must be made at the moment when those goods are presented to customs on the basis of the temporary storage declaration or the customs declaration or, where the customs declaration is made by any other act, the information available at the moment of the presentation. Article 187 of Implementing Regulation (EU) 2015/2447 should be amended so that it applies also to postal consignments and to consignments of an intrinsic value below EUR 22, by including the pertinent references to Commission Delegated Regulation (EU) 2015/2446 (6). |
|
(6) |
Economic operators should be given flexibility to provide, by forms or documents other than on a printout of a fishing logbook, the certification that sea-fishing products and goods transhipped and transported through a country or territory, which is not part of the customs territory of the Union, have not been manipulated. Nevertheless, in order to enable the allocation of the sea-fishing products and goods to the respective fishing logbook in cases where the certification of non-manipulation is provided by means of a form or document other than the printout of the fishing logbook, economic operators should include in that other form or document a reference to the respective fishing logbook. Article 214 of Implementing Regulation (EU) 2015/2447 should be amended accordingly. |
|
(7) |
In the context of the simplification whereby a customs declaration is lodged in the form of an entry into the declarant's records, the customs authorities may waive the obligation to present the goods. In order to allow for a proper customs control in specific situations, procedural rules should be laid down for cases where, due to a new serious financial risk or other specific situation, the supervising customs office requests that specific goods be presented to customs pursuant to the third subparagraph of Article 182(3) of the Code. Article 234 of Implementing Regulation (EU) 2015/2447 should be amended accordingly. |
|
(8) |
Article 302 of Implementing Regulation (EU) 2015/2447 provides for a waiver from sealing of the means of transport or the individual packages containing the goods for goods transported by air or by rail, provided certain conditions are fulfilled. Maritime transport is as secure as transport by air or by rail when it comes to ensuring that goods are delivered to the place of destination. Therefore, that waiver should be extended to goods transported by sea provided that a reference to the accompanying bill of lading is included in the electronic transport document used as customs declaration to place goods under the Union transit procedure. |
|
(9) |
Where the customs authority of a Member State involved in a transit operation obtains evidence that the events giving rise to the customs debt occurred in its territory, that authority should request the Member State of departure to transfer to it the responsibility to start the recovery. The Member State of departure should confirm within a certain period whether it transfers the competency to start the recovery to the requesting customs authority. Article 311 of Implementing Regulation (EU) 2015/2447 should therefore be amended to cover the specific case of a transit operation. |
|
(10) |
Article 324 of Implementing Regulation (EU) 2015/2447, concerning special cases of discharge of the inward processing procedure, and the corresponding codes in Annexes A and B should be amended to reflect the entry into force of Council Regulation (EU) 2018/581 (7). |
|
(11) |
When goods are taken out of the customs territory of the Union, the determination of the customs office of exit for goods loaded onto a vessel or onto an aircraft should be clarified. In addition, certain simplified arrangements for the determination of the customs office of exit should not apply to excise goods and non-Union goods. Article 329 of Implementing Regulation (EU) 2015/2447 should be amended accordingly. |
|
(12) |
Where, after having been released for export, goods are taken over under a single contract for transport of these goods out of the customs territory of the Union, the rules for ensuring customs supervision until the physical exit of these goods should be clarified. Article 332 of Implementing Regulation (EU) 2015/2447 should be amended accordingly. |
|
(13) |
The procedural rules laid down in Article 333 of Implementing Regulation (EU) 2015/2447 regarding the supervision of goods released for exit should be clarified to address situations whereby goods leave the customs territory of the Union in a different manner than initially foreseen, as well as to cover the exchange of information between customs authorities during the period until the deployment of the UCC Automated Export System referred to in the Annex to Implementing Decision (EU) 2016/578. |
|
(14) |
The procedural rules laid down in Articles 340 of Implementing Regulation (EU) 2015/2447 regarding the exit of goods should be clarified to address situations whereby goods are declared for export but eventually do not leave the customs territory of the Union. |
|
(15) |
Following the notification by North Macedonia to the United Nations and to the European Union of the entry into force of the Prespa Agreement as of 15 February 2019, the country previously denominated as ‘the former Yugoslav Republic of Macedonia’ has changed its name to ‘the Republic of North Macedonia’. That country should be referred to in the Annexes to Implementing Regulation (EU) 2015/2447 by that name, or, where appropriate, by the short form, ‘North Macedonia’. |
|
(16) |
In order to facilitate the use of the formats and codes of certain data requirements in the context of declarations and notifications in the various electronic systems, Annex B should be amended. |
|
(17) |
It is necessary to correct an editorial error in Annex 33-07 to Implementing Regulation (EU) 2015/2447 as regards a reference to Delegated Regulation (EU) 2015/2446. |
|
(18) |
Implementing Regulation (EU) 2015/2447 should therefore be amended and corrected accordingly. |
|
(19) |
The amendments to Annexes 21-01 and 21-02 to Implementing Regulation (EU) 2015/2447 laid down in this Regulation should apply from 1 January 2020 because that is the date from which Member States are to implement the information-exchange obligations imposed by Regulation (EU) No 904/2010. |
|
(20) |
The measures provided for in this Regulation are in accordance with the opinion of the Customs Code Committee, |
HAS ADOPTED THIS REGULATION:
Article 1
Amendments to Implementing Regulation (EU) 2015/2447
Implementing Regulation (EU) 2015/2447 is amended as follows:
|
(1) |
Article 55 is amended as follows:
|
|
(2) |
In Article 187, paragraph 5 is replaced by the following: ‘5. Where goods for which the obligation to lodge an entry summary declaration is waived in accordance with Article 104(1)(c) to (k), (m) and (n), (2), (3) and (4) of Commission Delegated Regulation (EU) 2015/2446 are brought into the customs territory of the Union, the risk analysis shall be carried out upon presentation of the goods where available on the basis of the temporary storage declaration or the customs declaration covering those goods.’. |
|
(3) |
In Article 214, the following paragraph is added: ‘3. The certification required in accordance with paragraph 1 may be provided by any relevant forms or documents other than on a printout of a fishing logbook including a reference to that fishing logbook.’. |
|
(4) |
In Article 234, the following paragraph is added: ‘3. Where the supervising customs office has requested, in accordance with the third sub-paragraph of Article 182(3) of the Code, that goods be presented to customs because the customs authorities have identified a new serious financial risk or another specific situation in relation to an authorisation to lodge a customs declaration in the form of an entry in the declarant's records with waiver of the obligation to present the goods, the supervising customs office shall indicate to the holder of such authorisation:
In these situations, the release of the goods shall take place in accordance with Article 194 of the Code.’. |
|
(5) |
In Article 302, paragraph 2, the following point is added:
|
|
(6) |
In Article 311, the following paragraphs are added: ‘3. Where the customs authority of a Member State involved in a transit operation obtains evidence, before the time-limit referred to in Article 77(a) of Delegated Regulation (EU) 2015/2446 expires, that the place where the events from which the customs debt arises occurred is in its territory, that authority shall immediately and in any event within that time-limit send a duly justified request to the customs authority of the Member State of departure to transfer the responsibility to start the recovery to the requesting customs authority. 4. The customs authority of the Member State of departure shall acknowledge the receipt of the request made in accordance with paragraph 3 and shall inform the requesting customs authority, within 28 days from the date on which the request was sent, whether it agrees to satisfy the request and to transfer to the requesting authority the responsibility to start the recovery.’. |
|
(7) |
In Article 324(1), point (e) is replaced by the following:
(*1) Council Regulation (EU) 2018/581 of 16 April 2018 temporarily suspending the autonomous Common Customs Tariff duties on certain goods of a kind to be incorporated in or used for aircraft, and repealing Regulation (EC) No 1147/2002 (OJ L 98, 18.4.2018, p.1).’." |
|
(8) |
Article 329 is amended as follows:
|
|
(9) |
In Article 332, paragraph 5, the second subparagraph is replaced by the following: ‘The obligation laid down in the first subparagraph shall not apply insofar as that information is available to the customs authorities through existing commercial, port or transport information systems, or in the situation covered by Article 329(7).’. |
|
(10) |
Article 333 is amended as follows:
|
|
(11) |
Article 340 is amended as follows:
|
|
(12) |
Annex A is amended as set out in Annex I to this Regulation. |
|
(13) |
Annex B is amended as set out in Annex II to this Regulation. |
|
(14) |
In Annex 21-01, after the row for D.E order No 3/39, the following row is inserted:
|
|
(15) |
In Annex 21-02, after the row for D.E order No 1/10, the following rows are inserted:
|
|
(16) |
In Annex 23-01, in the table, in the first column, the row Zone P is amended as follows:
|
|
(17) |
In Annex 32-01, point 1, the words ‘the former Yugoslav Republic of Macedonia’, are replaced by the words ‘the Republic of North Macedonia’. |
|
(18) |
In Annex 32-02, point 1, the words ‘the former Yugoslav Republic of Macedonia’, are replaced by the words ‘the Republic of North Macedonia’. |
|
(19) |
In Annex 32-03, point 1, the words ‘the former Yugoslav Republic of Macedonia’, are replaced by the words ‘the Republic of North Macedonia’. |
|
(20) |
In Annex 72-04, Part II is amended as follows:
|
Article 2
Corrections to Implementing Regulation (EU) 2015/2447
In Annex 33-07 to Implementing Regulation (EU) 2015/2447, in box 2, the words ‘[Delegated Regulation (EU) 2015/…]’ are replaced by the words ‘[Delegated Regulation (EU) 2015/2446]’.
Article 3
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
Points 14 and 15 of Article 1 shall apply from 1 January 2020.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 10 September 2019.
For the Commission
The President
Jean-Claude JUNCKER
(1) OJ L 269, 10.10.2013, p.1.
(2) Council Regulation (EU) No 904/2010 of 7 October 2010 on administrative cooperation and combating fraud in the field of value added tax (OJ L 268, 12.10.2010, p. 1).
(3) Council Directive 2006/112/EC of 28 November 2006 on the common system of value added tax (OJ L 347, 11.12.2006, p. 1).
(4) Regulation (EU) 2019/632 of the European Parliament and of the Council of 17 April 2019 amending Regulation (EU) No 952/2013 to prolong the transitional use of means other than the electronic data-processing techniques provided for in the Union Customs Code (OJ L 111, 25.4.2019, p. 54).
(5) Commission Implementing Decision (EU) 2016/578 of 11 April 2016 establishing the Work Programme relating to the development and deployment of the electronic systems provided for in the Union Customs Code (OJ L 99, 15.4.2016, p. 6).
(6) Commission Delegated Regulation (EU) 2015/2446 of 28 July 2015 supplementing Regulation (EU) No 952/2013 of the European Parliament and of the Council as regards detailed rules concerning certain provisions of the Union Customs Code (OJ L 343, 29.12.2015, p. 1).
(7) Council Regulation (EU) 2018/581 of 16 April 2018 temporarily suspending the autonomous Common Customs Tariff duties on certain goods of a kind to be incorporated in or used for aircraft, and repealing Regulation (EC) No 1147/2002 (OJ L 98, 18.4.2018, p. 1).
(*2) When Union code entered for (Calculation of taxes – Tax Type) is B00.’.
ANNEX I
Annex A to Implementing Regulation (EU) 2015/2447 is amended as follows:
|
(1) |
in Title I, the table ‘Formats of the common data requirements for applications and decisions’ is amended as follows:
|
|
(2) |
in Title II, in heading ‘CODES’, in subheading ‘6/2. Economic conditions’, in row for Code 14, the text is replaced by the following: ‘the processing into products to be incorporated in or used for aircraft for which an authorised release certificate EASA Form 1 or an equivalent certificate has been issued,’. |
ANNEX II
Annex B to Implementing Regulation (EU) 2015/2447 is amended as follows:
|
(1) |
In Title I, the table ‘Formats and cardinality of the common data requirements for declarations and notifications’ is amended as follows:
|
|
(2) |
In Title II, Section ‘2. CODES’ is amended as follows,
|
|
11.9.2019 |
EN |
Official Journal of the European Union |
L 234/14 |
COMMISSION IMPLEMENTING REGULATION (EU) 2019/1395
of 10 September 2019
amending Annex I to Regulation (EC) No 798/2008 as regards the entries for Bosnia and Herzegovina and Israel and the name of the Republic of North Macedonia in the list of third countries, territories, zones or compartments from which certain poultry commodities may be imported into and transit through the Union and amending the model veterinary certificate for egg products
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Council Directive 2002/99/EC of 16 December 2002 laying down the animal health rules governing the production, processing, distribution and introduction of products of animal origin for human consumption (1), and in particular the introductory phrase of Article 8, the first subparagraph of point 1 of Article 8, point 4 of Article 8 and Article 9(4) thereof,
Having regard to Council Directive 2009/158/EC of 30 November 2009 on animal health conditions governing intra-Community trade in, and imports from third countries of, poultry and hatching eggs (2), and in particular Articles 23(1), 24(2), 25(2) and 26(2) thereof,
Whereas:
|
(1) |
Commission Regulation (EC) No 798/2008 (3) lays down veterinary certification requirements for imports into and transit, including storage during transit, through the Union of poultry and poultry products (the commodities). It provides that the commodities are only to be imported into and transit through the Union from the third countries, territories, zones or compartments listed in columns 1 and 3 of the table in Part 1 of Annex I thereto. |
|
(2) |
Regulation (EC) No 798/2008 also lays down the conditions for a third country, territory, zone or compartment to be considered as free from highly pathogenic avian influenza (HPAI). |
|
(3) |
Bosnia and Herzegovina is listed in Part 1 of Annex I to Regulation (EC) No 798/2008 as a third country from which imports into and transit through the Union of meat of poultry is authorised from the whole of its territory. |
|
(4) |
Bosnia and Herzegovina has requested to be authorised also for imports into and transit through the Union of eggs and egg products. Based on the information gathered during the Commission audit in Bosnia and Herzegovina to evaluate the animal health controls in place for meat of poultry intended for export to the Union and given the favourable outcome of that audit, the Commission has concluded that Bosnia and Herzegovina satisfies the animal health requirements laid down in Regulation (EC) No 798/2008 for the import into and transit through the Union of eggs and egg products. It is therefore appropriate to amend the entry for Bosnia and Herzegovina in the table in Part 1 of Annex I to Regulation (EC) No 798/2008 to authorise that third country for imports into and transit through the Union of eggs and egg products. |
|
(5) |
In addition, Bosnia and Herzegovina has submitted to the Commission its national control programme for Salmonella in laying hens of Gallus gallus. However, that programme was not found to provide guarantees equivalent to the guarantees set out in Regulation (EC) No 2160/2003 of the European Parliament and of the Council (4) and the approval of that programme has not been finalised. Therefore, only imports of the type of eggs of Gallus gallus indicated under ‘S4’ in Part 2 of Annex I to Regulation (EC) No 798/2008 are permitted from Bosnia and Herzegovina. |
|
(6) |
Israel is listed in Part 1 of Annex I to Regulation (EC) No 798/2008 as a third country from which imports into and transit through the Union of certain poultry commodities are authorised from certain parts of its territory depending on the implementation of the stamping-out policy for Newcastle disease. That regionalisation was set out in Part 1 of Annex I to Regulation (EC) No 798/2008. |
|
(7) |
On 24 April 2019, Israel confirmed the presence of HPAI of subtype H5N8 in a poultry holding on its territory. Due to that confipormed outbreak of HPAI since April 2019 the territory of Israel can no longer be considered as free from that disease and the veterinary authorities of Israel can therefore no longer certify consignments of meat of poultry for human consumption for import into or transit through the Union. |
|
(8) |
The veterinary authorities of Israel have submitted preliminary information to the Commission on the outbreak of HPAI and have confirmed that since the date of confirmation of the outbreak of HPAI they have suspended issuing veterinary certificates for consignments of meat of poultry intended for import into, or transit through, the Union. |
|
(9) |
Therefore, no consignments of such products originating in Israel have been introduced into the Union since that date. For reasons of clarity and legal certainty, it is appropriate to document this situation and introduce in the table in Part 1 of Annex I to Regulation (EC) No 798/2008 the relevant closing date. This also ensures that, where Israel regains freedom from HPAI and an opening date is established, consignments of such products produced after the closing date and before that opening date will not be eligible for introduction into the Union. |
|
(10) |
The entry for Israel in the table in Part 1 of Annex I to Regulation (EC) No 798/2008 should therefore be amended to take account of the current epidemiological situation in that third country. |
|
(11) |
Following United nations (UN) facilitation, Athens and Skopje reached a bilateral agreement (‘Prespa agreement’) in June 2018, to change the UN provisional reference for the former Yugoslav Republic of Macedonia. This agreement has now been ratified by both countries and the Republic of North Macedonia has formally notified the EU about its entry into force. It is therefore appropriate to amend the name of that third country in the table in Part 1 of Annex I to Regulation (EC) No 798/2008. |
|
(12) |
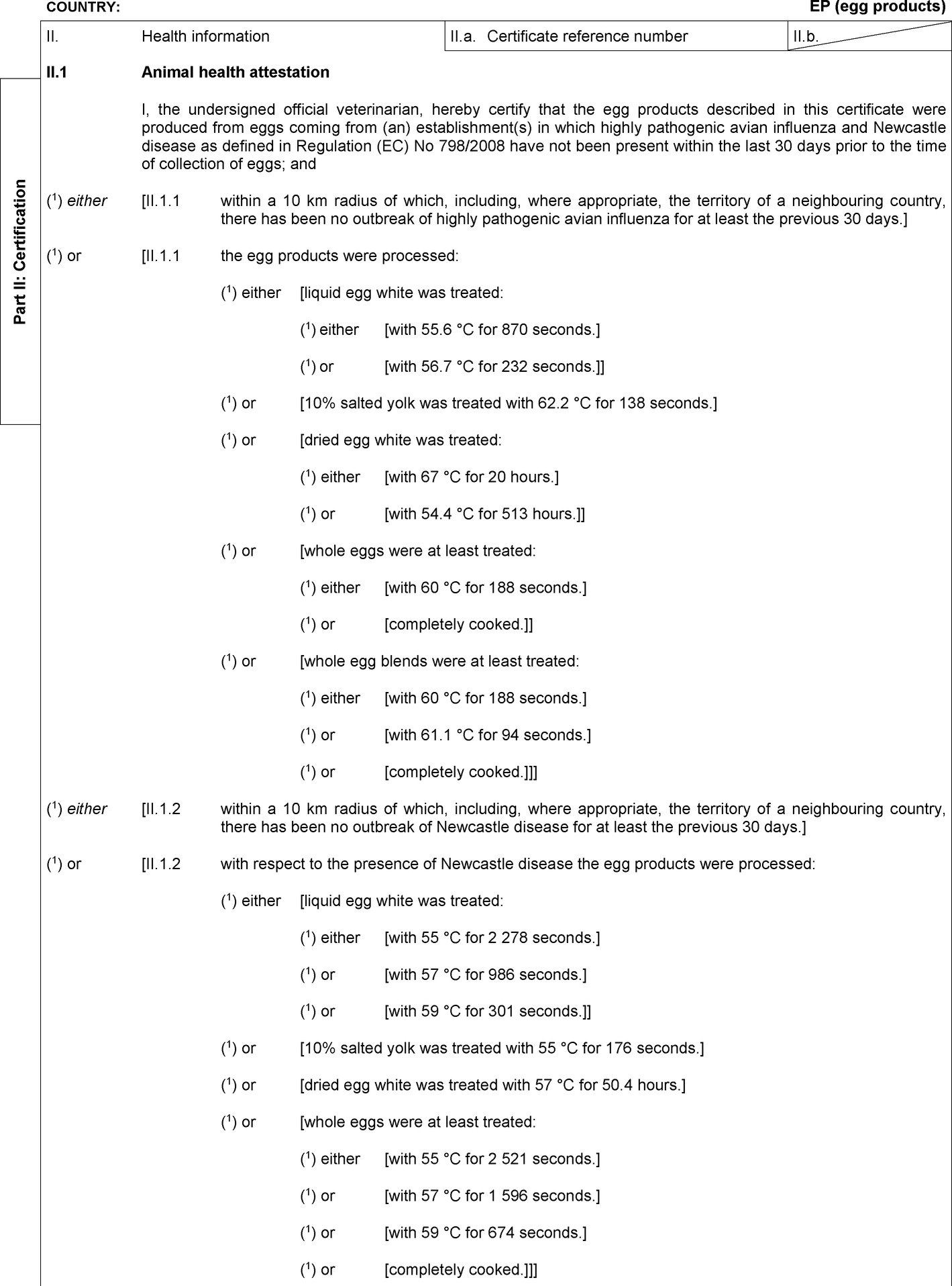
In Part 2 of Annex I to Regulation (EC) No 798/2008, a model veterinary certificate is set out for egg products (EP). In that model veterinary certificate, Part I of the Notes refers to the Harmonised System (HS) codes that are to be indicated in Box I.19. of Part I of that certificate. |
|
(13) |
Enzymes deriving from eggs, such as lysozyme, are considered egg products and the relevant HS codes for those enzymes should be added to the HS codes to be indicated in Box I.19. of Part I of the model veterinary certificate for egg products. It is appropriate, therefore, to amend the model veterinary certificate for egg products (EP) accordingly. |
|
(14) |
Annex I to Regulation (EC) No 798/2008 should therefore be amended accordingly. |
|
(15) |
A reasonable transitional period of two months should be allowed to elapse before the amended model veterinary certificate becomes mandatory in order to allow Member States and the industry to adapt to the new requirements set out in the amended model veterinary certificate. |
|
(16) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on Plants, Animals, Food and Feed, |
HAS ADOPTED THIS REGULATION:
Article 1
Annex I to Regulation (EC) No 798/2008 is amended in accordance with the Annex to this Regulation.
Article 2
For a transitional period until 11 November 2019, Member States shall continue to authorise the introduction into the Union of consignments of the commodities covered by the model veterinary certificate for egg products (EP), as set out in Part 2 of Annex I to Regulation (EC) No 798/2008, in its version before the amendment made to that model by this Regulation, provided that it is signed before 11 October 2019.
Article 3
This Regulation shall enter into force on the third day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 10 September 2019.
For the Commission
The President
Jean-Claude JUNCKER
(1) OJ L 18, 23.1.2003, p. 11.
(2) OJ L 343, 22.12.2009, p. 74.
(3) Commission Regulation (EC) No 798/2008 of 8 August 2008 laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements (OJ L 226, 23.8.2008, p. 1).
(4) Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of salmonella and other specified food-borne zoonotic agents (OJ L 325, 12.12.2003, p. 1).
ANNEX
Annex I to Regulation (EC) No 798/2008 is amended as follows:
|
(1) |
Part 1 is amended as follows:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
(2) |
In Part 2, the model veterinary certificate for egg products (EP) is replaced by the following: ‘Model veterinary certificate for egg products (EP)  Text of image
Text of image
 Text of image
Text of image
 Text of image
Text of image
 Text of image
’
Text of image
’ |
DECISIONS
|
11.9.2019 |
EN |
Official Journal of the European Union |
L 234/23 |
COMMISSION IMPLEMENTING DECISION (EU) 2019/1396
of 10 September 2019
laying down the rules for the application of Regulation (EU) 2017/745 of the European Parliament and of the Council as regards the designation of expert panels in the field of medical devices
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC (1), and in particular Article 106(1) thereof
Whereas:
|
(1) |
Expert panels are to be designated in order to provide scientific, technical and clinical assistance to the Commission, the Medical Device Coordination Group (MDCG), Member States, notified bodies and manufacturers in relation to the implementation of Regulation (EU) 2017/745 and in order to provide views in accordance with Article 48(6) of Regulation (EU) 2017/746 of the European Parliament and of the Council (2). |
|
(2) |
In particular, notified bodies are required to carry out consultations of expert panels on clinical evaluations of certain high-risk medical devices in the context of Regulation (EU) 2017/745 and on performance evaluations of certain high-risk in vitro diagnostic medical devices in the context of Regulation (EU) 2017/746. |
|
(3) |
The Commission has, in consultation with the MDCG, identified areas in which the provision of consistent scientific, technical and/or clinical advice is needed. Expert panels should be designated in those areas and the principles of their organisation and operation should be defined, including the procedures for the selection and appointment of their members, as to ensure that they work according to the highest scientific competence, impartiality, independence and transparency. The list of designated expert panels can be revised, based on experience or newly identified needs. |
|
(4) |
The advisors in the expert panels should be appointed on the basis of objective criteria and following a public call for expression of interest. The selection criteria included in the call for expression of interest should ensure that highly qualified advisors with a sufficient level of up-to-date clinical, scientific or technical expertise in the relevant identified areas are selected, and that advisors are able to act independently and in the public interest. The selection criteria should also ensure that the collective expertise of all advisors selected adequately covers all the identified areas and that the geographical origin of the advisors reflects the diversity of scientific and clinical approaches in the Union. |
|
(5) |
The number of advisors to be appointed to each expert panel or included in the central list of available experts should be specified in the call for expression of interest, based on expected workload and necessary expertise. |
|
(6) |
The organisation of the expert panels should ensure flexibility so that specialised knowledge can be deployed based on requisite needs. In addition to advisors appointed to expert panels, a central list of advisors who are not members of expert panels should therefore be established. Advisors on that list should be available to support the work of the expert panels as needed. |
|
(7) |
In order to ensure timely and efficient exercise of their tasks, the expert panels should be able to establish sub-groups entrusted with specific tasks and composed of a certain number of their members. |
|
(8) |
In order to facilitate the organisation of the expert panels and the communication between them, a coordination committee composed of the Chairs and Vice-Chairs of the panels should be established. In order to ensure the support necessary for the efficient functioning of the expert panels, the Commission should provide a secretariat for the expert panels and for the coordination committee. |
|
(9) |
Expert panels should operate in a transparent and harmonised manner. To this end, common rules of procedure, internal guidance and methodologies for their operation should be established by the coordination committee and be publicly accessible. The common rules of procedure, the internal guidance and the methodologies should be reviewed regularly to ensure that they take account of the latest scientific developments and reflect state-of-the-art practice. |
|
(10) |
Any personal data treated by the expert panels, the Secretariat or the coordination committee is to be processed in accordance with Regulation (EU) 2018/1725 of the European Parliament and of the Council (3). |
|
(11) |
Advisors should comply with the rules on security regarding the protection of Union classified information and sensitive non-classified information, laid down in Commission Decisions (EU, Euratom) 2015/443 (4) and (EU, Euratom) 2015/444 (5). |
|
(12) |
Given the contribution of expert panels to achieve the objectives of Union policies, by providing scientific, technical and clinical assistance to the Commission, the MDCG, manufacturers and notified bodies in relation to the implementation of Regulation (EU) 2017/745 and of Regulation (EU) 2017/746, and taking into account the principle of cost-effectiveness, advisors should receive adequate remuneration for their activities, beyond reimbursement of expenses. The level of remuneration should reflect the extent of work required from the advisors, notably in relation to the duration and nature of their tasks. |
|
(13) |
The financing of the expert panel activities should be provided by the relevant budget line of the Commission, |
HAS ADOPTED THIS DECISION:
Article 1
Designation of expert panels
1. One expert panel is designated in each of the following areas to fulfil the tasks set out in paragraphs 9 and 10 of Article 106 of Regulation (EU) 2017/745 and in paragraph 6 of Article 48 of Regulation (EU) 2017/746:
|
(1) |
Orthopaedics, traumatology, rehabilitation, rheumatology; |
|
(2) |
Circulatory system; |
|
(3) |
Neurology; |
|
(4) |
Respiratory system, anaesthesiology, intensive care; |
|
(5) |
Endocrinology and diabetes; |
|
(6) |
General and plastic surgery and dentistry; |
|
(7) |
Obstetrics and gynaecology, including reproductive medicine; |
|
(8) |
Gastroenterology and hepatology; |
|
(9) |
Nephrology and urology; |
|
(10) |
Ophthalmology; |
|
(11) |
In-vitro diagnostic medical devices (IVD). |
2. An additional expert panel is designated to be in charge of the decision referred to in point (c) of Section 5.1 of Annex IX to Regulation (EU) 2017/745.
Article 2
Appointment of advisors and establishment of the central list
1. For the purpose of Article 106(5) of Regulation (EU) 2017/745, advisors shall be appointed to the expert panels following a call for expression of interest and consultation with the Medical Device Coordination Group (‘MDCG’), based on selection criteria stipulated in that call for expression of interest.
2. The number of members of each expert panel shall be determined in the call for expression of interest referred to in paragraph 1.
3. For the purpose of Article 106(6) of Regulation (EU) 2017/745 and following consultation with the MDCG, advisors who satisfy the criteria stipulated in the call but who are not appointed to an expert panel shall be included in a central list of available experts (the ‘central list’).
4. Advisors shall be selected with regard to the need to ensure:
|
(a) |
adequate and up-to-date clinical, scientific or technical expertise in the areas referred to in Article 1(1); |
|
(b) |
independence, impartiality, objectivity and absence of conflict of interest as outlined in Article 107 of Regulation (EU) 2017/745; |
|
(c) |
balanced geographical representation. |
5. Where it is necessary due to the workload of a certain expert panel or the need to provide the required expertise to a certain expert panel, additional advisors may be appointed to that expert panel from the central list.
6. Where it is necessary due to the workload of a certain expert panel or the need to provide the required expertise to a certain expert panel, advisors on the central list or in another expert panel may be assigned to that expert panel for specific tasks and for a limited period of time.
7. The central list may be updated by launching subsequent calls for expression of interest.
Article 3
Sub-groups
1. An expert panel may, in agreement with the Commission, establish permanent or ad-hoc sub-groups entrusted with specific tasks and composed of a certain number of its members.
2. Sub-groups shall operate in accordance with the common rules of procedure for the expert panels, referred to in Article 9(1).
Article 4
Term of office
1. Advisors shall be appointed as members of an expert panel for a term of three years, with the possibility of renewal.
2. Where an advisor no longer complies with the conditions set out in Articles 12 and 15 or in Article 339 of the Treaty on the Functioning of the European Union, resigns or is no longer capable of contributing effectively to the expert panel's work, the Commission may dismiss that advisor.
3. Where an advisor is dismissed during his or her term of office, a replacement for that advisor shall be appointed for the remainder of the term from the central list.
Article 5
Election of the Chair and Vice-Chair
1. At the beginning of each term of office referred to in Article 4, each panel and its sub-groups shall, acting by simple majority, elect a Chair and a Vice-Chair from among its members.
2. The term of office of the Chair and Vice-Chair shall be three years, and shall be renewable. Any replacement of the Chair or Vice-Chair during that term of office shall take place according to the procedure referred to in paragraph 1 and shall be valid for the remainder of the term.
3. As regards sub-groups, the term of office of the Chair and Vice-Chair shall run from the moment of their election until the expiration of the mandate of the sub-group.
Article 6
Voting rules
When adopting scientific opinions or views, as applicable, in the context of Articles 54(1) and 61(2) of Regulation (EU) 2017/745 and Article 48(6) of Regulation (EU) 2017/746, the expert panel shall make decisions in accordance with Article 106(12) of Regulation (EU) 2017/745.
Article 7
Coordination committee
1. A coordination committee (the ‘Committee’) composed of the Chairs and Vice-Chairs of all expert panels shall be established following the election referred to in Article 5.
2. The Committee shall, inter alia:
|
— |
ensure effective exchange of information between expert panels; |
|
— |
adopt and review the common rules of procedure for the expert panels in accordance with Article 9; |
|
— |
adopt and review internal guidance and methodologies to be used by the expert panels. |
3. The Committee shall operate in accordance with the common rules of procedure referred to in Article 9(1).
Article 8
Preparation of opinions, views or positions
1. For each opinion, view or position under preparation the Chair of the expert panel or the sub-group may appoint a rapporteur and co-rapporteur. In that context, all other members shall be reviewing members.
2. The expert panels shall follow the common rules of procedure referred to in Article 9 and any relevant guidance adopted by the Committee as referred to in Article 7(2) third indent.
3. In the context of the activities of expert panels referred to in Article 54(1) of Regulation (EU) 2017/745, the expert panels shall use the guidance to be provided by the Commission as set out in point (h) of Section 5.1 of Annex IX to Regulation (EU) 2017/745.
Article 9
Common rules of procedure
1. On a proposal by and in agreement with the Commission services, the Committee shall adopt common rules of procedure for all expert panels by simple majority of its members.
The Chairs shall consult their respective expert panels on the content of the common rules of procedure prior to adoption.
2. The common rules of procedure for the expert panels shall provide for, inter alia:
|
(a) |
procedures for carrying out the tasks of the expert panels as referred to in paragraphs 9 and 10 of Article 106 of Regulation (EU) 2017/745; |
|
(b) |
rules ensuring the application of the principles laid down in Articles 12 to 15. |
3. The Committee shall, in agreement with the Commission services, review the common rules of procedure at least every 3 years and update them to ensure that they take account of the latest scientific developments and that they reflect state-of-the-art practice.
4. The common rules of procedure shall be publicly available on a dedicated Commission website.
Article 10
Secretariat
1. The Commission shall provide a secretariat (the ‘Secretariat’) for the expert panels and for the Committee.
2. The Secretariat shall be responsible for the support necessary for the efficient functioning of the expert panels. The Secretariat shall, in particular:
|
— |
identify and manage potential conflicts of interests; |
|
— |
supervise the consistent application of the criteria set out in point (c) of Section 5.1 of Annex IX to Regulation (EU) 2017/745 by the relevant expert panel in accordance with the Commission guidance referred to in Article 8(3); |
|
— |
supervise the work of the expert panel referred to in Article 1(2); |
|
— |
monitor compliance with the common rules of procedure referred to in Article 9, the guidance and methodologies referred to in Article 7(2) third indent and the requests for opinions, views and positions; |
|
— |
publish their opinions, views and positions in accordance with the second subparagraph of Article 106(12) of Regulation (EU) 2017/745; |
|
— |
process requests from expert panels for additional expertise. |
Article 11
Remuneration
1. Advisors shall be remunerated for their preparatory work and participation (in person or by electronic means) in the meetings of the expert panel and in other activities of the expert panels governed by this Decision. The remuneration shall be established according to the criteria set out in the Annex.
2. Travel and, where appropriate, subsistence expenses of advisors in connection with the activities of the expert panels governed by this Decision shall be reimbursed by the Commission in accordance with the provisions in force at the Commission. Those expenses shall be reimbursed within the limits of the available appropriations allocated to the Commission departments under the annual procedure for the allocation of resources.
Article 12
Independence, impartiality and objectivity
1. Advisors shall be appointed or assigned in their personal capacity. They shall not delegate their responsibilities to any other person.
2. Advisors shall not have financial or other interests in the medical device industry or in a notified body or any other organisation or sector, which could affect their independence, impartiality and objectivity. They shall make a declaration of interests indicating any interest which may compromise or may reasonably be perceived to compromise their independence, impartiality and objectivity, including any relevant circumstances relating to their close family members.
3. Declarations of interests shall be submitted in writing, when applying to the call for expression of interest.
4. Advisors shall update their declarations of interest:
|
— |
prior to the appointment to an expert panel or prior to inclusion on the central list; |
|
— |
whenever a change of circumstances so requires; |
|
— |
prior to commencement of a specific task in the expert panel. |
5. Where the obligations referred to in paragraphs 1 to 4 are not met, the Commission may take all appropriate measures.
Article 13
Commitment
1. Advisors shall commit to acting in the public interest and observing the principles listed in Articles 12 to 15. For that purpose, they shall sign a declaration of commitment.
2. Advisors shall respond to requests and other communications from the Chair of their respective expert panel or sub-group and from the Secretariat. They shall dedicate the necessary effort to complete the assigned tasks to the best of their ability and within the timelines as described in the common rules of procedure referred to in Article 9.
Article 14
Transparency
The activities of the expert panels shall be carried out in a transparent manner. The Secretariat shall in particular make available to the public on a dedicated Commission website, without undue delay:
|
(a) |
the names of the advisors appointed or assigned to the expert panels or included in the central list of available experts; |
|
(b) |
the curriculum vitae and the declarations of interests, confidentiality and commitment of advisors appointed or assigned to the expert panels; |
|
(c) |
the common rules of procedure of the expert panels referred to in Article 9; |
|
(d) |
opinions, views and positions in accordance with Article 8. |
Article 15
Confidentiality
1. Advisors shall not divulge any information of confidential nature acquired as part of their work in the expert panels or as a result of other activities governed by this Decision. For that purpose, they shall sign a declaration of confidentiality.
2. Advisors shall comply with the rules on security regarding the protection of Union classified information and sensitive non-classified information, laid down in Decisions (EU, Euratom) 2015/443 and (EU, Euratom) 2015/444.
3. Where the obligations referred to in paragraphs 1 and 2 are not met, the Commission may take all appropriate measures.
Article 16
Entry into force and date of application
This Decision shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
Done at Brussels, 10 September 2019.
For the Commission
The President
Jean-Claude JUNCKER
(2) Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU (OJ L 117, 5.5.2017, p. 176).
(3) Regulation (EU) 2018/1725 of the European Parliament and of the Council of 23 October 2018 on the protection of natural persons with regard to the processing of personal data by the Union institutions, bodies, offices and agencies and on the free movement of such data, and repealing Regulation (EC) No 45/2001 and Decision No 1247/2002/EC (OJ L 295, 21.11.2018, p. 39).
(4) Commission Decision (EU, Euratom) 2015/443 of 13 March 2015 on Security in the Commission (OJ L 72, 17.3.2015, p. 41).
(5) Commission Decision (EU, Euratom) 2015/444 of 13 March 2015 on the security rules for protecting EU classified information (OJ L 72, 17.3.2015, p. 53).
ANNEX
REMUNERATION OF ADVISORS
|
1. |
The remuneration of advisors is 450 EUR for each full working day. |
|
2. |
The total working time is calculated and rounded to the nearest half working day. |
|
3. |
For tasks referred to in Article 54(1) of Regulation (EU) 2017/745 and Article 48(6) of Regulation (EU) 2017/746, the maximum number of working days for which experts may be remunerated is set out in Table 1.
Table 1 Maximum number of working days for which experts may be remunerated for tasks referred to in Article 54(1) of Regulation (EU) 2017/745 and Article 48(6) of Regulation (EU) 2017/746
|
|||||||||||||||||||||||||||||
|
4. |
For tasks referred to in Articles 55(3), 61(2), 106(10)(a) to (f) and 106(11) of Regulation (EU) 2017/745 and Article 50(3) of Regulation (EU) 2017/746, divided into categories depending on their level of complexity, the maximum number of working days is specified in Table 2.
Table 2 Maximum number of working days for which experts may be remunerated for tasks under Articles 55(3), 61(2), 106(10)(a) to (f) and 106(11) of Regulation (EU) 2017/745 and Article 50(3) of Regulation (EU) 2017/746
|
|
5. |
The remuneration shall be conditional on the completion of the relevant tasks as per the common rules of procedure. |
(*1) advisors of the respective expert panel or sub-groups who validate the opinion or view produced by the rapporteur and the co-rapporteur
(*2) Each of these criteria may be applied independently
Corrigenda
|
11.9.2019 |
EN |
Official Journal of the European Union |
L 234/31 |
Corrigendum to Council Implementing Regulation (EU) 2019/798 of 17 May 2019 implementing Regulation (EU) No 36/2012 concerning restrictive measures in view of the situation in Syria
( Official Journal of the European Union L 132 of 20 May 2019 )
On page 3, the Annex, entry 36, column ‘Name’:
for:
‘Nizar ( ) al-Asaad (
) al-Asaad (![]() ) (a.k.a. Nizar Asaad)’,
) (a.k.a. Nizar Asaad)’,
read:
‘Nizar (![]() ) Al-Assad (
) Al-Assad ( ) (a.k.a. Al-Asad; Assad; Asad)’.
) (a.k.a. Al-Asad; Assad; Asad)’.
|
11.9.2019 |
EN |
Official Journal of the European Union |
L 234/31 |
Corrigendum to Council Decision (CFSP) 2019/806 of 17 May 2019 amending Decision 2013/255/CFSP concerning restrictive measures against Syria
( Official Journal of the European Union L 132 of 20 May 2019 )
On page 38, the Annex, entry 36, column ‘Name’:
for:
‘Nizar ( ) al-Asaad (
) al-Asaad (![]() ) (a.k.a. Nizar Asaad)’,
) (a.k.a. Nizar Asaad)’,
read:
‘Nizar (![]() ) Al-Assad (
) Al-Assad ( ) (a.k.a. Al-Asad; Assad; Asad)’.
) (a.k.a. Al-Asad; Assad; Asad)’.