ISSN 1977-0677
doi:10.3000/19770677.L_2013.033.eng
Official Journal
of the European Union
L 33

English edition
Legislation
Volume 56
2 February 2013
|
ISSN 1977-0677 doi:10.3000/19770677.L_2013.033.eng |
||
|
Official Journal of the European Union |
L 33 |
|

|
||
|
English edition |
Legislation |
Volume 56 |
|
|
|
|
|
(1) Text with EEA relevance |
|
EN |
Acts whose titles are printed in light type are those relating to day-to-day management of agricultural matters, and are generally valid for a limited period. The titles of all other Acts are printed in bold type and preceded by an asterisk. |
II Non-legislative acts
INTERNATIONAL AGREEMENTS
|
2.2.2013 |
EN |
Official Journal of the European Union |
L 33/1 |
Notice concerning the entry into force of an Agreement on Trade in Bananas between the European Union and the United States of America
The Agreement on Trade in Bananas between the European Union and the United States of America, signed in Geneva on 8 June 2010 (1), has entered into force on 24 January 2013.
REGULATIONS
|
2.2.2013 |
EN |
Official Journal of the European Union |
L 33/2 |
COMMISSION IMPLEMENTING REGULATION (EU) No 91/2013
of 31 January 2013
laying down specific conditions applicable to the import of groundnuts from Ghana and India, okra and curry leaves from India and watermelon seeds from Nigeria and amending Regulations (EC) No 669/2009 and (EC) No 1152/2009
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety (1), and in particular Article 53(1)(b)(ii) thereof,
Having regard to Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules (2), and in particular Article 15(5) thereof,
Whereas:
|
(1) |
Article 53 of Regulation (EC) No 178/2002 provides for the possibility to adopt appropriate Union emergency measures for feed and food imported from a third country in order to protect human health, animal health and the environment, where the risk cannot be contained satisfactorily by means of measures taken by the Member States individually. |
|
(2) |
Commission Regulation (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non-animal origin and amending Decision 2006/504/EC (3), establishes an increased level of official controls on imports of certain feed and food of non-animal origin. |
|
(3) |
Amongst other, an increased frequency of official controls on import has been established for more than two years on groundnuts from India as regards aflatoxins, curry leaves from India as regards pesticide residues, groundnuts from Ghana as regards aflatoxins and watermelon seeds from Nigeria as regards aflatoxins and for nearly two years on okras from India as regards pesticide residues. |
|
(4) |
The results from the of increased frequency of controls show a continuous high frequency of non-compliance with maximum levels of aflatoxins and maximum residue levels of pesticide residues established in Union legislation and several times very high levels were observed. These results provide evidence that the import of these foods and feeds constitute a risk for animal and human health. No improvement of the situation could be observed after this period of increased frequency of controls at Union borders. Furthermore, no concrete and satisfactory action plan to remediate the shortcomings and deficiencies in the production and control systems was received from the Indian, Nigerian and Ghanaian authorities, despite the explicit request from the European Commission. |
|
(5) |
To protect human and animal health in the Union, it is necessary to provide for additional guarantees in relation to those food and feed from India, Ghana and Nigeria. All consignments of groundnuts from India and Ghana, watermelon seeds from Nigeria and curry leaves and okras from India should therefore be accompanied by a certificate stating that the products have been sampled and analysed for the presence of, according to the case, aflatoxins or pesticide residues and have been found compliant with Union legislation. |
|
(6) |
For the protection of public and animal health compound feed and food containing to a significant amount the feed and food covered by this Regulation should also be included in the scope of the Regulation. |
|
(7) |
The sampling and the analysis of consignments should be performed in accordance with the relevant Union legislation. The maximum levels of aflatoxins in food are established by Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (4) and in feed by Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed (5). The maximum residue levels for pesticide residues are established by Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC (6). The provisions on sampling and analysis for the control of aflatoxins in food are established by Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the offcial control of the levels of mycotoxins in foodstuffs (7) and in feed by Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed (8). The provisions on sampling for the official control of pesticide residues are established by Commission Directive 2002/63/EC of 11 July 2002 establishing Community methods of sampling for the official control of pesticide residues in and on products of plant and animal origin and repealing Directive 79/700/EEC (9). |
|
(8) |
In order to ensure an efficient organisation and ensure a degree of uniformity at the Union level of the controls at import, it is appropriate to provide in this Regulation measures which are equivalent to the existing measures as provided for in Regulation (EC) No 669/2009 for the physical control on pesticide residues on curry leaves and okra from India and Commission Regulation (EC) No 1152/2009 of 27 November 2009 imposing special conditions governing the import of certain foodstuffs from certain third countries due to contamination risk by aflatoxins and repealing Decision 2006/504/EC (10) for the control of aflatoxins in groundnuts from India and Ghana and watermelon seeds from Nigeria. |
|
(9) |
In order to ensure an efficient organisation of the official controls, it is furthermore appropriate that the ‘first point of introduction’ referred to in Regulation (EC) No 1152/2009 is replaced by ‘designated point of entry’ as defined in Regulation (EC) No 669/2009. |
|
(10) |
With a view to minimise negative effects on trade and to enable the competent authorities of India, Ghana and Nigeria to set up an appropriate control system, it is appropriate to provide that the requirement of a health certificate only applies to consignments of products covered by this Regulation which have left the country of origin after a certain date. It is important for the protection of human and animal health to keep this period as short as possible. |
|
(11) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS REGULATION:
Article 1
Scope
1. This Regulation shall apply to consignments of the following food and feed falling within the CN codes and TARIC classifications set out in Annex I:
|
(a) |
Groundnuts (peanuts) in shell and shelled and peanut butter (feed and food) originating in or consigned from Ghana; |
|
(b) |
Okra (food, fresh) originating in or consigned from India; |
|
(c) |
Curry leaves (food, herbs) originating in or consigned from India; |
|
(d) |
Groundnuts (peanuts) in shell and shelled, peanut butter, groundnuts (peanuts) otherwise prepared or preserved (feed and food) originating in or consigned from India; |
|
(e) |
Watermelon seeds and derived products (food) originating in or consigned from Nigeria. |
2. This Regulation shall also apply to compound food and feed, containing any of the food or feed referred to in paragraph 1 in a quantity above 20 %.
3. This Regulation shall not apply to consignments of food and feed referred to in paragraphs 1 and 2 which are destined to a private person for personal consumption and use only. In case of doubt, the burden of proof lies with the recipient of the consignment.
Article 2
Definitions
For the purposes of this Regulation, the definitions laid down in Articles 2 and 3 of Regulation (EC) No 178/2002, Article 2 of Regulation (EC) No 882/2004, Article 2 of Regulation (EC) No 1152/2009 and Article 3 of Regulation (EC) No 669/2009 shall apply.
For the purpose of this Regulation, a consignment corresponds to a lot as referred to in Regulations (EC) No 401/2006 and (EC) No 152/2009 and Directive 2002/63/EC.
Article 3
Import into the Union
Consignments of food and feed referred to in Article 1(1) and (2) may only be imported into the Union in accordance with the procedures laid down in this Regulation.
Article 4
Results of sampling and analysis
1. Consignments of the food and feed referred to in Article 1(1) and (2) shall be accompanied by the results of sampling and analysis performed by the competent authorities of the country of origin, or of the country where the consignment is consigned from if that country is different from the country of origin, to ascertain compliance with:
|
(a) |
Union legislation on maximum levels of aflatoxins, for the food and feed referred to in Article 1(1)(a),(d) and (e), including compounds containing such food or feed in a quantity above 20 %; |
|
(b) |
Union legislation on maximum residue levels of pesticides, for the food referred to in Article 1(1)(b) and (c) including compound food containing such food in a quantity above 20 %. |
2. The sampling and the analysis referred to in paragraph 1 must be performed in accordance with Regulation (EC) No 401/2006, for aflatoxins in food, with Regulation (EC) No 152/2009, for aflatoxins in feed, and with Directive 2002/63/EC for pesticide residues.
Article 5
Health certificate
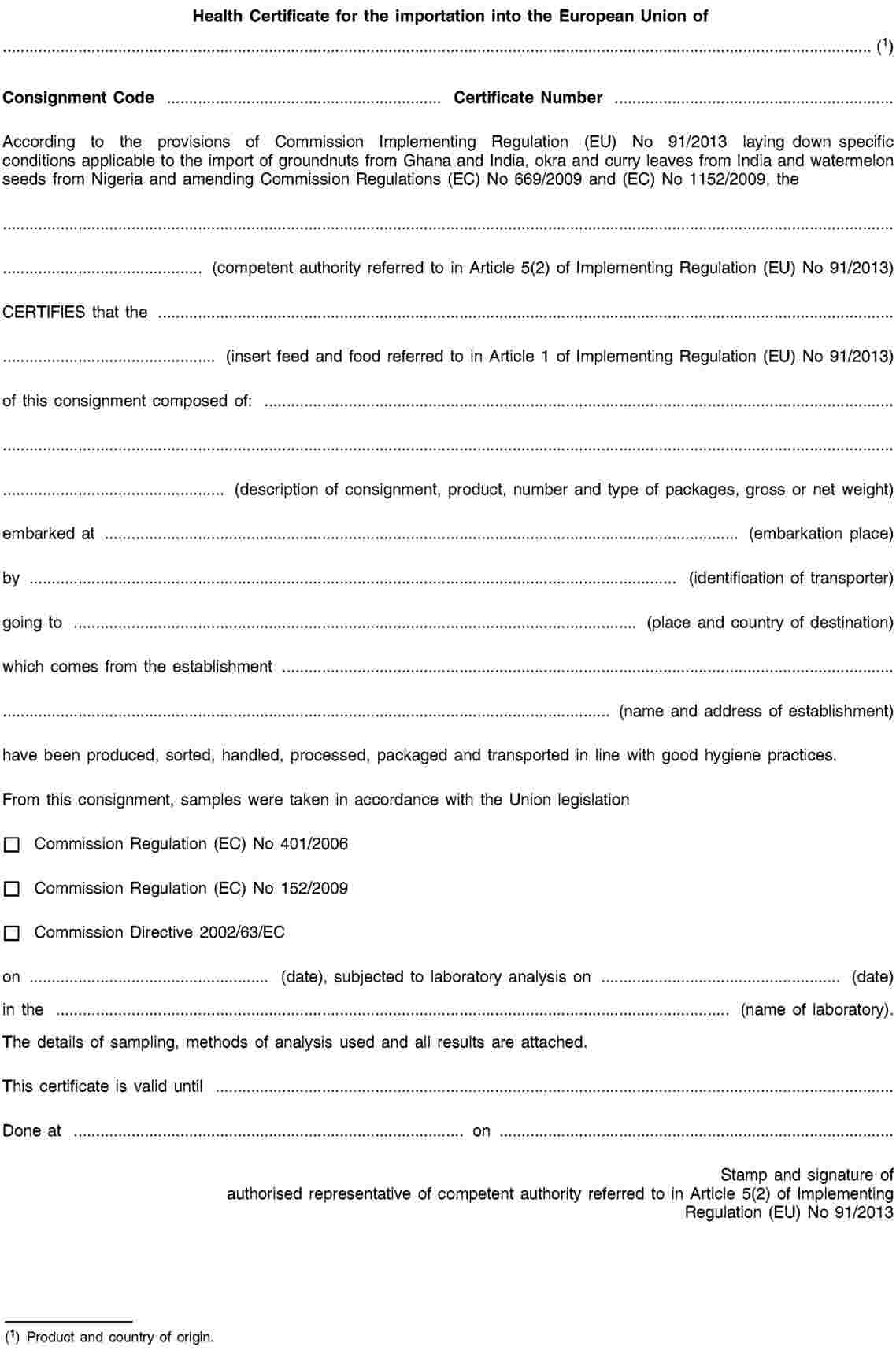
1. The consignments shall also be accompanied by a health certificate in accordance with the model set out in Annex II.
2. The health certificate shall be completed, signed and verified by an authorised representative of the competent authority of the country of origin or the competent authority of the country where the consignment is consigned from if that country is different from the country of origin.
3. The health certificate shall be drawn up in an official language of the Member State of arrival, or in another language that the competent authorities of that Member State have decided to accept.
4. The health certificate shall only be valid during four months from the date of issue.
Article 6
Identification
Each consignment of the food and feed referred to in Article 1(1) and (2) shall be identified with an identification code which corresponds to the identification code mentioned on the results of the sampling and analysis referred to in Article 4 and the health certificate referred to in Article 5. Each individual bag, or other packaging form, of the consignment shall be identified with that identification code.
Article 7
Prior notification of consignments
1. Feed and food business operators or their representatives shall give prior notification of the estimated date and time of physical arrival of consignments of the food and feed referred to in Article 1(1) and (2) to the competent authorities at the Designated Point of Entry and of the nature of the consignment.
2. For the purpose of prior notification, they shall complete Part I of the common entry document (CED) and transmit that document to the competent authority at the Designated Point of Entry, at least one working day prior to the physical arrival of the consignment.
3. For the completion of the CED in application of this Regulation, feed and food business operators shall take into account:
|
(a) |
for the food a referred to in Article 1(1)(b) and (c) of this Regulation including compound food containing such food in a quantity above 20 %, the notes for guidance for the CED laid down in Annex II to Regulation (EC) No 669/2009; |
|
(b) |
for the food and feed referred to in Article 1(1)(a), (d) and (e) of this Regulation, including compounds containing such food or feed in a quantity above 20 % the notes for guidance for the CED laid down in Annex II to Regulation (EC) No 1152/2009. |
Article 8
Official controls
1. The competent authority at the DPE shall carry out documentary checks on each consignment of the food and feed referred to in Article 1(1) and (2) to ascertain compliance with the requirements laid down in Articles 4 and 5.
2. The identity and physical checks on the food referred to in Article 1(1)(a), (d) and (e) and the related compound food referred to in Article 1(2) of this Regulation shall be carried out in accordance with Article 7 of Regulation (EC) No 1152/2009 at the frequency set out in Annex I of this Regulation. The provisions of Article 7 of Regulation (EC) No 1152/2009 are applied for the identity and physical checks on the feed referred to in Article 1(1)(a), (d) and (e) and the related compound feed referred to in Article 1(2) of this Regulation, whereby the sample for analysis of aflatoxin B1 is taken in accordance with Regulation (EC) No 152/2009 at the frequency set out in Annex I of this Regulation.
3. The identity and physical checks on the food referred to in Article 1(1)(b) and (c) and the related compound food referred to in Article 1(2) of this Regulation shall be carried out in accordance with Articles 8, 9 and 19 of Regulation (EC) No 669/2009 at the frequency set out in Annex I of this Regulation.
4. After completion of the checks, the competent authorities shall,
|
(a) |
complete the relevant entries of Part II of the CED; |
|
(b) |
join the results of sampling and analysis carried out in accordance with paragraphs 2 and 3 of this Article; |
|
(c) |
provide and fill the CED reference number on the CED; |
|
(d) |
stamp and sign the original of the CED; |
|
(e) |
make and retain a copy of the signed and stamped CED. |
5. The original of the CED and of the health certificate with the accompanying results of sampling and analysis shall accompany the consignment during its transport until it is released for free circulation. For food referred to in Article 1(1)(b) and (c), in case of authorisation of onward transportation of the consignments pending the results of the physical checks, a certified copy of the original CED shall be issued for that purpose.
Article 9
Splitting of a consignment
1. Consignments shall not be split until all official controls have been completed, and the CED has been fully completed by the competent authorities as provided for in Article 8.
2. In the case of subsequent splitting of the consignment, an authenticated copy of the CED shall accompany each part of the consignment during its transport until it is released for free circulation.
Article 10
Release for free circulation
The release for free circulation of consignments shall be subject to the presentation by the feed and food business operator or their representative to the custom authorities of a CED duly completed by the competent authority once all official controls have been carried out and favourable results from physical checks, where such checks are required, are known.
Article 11
Non-compliance
If the official controls establish non-compliance with the relevant Union legislation, the competent authority shall complete Part III of the CED and action shall be taken pursuant to Articles 19, 20 and 21 of Regulation (EC) No 882/2004.
Article 12
Reports
Member States shall submit to the Commission every three months a report of all analytical results of official controls on consignments of feed and food pursuant to this Regulation. That report shall be submitted during the month following each quarter.
The report shall include the following information:
|
— |
the number of consignments imported; |
|
— |
the number of consignments subjected to sampling for analysis; |
|
— |
the results of the checks as provided for in Article 8(2) and (3). |
Article 13
Costs
All costs resulting from the official controls including sampling, analysis, storage and any measures taken following non-compliance, shall be borne by the feed and food business operators.
Article 14
Amendment to Regulation (EC) No 669/2009
Regulation (EC) No 669/2009 is amended in accordance with Annex III to this Regulation
Article 15
Amendment to Regulation (EC) No 1152/2009
Regulation (EC) No 1152/2009 is amended as follows:
|
1. |
In Article 2, the following point (b) is deleted:
and replaced by:
|
|
2. |
In Article 5, first and second paragraph, Article 7(2) and (3) and in Annex II, under General, Box I.4, Box I.9, Box II.5, Box II.6, Box II.8, Box II.9 and Box III.1 the term ‘first point of introduction’ is replaced by ‘DPE’. |
Article 16
Transitional measures
By way of derogation from Articles 4(1) and 5 (1), Member States shall authorise the imports of consignments of feed and food referred to in Article 1(1) and (2) which left the country of origin prior to 18 February 2013 without being accompanied by a health certificate and the results of sampling and analysis.
Article 17
Entry into force
This Regulation shall enter into force on the third day following that of its publication in the Official Journal of the European Union.
It shall apply from 18 February 2013.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 31 January 2013.
For the Commission
The President
José Manuel BARROSO
(2) OJ L 165, 30.4.2004, p. 1.
(3) OJ L 194, 25.7.2009, p. 11.
(4) OJ L 364, 20.12.2006, p. 5.
(5) OJ L 140, 30.5.2002, p. 10.
(9) OJ L 187, 16.7.2002, p. 30.
(10) OJ L 313, 28.11.2009, p. 40.
ANNEX I
Feed and food of non-animal origin subject to the measures provided for in this Regulation:
|
Feed and food (intended use) |
CN code (1) |
TARIC sub-division |
Country of origin |
Hazard |
Frequency of physical and identity checks (%) at import |
||||
|
|
|
Ghana (GH) |
Aflatoxins |
50 |
||||
|
|
||||||||
|
|
||||||||
|
(Feed and Food) |
|
||||||||
|
Okra |
ex 0709 99 90 |
20 |
India (IN) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single-residue methods (2) |
20 |
||||
|
(Food — fresh) |
|
||||||||
|
Curry leaves (Bergera/Murraya koenigii) |
ex 1211 90 86 |
10 |
India (IN) |
Pesticide residues analysed with multi-residue methods based on GC-MS and LC-MS or with single residue methods (3) |
20 |
||||
|
(Food — herbs) |
|
||||||||
|
|
|
India (IN) |
Aflatoxins |
20 |
||||
|
|
||||||||
|
|
||||||||
|
|
||||||||
|
(Feed and food) |
|
||||||||
|
Watermelon (egusi, Citrullus lanatus) seeds and derived products |
ex 1207 70 00; |
10 |
Nigeria (NG) |
Aflatoxins |
50 |
||||
|
ex 1106 30 90; |
30 |
||||||||
|
ex 2008 99 99; |
50 |
||||||||
|
(Food) |
|
|
(1) Where only certain products under any CN code are required to be examined and no specific subdivision under that code exists in the goods nomenclature, the CN code is marked ‘ex’.
(2) Certification by the country of origin and control at import by the Member States to ensure compliance with Regulation (EC) No 396/2005 in particular residues of: Acephate, Methamidophos, Triazophos, Endosulfan, Monocrotophos, Methomyl, Thiodicarb, Diafenthiuron, Thiamethoxam, Fipronil, Oxamyl, Acetamiprid, Indoxacarb, Mandipropamid.
(3) Certification by the country of origin and control at import by the Member States to ensure compliance with Regulation (EC) No 396/2005 in particular residues of: Triazophos, Oxydemeton-methyl, Chlorpyriphos, Acetamiprid, Thiamethoxam, Clothianidin, Methamidophos, Acephate, Propargite, Monocrotophos.
ANNEX II
ANNEX III
Regulation (EC) No 669/2009 is amended as follows:
|
1. |
In Annex I, the following entries are deleted:
|
|
2. |
In Annex I, the following endnote (2) is deleted:
|
|
3. |
In Annex I, the following endnote (5) is deleted:
|
|
2.2.2013 |
EN |
Official Journal of the European Union |
L 33/11 |
COMMISSION IMPLEMENTING REGULATION (EU) No 92/2013
of 1 February 2013
amending Implementing Regulation (EU) No 700/2012 as regards deductions from the Portuguese fishing quotas available for cod, Greenland halibut and redfish and the Spanish fishing quota available for red seabream in certain areas
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Council Regulation (EC) No 1224/2009 of 20 November 2009 establishing a Community control system for ensuring compliance with the rules of the common fisheries policy, amending Regulations (EC) No 847/96, (EC) No 2371/2002, (EC) No 811/2004, (EC) No 768/2005, (EC) No 2115/2005, (EC) No 2166/2005, (EC) No 388/2006, (EC) No 509/2007, (EC) No 676/2007, (EC) No 1098/2007, (EC) No 1300/2008, (EC) No 1342/2008 and repealing Regulations (EEC) No 2847/93, (EC) No 1627/94 and (EC) No 1966/2006 (1), and in particular Article 105(1), (2) and (3) thereof,
Whereas:
|
(1) |
Firstly, following the publication of Commission Implementing Regulation (EU) No 700/2012 of 30 July 2012 operating deductions from fishing quotas available for certain stocks in 2012 on account of overfishing in the previous years (2), the Portuguese fisheries authorities discovered and notified a mistake in the declarations of catches landed by Spain in their country. |
|
(2) |
Having been consulted, the Spanish fisheries authorities confirmed and corrected the reporting mistake. |
|
(3) |
After correction, it appears that the Portuguese quotas of the 2011 stocks for cod in NAFO area 3M (COD/N3M.), Greenland halibut in NAFO area 3LMNO (GHL/N3LMNO), redfish in NAFO area 3LN (RED/N3LN.), and in EU and international waters of V; international waters of XII and XIV (RED/51214D) were not overfished. |
|
(4) |
The deductions to these 2012 Portuguese fishing quotas as set out in the Annex to Implementing Regulation (EU) No 700/2012 should therefore no longer be applicable. |
|
(5) |
Secondly, Council Regulation (EU) No 1225/2010 of 13 December 2010 fixing for 2011 and 2012 the fishing opportunities for EU vessels for fish stocks of certain deep-sea fish species (3) stipulates, for the stock of red seabream in EU and international waters of IX (SBR/09-), that a maximum of 8 % may be fished in EU and international waters of VI, VII and VIII (SBR/*678-). |
|
(6) |
In the framework of Commission Implementing Regulation (EU) No 700/2011 of 20 July 2011 adding to the 2011 fishing quotas certain quantities withheld in the year 2010 pursuant to Article 4(2) of Council Regulation (EC) No 847/96 (4), the 2011 Spanish quota of red seabream in EU and international waters of IX (SBR/09-) has been increased from 614 to 684 tonnes. |
|
(7) |
Another 30 more tonnes of that stock have been added to the 2011 Spanish quota following an exchange concluded with Portugal on 2 August 2011. |
|
(8) |
Considering the 2011 Spanish final adapted quota for red seabream in EU and international waters of IX (SBR/09-) amounting to 714 tonnes, a maximum of 57,12 tonnes, corresponding to 8 %, of red seabream may have been fished by Spain in EU and international waters of VI, VII and VIII (SBR/*678-). It is this amount that should have been taken into account to fix the Spanish final quota of SBR/*678- to be deducted according to Implementing Regulation (EU) No 700/2012. |
|
(9) |
The deduction applied to the Spanish quota of SBR/*678- should be reduced accordingly. |
|
(10) |
Implementing Regulation (EU) No 700/2012 should therefore be amended accordingly and the amendments should have retroactive effect to the date of entry into force of that Regulation, |
HAS ADOPTED THIS REGULATION:
Article 1
The Annex to Implementing Regulation (EU) No 700/2012 is amended in accordance with the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the day following that of its publication in the Official Journal of the European Union.
It shall apply from 7 August 2012.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 1 February 2013.
For the Commission
The President
José Manuel BARROSO
(1) OJ L 343, 22.12.2009, p. 1.
(2) OJ L 203, 31.7.2012, p. 52.
(3) OJ L 336, 21.12.2010, p. 1.
(4) OJ L 190, 21.7.2011, p. 2.
ANNEX
The Annex to Implementing Regulation (EU) No 700/2012 is amended as follows:
|
(a) |
on page 56, the line hereunder
is replaced by the following:
|
|
(b) |
on page 58, the four lines hereunder are deleted
|
|
2.2.2013 |
EN |
Official Journal of the European Union |
L 33/14 |
COMMISSION REGULATION (EU) No 93/2013
of 1 February 2013
laying down detailed rules for the implementation of Council Regulation (EC) No 2494/95 concerning harmonised indices of consumer prices, as regards establishing owner-occupied housing price indices
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Council Regulation (EC) No 2494/95 of 23 October 1995 concerning harmonised indices of consumer prices (1), and in particular the third paragraph of Article 4 and Article 5(3) thereof,
Having regard to the opinion of the European Central Bank (2),
Whereas:
|
(1) |
Regulation (EC) No 2494/95 establishes the production of harmonised indices of consumer prices (HICP). |
|
(2) |
Establishing price indices for dwellings and in particular for owner-occupied housing should be explored to improve the relevance and comparability of the HICP. |
|
(3) |
With a view to compiling owner-occupied housing indices it is necessary to produce house price indices. House price indices are also important indicators in their own right. |
|
(4) |
Methodological guidance on compiling owner-occupied housing and house price indices is necessary to ensure reliable and comparable results from all Member States. |
|
(5) |
The measures provided for in this Regulation are in accordance with the opinion of the European Statistical System Committee (ESS Committee), |
HAS ADOPTED THIS REGULATION:
Article 1
Subject matter
This Regulation establishes owner-occupied housing price indices with a view to improving the relevance and comparability of harmonised indices of consumer prices (‘HICP’).
Article 2
Definitions
For the purposes of this Regulation, the following definitions shall apply:
|
1. |
‘owner-occupied housing price index’ means an index that measures the changes in the transaction prices of dwellings new to the household sector and other goods and services that households acquire in their role as owner-occupiers; |
|
2. |
‘house price index’ means an index that measures the changes in the transaction prices of dwellings purchased by households. |
Article 3
Coverage
1. The following expenditure categories shall be covered in the owner-occupied housing price index:
|
O.1. |
Owner-occupiers’ housing expenditures |
|
O.1.1. |
Acquisitions of dwellings |
|
O.1.1.1. |
New dwellings |
|
O.1.1.1.1. |
Purchases of new dwellings |
|
O.1.1.1.2. |
Self-build dwellings and major renovations |
|
O.1.1.2. |
Existing dwellings new to households |
|
O.1.1.3. |
Other services related to the acquisition of dwellings |
|
O.1.2. |
Ownership of dwellings |
|
O.1.2.1. |
Major repairs and maintenance |
|
O.1.2.2. |
Insurance connected with dwellings |
|
O.1.2.3. |
Other services related to ownership of dwellings |
2. The following expenditure categories shall be covered in the house price index:
|
H.1. |
Purchases of dwellings |
|
H.1.1. |
Purchases of new dwellings |
|
H.1.2. |
Purchases of existing dwellings |
3. The owner-occupied housing price index shall be based on the ‘Net acquisitions’ approach, which measures changes in actual prices paid by consumers for the acquisition of dwellings that are new to the household sector as well as changes in other costs related to the ownership, and transfer of ownership, of dwellings.
4. Any housing expenditure category as defined in Article 3(1) with a weight of at least one part per hundred of the total housing expenditure O.1 shall be covered. Any housing expenditure category as defined in Article 3(2) with a weight of at least one part per hundred of the total housing expenditure H.1, shall be covered.
Article 4
Methodological manual
1. The Commission (Eurostat), in close cooperation with the Member States, shall establish a manual which provides a methodological framework for owner-occupied housing and house price indices produced pursuant to this Regulation (hereinafter referred to as the ‘OOH-HPI manual’). When duly justified, the Commission (Eurostat) shall update the manual, in accordance with procedural arrangements approved by the ESS Committee.
2. The quality criteria referred to in Article 12(1) of Regulation (EC) No 223/2009 of the European Parliament and of the Council (3) shall apply to the compilation of the owner-occupied housing and house price indices.
3. Member States shall provide the Commission (Eurostat), at its request, with the necessary information to assess the compliance of owner-occupied housing and house price indices with the provisions of this Regulation.
Article 5
Data requirements
1. Member States shall compile and provide the Commission (Eurostat) with price indices for the categories laid down in Article 3 in accordance with the OOH-HPI manual.
2. Member States shall provide quarterly price indices. In addition to quarterly indices, Member States may also provide monthly indices.
3. The indices referred to in Article 3(1) shall be provided from the third quarter of 2014 relating to the second quarter of 2014. The indices referred to in Article 3(2) shall be provided from the third quarter of 2012 relating to the second quarter of 2012.
4. Member States shall provide the Commission (Eurostat) with the quarterly indices within a deadline which shall not exceed eighty-five days from the end of the quarter to which the indices relate. Those Member States that choose to provide also monthly indices shall provide them within thirty days from the end of the month to which the indices relate.
5. Each year, Member States shall compile and provide the Commission (Eurostat) with one set of expenditure weights for owner-occupied housing price indices and one set of expenditure weights for house price indices, as defined in the OOH-HPI manual and in accordance with the Commission Regulation (EU) No 1114/2010 (4).
The weights for the quarterly indices shall be provided no later than 15 June of the year following the year to which the weights relate.
Member States providing monthly indices shall provide the corresponding weights no later than 20 February of the year following the year to which the weights relate.
6. Member States shall compile and provide the Commission (Eurostat) with estimated back data starting with the index for the first quarter of 2010 for the indices referred to in Article 3(1), within the deadlines referred to in Articles 5(3) and (4) at the latest. Member States shall compile and provide the Commission (Eurostat) with estimated back data starting with the index for the first quarter of 2008 for the indices referred to in Article 3(2), within the deadlines referred to in Articles 5(3) and (4) at the latest.
7. Member States shall provide the data required by this Regulation and the associated metadata in accordance with an exchange standard specified by the Commission (Eurostat). Data and metadata shall be provided to the Commission (Eurostat) via the Single Entry Point services or in such a way that the Commission can retrieve them via the Single Entry Point services.
Article 6
Transitional measures
1. One year and three years respectively after the date of entry into force of this Regulation, Member States shall provide the Commission (Eurostat) with reports on the quality of the data, on the basis of the standards defined within the European Statistical System and in the OOH-HPI manual.
2. The Commission (Eurostat) shall, within five years of the date of entry into force of this Regulation, prepare a report on the indices established pursuant to this Regulation and in particular on their degree of compliance with Commission Regulation (EC) No 1749/96 (5) and Regulation (EU) No 1114/2010. The report shall also address the suitability of the owner-occupied housing indices for integration into HICP coverage.
Article 7
Entry into force
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
It shall apply from 1 September 2012. This Regulation shall be binding in its entirety and directly applicable in the Member States in accordance with the Treaties.
Done at Brussels, 1 February 2013.
For the Commission
The President
José Manuel BARROSO
(1) OJ L 257, 27.10.1995, p. 1.
(2) Opinion of 19 October 2012 (not yet published in the Official Journal).
(3) OJ L 87, 31.3.2009, p. 164.
(4) OJ L 316, 2.12.2010, p. 4.
(5) OJ L 229, 10.9.1996, p. 3.
|
2.2.2013 |
EN |
Official Journal of the European Union |
L 33/17 |
COMMISSION IMPLEMENTING REGULATION (EU) No 94/2013
of 1 February 2013
amending Regulation (EU) No 162/2011 as regards the intervention centres for rice in Spain
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Council Regulation (EC) No 1234/2007 of 22 October 2007 establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products (‘Single CMO’ Regulation) (1), and in particular Article 41 in conjunction with Article 4 thereof,
Whereas:
|
(1) |
The Annex to Commission Regulation (EU) No 162/2011 of 21 February 2011 determining the intervention centres for rice (2) designates the intervention centres for rice. |
|
(2) |
In accordance with Article 55(1) of Commission Regulation (EU) No 1272/2009 of 11 December 2009 laying down common detailed rules for the implementation of Council Regulation (EC) No 1234/2007 as regards buying-in and selling of agricultural products under public intervention (3), Spain has communicated to the Commission the amended list of its intervention centres for rice and the list of storage premises (4) attached to those centres which have been approved as fulfilling the minimum standards required by EU legislation. |
|
(3) |
Regulation (EU) No 162/2011 should therefore be amended accordingly, and the list of storage premises attached thereto should be published on the internet, together with all the information required by the operators involved in public intervention. |
|
(4) |
The measures provided for in this Regulation are in accordance with the opinion of the Management Committee for the Common Organisation of Agricultural Markets, |
HAS ADOPTED THIS REGULATION:
Article 1
The Annex to Regulation (EU) No 162/2011 is amended in accordance with the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the third day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 1 February 2013.
For the Commission
The President
José Manuel BARROSO
(1) OJ L 299, 16.11.2007, p. 1.
(2) OJ L 47, 22.2.2011, p. 11.
(3) OJ L 349, 29.12.2009, p. 1.
(4) The addresses of the storage premises of the intervention centres are available on the European Commission website EUROPA/agriculture http://ec.europa.eu/agriculture/cereals/legislation/index_en.htm
ANNEX
In the Annex to Regulation (EU) No 162/2011, the section entitled ‘SPAIN’ is replaced by the following:
‘SPAIN
|
|
Andalucía |
|
|
Aragón |
|
|
Castilla y León |
|
|
Castilla-La Mancha |
|
|
Extremadura |
|
|
Navarra’ |
|
2.2.2013 |
EN |
Official Journal of the European Union |
L 33/19 |
COMMISSION IMPLEMENTING REGULATION (EU) No 95/2013
of 1 February 2013
concerning the authorisation of a preparation of Pediococcus acidilactici CNCM MA 18/5M as a feed additive for all fish other than salmonids (holder of authorisation Lallemand SAS)
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition (1), and in particular Article 9(2) thereof,
Whereas:
|
(1) |
Regulation (EC) No 1831/2003 provides for the authorisation of additives for use in animal nutrition and for the grounds and procedures for granting such authorisation. |
|
(2) |
In accordance with Article 7 of Regulation (EC) No 1831/2003, an application was submitted for a new use of a preparation of Pediococcus acidilactici CNCM MA 18/5M. That application was accompanied by the particulars and documents required under Article 7(3) of Regulation (EC) No 1831/2003. |
|
(3) |
The application concerns the authorisation of a new use of Pediococcus acidilactici CNCM MA 18/5M as a feed additive for all fish, to be classified in the additive category ‘zootechnical additives’. |
|
(4) |
The use of Pediococcus acidilactici CNCM MA 18/5M was authorised without a time limit for chickens for fattening by Commission Regulation (EC) No 1200/2005 (2) and for pigs for fattening by Commission Regulation (EC) No 2036/2005 (3), for 10 years for salmonids and shrimps by Commission Regulation (EC) No 911/2009 (4), for weaned piglets by Commission Regulation (EU) No 1120/2010 (5) and for laying hens by Commission Regulation (EU) No 212/2011 (6). |
|
(5) |
The European Food Safety Authority (‘the Authority’) concluded in its opinion of 11 September 2012 (7) that, under the proposed conditions of use, Pediococcus acidilactici CNCM MA 18/5M has the potential to produce beneficial effect in development for all fish increasing the proportion of well conformed fish, reducing bone deformation. |
|
(6) |
The assessment of Pediococcus acidilactici CNCM MA 18/5M shows that the conditions for authorisation, as provided for in Article 5 of Regulation (EC) No 1831/2003, are satisfied. Accordingly, the use of this preparation should be authorised as specified in the Annex to this Regulation. |
|
(7) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS REGULATION:
Article 1
The preparation specified in the Annex, belonging to the additive category ‘zootechnical additives’ and to the functional group ‘other zootechnical additives’, is authorised as an additive in animal nutrition subject to the conditions laid down in that Annex.
Article 2
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 1 February 2013.
For the Commission
The President
José Manuel BARROSO
(1) OJ L 268, 18.10.2003, p. 29.
(2) OJ L 195, 27.7.2005, p. 6.
(3) OJ L 328, 15.12.2005, p. 13.
(4) OJ L 257, 30.9.2009, p. 10.
(5) OJ L 317, 3.12.2010, p. 12.
(7) EFSA Journal 2012; 10(9):2886.
ANNEX
|
Identification number of the additive |
Name of the holder of authorisation |
Additive |
Composition, chemical formula, description, analytical method |
Species or category of animal |
Maximum age |
Minimum content |
Maximum content |
Other provisions |
End of period of authorisation |
||||||||||||||
|
Units of activity/kg of complete feedingstuff with a moisture content of 12 % |
|||||||||||||||||||||||
|
Category of zootechnical additives. Functional group: other zootechnical additives (favourably affect growth). |
|||||||||||||||||||||||
|
4d1712 |
Lallemand SAS |
Pediococcus acidilactici CNCM MA 18/5M |
|
All fish other than salmonids |
— |
1 × 109 |
— |
|
22 February 2023 |
||||||||||||||
(1) Details of the analytical methods are available at the following address of the Reference Laboratory: http://irmm.jrc.ec.europa.eu/EURLs/EURL_feed_additives/Pages/index.aspx
|
2.2.2013 |
EN |
Official Journal of the European Union |
L 33/21 |
COMMISSION IMPLEMENTING REGULATION (EU) No 96/2013
of 1 February 2013
concerning the authorisation of a preparation of Lactobacillus buchneri NCIMB 30139 and of a preparation of Lactobacillus casei ATTC PTA 6135 as feed additives for all animal species
(Text with EEA relevance)
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition (1), and in particular Article 9(2) thereof,
Whereas:
|
(1) |
Regulation (EC) No 1831/2003 provides for the authorisation of additives for use in animal nutrition and for the grounds and procedures for granting such authorisation. Article 10(7) of Regulation (EC) No 1831/2003 in conjunction with Article 10(1) to (4) thereof sets out specific provisions for the evaluation of products used in the Union as silage additives at the date that Regulation became applicable. |
|
(2) |
In accordance with Article 10(1) of Regulation (EC) No 1831/2003, a preparation of Lactobacillus buchneri NCIMB 30139 and a preparation of Lactobacillus casei ATTC PTA 6135 were entered in the Community Register of Feed Additives as existing products belonging to the functional group of silage additives, for all animal species. |
|
(3) |
In accordance with Article 10(2) of Regulation (EC) No 1831/2003 in conjunction with Article 7 thereof, applications were submitted for the authorisation of those preparations as feed additives for all animal species, requesting those additives to be classified in the category ‘technological additives’ and in the functional group ‘silage additives’. Those applications were accompanied by the particulars and documents required under Article 7(3) of Regulation (EC) No 1831/2003. |
|
(4) |
The European Food Safety Authority (‘the Authority’) concluded in its opinions of 11 September 2012 (2) and 12 September 2012 (3) that, under the proposed conditions of use, the preparations concerned do not have an adverse effect on animal health, human health or the environment. The Authority also concluded that the preparation of Lactobacillus buchneri NCIMB 30139 has the potential to improve the preservation of easy to ensile material by increasing acetic acid production and the preparation of Lactobacillus casei ATTC PTA 6135 has the potential to improve the production of silage from easy to ensile material by reducing the pH and increasing the preservation of dry matter. The Authority does not consider that there is a need for specific requirements of post-market monitoring. It also verified the report on the methods of analysis of the feed additives in feed submitted by the Reference Laboratory set up by Regulation (EC) No 1831/2003. |
|
(5) |
The assessment of the preparations concerned shows that the conditions for authorisation, as provided for in Article 5 of Regulation (EC) No 1831/2003, are satisfied. Accordingly, the use of those preparations should be authorised as specified in the Annex to this Regulation. |
|
(6) |
Since safety reasons do not require the immediate application of the modifications to the conditions of authorisation, it is appropriate to allow a transitional period for interested parties to prepare themselves to meet the new requirements resulting from the authorisation. |
|
(7) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS REGULATION:
Article 1
Authorisation
The preparations specified in the Annex belonging to the additive category ‘technological additives’ and to the functional group ‘silage additives’, are authorised as additives in animal nutrition, subject to the conditions laid down in that Annex.
Article 2
Transitional measures
The preparations specified in the Annex and feed containing them, which are produced and labelled before 22 August 2013 in accordance with the rules applicable before 22 February 2013, may continue to be placed on the market and used until the existing stocks are exhausted.
Article 3
Entry into force
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 1 February 2013.
For the Commission
The President
José Manuel BARROSO
(1) OJ L 268, 18.10.2003, p. 29.
(2) EFSA Journal 2012; 10(9):2883.
(3) EFSA Journal 2012; 10(9):2884.
ANNEX
|
Identification number of the additive |
Name of the holder of authorisation |
Additive |
Composition, chemical formula, description, analytical method |
Species or category of animal |
Maximum age |
Minimum content |
Maximum content |
Other provisions |
End of period of authorisation |
||||||||||||||||||
|
CFU/kg of fresh material |
|||||||||||||||||||||||||||
|
Category of technological additives. Functional group: silage additives |
|||||||||||||||||||||||||||
|
1k20734 |
— |
Lactobacillus buchneri NCIMB 30139 |
|
All animal species |
— |
— |
— |
|
22 February 2023 |
||||||||||||||||||
|
1k20735 |
— |
Lactobacillus casei ATTC PTA 6135 |
|
All animal species |
— |
— |
— |
|
22 February 2023 |
||||||||||||||||||
(1) Details of the analytical methods are available at the following address of the Reference Laboratory: http://irmm.jrc.ec.europa.eu/EURLs/EURL_feed_additives/Pages/index.aspx
(2) Easy to ensile forage: > 3 % soluble carbohydrates in fresh material. As defined in Commission Regulation (EC) No 429/2008 (OJ L 133, 22.5.2008, p. 1).
|
2.2.2013 |
EN |
Official Journal of the European Union |
L 33/24 |
COMMISSION IMPLEMENTING REGULATION (EU) No 97/2013
of 1 February 2013
establishing the standard import values for determining the entry price of certain fruit and vegetables
THE EUROPEAN COMMISSION,
Having regard to the Treaty on the Functioning of the European Union,
Having regard to Council Regulation (EC) No 1234/2007 of 22 October 2007 establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products (Single CMO Regulation) (1),
Having regard to Commission Implementing Regulation (EU) No 543/2011 of 7 June 2011 laying down detailed rules for the application of Council Regulation (EC) No 1234/2007 in respect of the fruit and vegetables and processed fruit and vegetables sectors (2), and in particular Article 136(1) thereof,
Whereas:
|
(1) |
Implementing Regulation (EU) No 543/2011 lays down, pursuant to the outcome of the Uruguay Round multilateral trade negotiations, the criteria whereby the Commission fixes the standard values for imports from third countries, in respect of the products and periods stipulated in Annex XVI, Part A thereto. |
|
(2) |
The standard import value is calculated each working day, in accordance with Article 136(1) of Implementing Regulation (EU) No 543/2011, taking into account variable daily data. Therefore this Regulation should enter into force on the day of its publication in the Official Journal of the European Union, |
HAS ADOPTED THIS REGULATION:
Article 1
The standard import values referred to in Article 136 of Implementing Regulation (EU) No 543/2011 are fixed in the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the day of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 1 February 2013.
For the Commission, On behalf of the President,
José Manuel SILVA RODRÍGUEZ
Director-General for Agriculture and Rural Development
(1) OJ L 299, 16.11.2007, p. 1.
(2) OJ L 157, 15.6.2011, p. 1.
ANNEX
Standard import values for determining the entry price of certain fruit and vegetables
|
(EUR/100 kg) |
||
|
CN code |
Third country code (1) |
Standard import value |
|
0702 00 00 |
MA |
50,1 |
|
PS |
161,2 |
|
|
TN |
64,9 |
|
|
TR |
115,9 |
|
|
ZZ |
98,0 |
|
|
0707 00 05 |
EG |
206,0 |
|
MA |
124,7 |
|
|
TR |
169,6 |
|
|
ZZ |
166,8 |
|
|
0709 91 00 |
EG |
113,1 |
|
ZZ |
113,1 |
|
|
0709 93 10 |
EG |
194,1 |
|
MA |
54,6 |
|
|
TR |
135,1 |
|
|
ZZ |
127,9 |
|
|
0805 10 20 |
EG |
53,5 |
|
MA |
56,2 |
|
|
TN |
52,3 |
|
|
TR |
62,1 |
|
|
ZZ |
56,0 |
|
|
0805 20 10 |
MA |
88,2 |
|
ZZ |
88,2 |
|
|
0805 20 30, 0805 20 50, 0805 20 70, 0805 20 90 |
CN |
153,7 |
|
IL |
126,3 |
|
|
KR |
135,0 |
|
|
MA |
114,4 |
|
|
TR |
86,2 |
|
|
ZZ |
123,1 |
|
|
0805 50 10 |
TR |
70,3 |
|
ZZ |
70,3 |
|
|
0808 10 80 |
AR |
86,6 |
|
CN |
99,8 |
|
|
MK |
36,4 |
|
|
US |
178,4 |
|
|
ZZ |
100,3 |
|
|
0808 30 90 |
CN |
58,9 |
|
TR |
177,0 |
|
|
US |
136,7 |
|
|
ZA |
107,8 |
|
|
ZZ |
120,1 |
|
(1) Nomenclature of countries laid down by Commission Regulation (EC) No 1833/2006 (OJ L 354, 14.12.2006, p. 19). Code ‘ZZ’ stands for ‘of other origin’.