ISSN 1725-2555
Official Journal
of the European Union
L 97

English edition
Legislation
Volume 50
12 April 2007
|
ISSN 1725-2555 |
||
|
Official Journal of the European Union |
L 97 |
|

|
||
|
English edition |
Legislation |
Volume 50 |
|
Contents |
|
I Acts adopted under the EC Treaty/Euratom Treaty whose publication is obligatory |
page |
|
|
|
REGULATIONS |
|
|
|
|
||
|
|
* |
||
|
|
* |
||
|
|
* |
||
|
|
* |
||
|
|
|
||
|
|
|
DIRECTIVES |
|
|
|
* |
Commission Directive 2007/21/EC of 10 April 2007 amending Council Directive 91/414/EEC as regards the expiry dates for inclusion in Annex I of the active substances azoxystrobin, imazalil, kresoxim-methyl, spiroxamin, azimsulfuron, prohexadion-calcium and fluroxypyr ( 1 ) |
|
|
|
II Acts adopted under the EC Treaty/Euratom Treaty whose publication is not obligatory |
|
|
|
|
DECISIONS |
|
|
|
|
Commission |
|
|
|
|
2007/226/EC |
|
|
|
* |
|
|
|
Corrigenda |
|
|
|
* |
||
|
|
* |
|
|
|
|
|
(1) Text with EEA relevance |
|
EN |
Acts whose titles are printed in light type are those relating to day-to-day management of agricultural matters, and are generally valid for a limited period. The titles of all other Acts are printed in bold type and preceded by an asterisk. |
I Acts adopted under the EC Treaty/Euratom Treaty whose publication is obligatory
REGULATIONS
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/1 |
COMMISSION REGULATION (EC) No 387/2007
of 11 April 2007
establishing the standard import values for determining the entry price of certain fruit and vegetables
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Commission Regulation (EC) No 3223/94 of 21 December 1994 on detailed rules for the application of the import arrangements for fruit and vegetables (1), and in particular Article 4(1) thereof,
Whereas:
|
(1) |
Regulation (EC) No 3223/94 lays down, pursuant to the outcome of the Uruguay Round multilateral trade negotiations, the criteria whereby the Commission fixes the standard values for imports from third countries, in respect of the products and periods stipulated in the Annex thereto. |
|
(2) |
In compliance with the above criteria, the standard import values must be fixed at the levels set out in the Annex to this Regulation, |
HAS ADOPTED THIS REGULATION:
Article 1
The standard import values referred to in Article 4 of Regulation (EC) No 3223/94 shall be fixed as indicated in the Annex hereto.
Article 2
This Regulation shall enter into force on 12 April 2007.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 11 April 2007.
For the Commission
Jean-Luc DEMARTY
Director-General for Agriculture and Rural Development
(1) OJ L 337, 24.12.1994, p. 66. Regulation as last amended by Regulation (EC) No 386/2005 (OJ L 62, 9.3.2005, p. 3).
ANNEX
to Commission Regulation of 11 April 2007 establishing the standard import values for determining the entry price of certain fruit and vegetables
|
(EUR/100 kg) |
||
|
CN code |
Third country code (1) |
Standard import value |
|
0702 00 00 |
MA |
104,8 |
|
TN |
143,7 |
|
|
TR |
158,1 |
|
|
ZZ |
135,5 |
|
|
0707 00 05 |
JO |
171,8 |
|
TR |
67,0 |
|
|
ZZ |
119,4 |
|
|
0709 90 70 |
MA |
69,8 |
|
TR |
77,1 |
|
|
ZZ |
73,5 |
|
|
0709 90 80 |
IL |
84,1 |
|
ZZ |
84,1 |
|
|
0805 10 20 |
EG |
48,8 |
|
IL |
51,4 |
|
|
MA |
45,1 |
|
|
TN |
55,3 |
|
|
TR |
74,9 |
|
|
ZZ |
55,1 |
|
|
0805 50 10 |
IL |
65,6 |
|
TR |
68,4 |
|
|
ZZ |
67,0 |
|
|
0808 10 80 |
AR |
85,0 |
|
BR |
80,2 |
|
|
CA |
124,4 |
|
|
CL |
89,4 |
|
|
CN |
82,4 |
|
|
NZ |
122,4 |
|
|
US |
118,1 |
|
|
UY |
68,4 |
|
|
ZA |
94,3 |
|
|
ZZ |
96,1 |
|
|
0808 20 50 |
AR |
78,7 |
|
CL |
104,4 |
|
|
ZA |
87,1 |
|
|
ZZ |
90,1 |
|
(1) Country nomenclature as fixed by Commission Regulation (EC) No 1833/2006 (OJ L 354, 14.12.2006, p. 19). Code ‘ZZ’ stands for ‘of other origin’.
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/3 |
COMMISSION REGULATION (EC) No 388/2007
of 11 April 2007
amending Regulation (EC) No 1622/2000 laying down certain detailed rules for implementing Regulation (EC) No 1493/1999 on the common organisation of the market in wine and establishing a Community code of oenological practices and processes
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Regulation (EC) No 1493/1999 of 17 May 1999 on the common organisation of the market in wine (1), and in particular Article 46(1) thereof,
Whereas:
|
(1) |
Under Point A(3) of Annex V to Regulation (EC) No 1493/1999, the maximum permissible total sulphur dioxide levels of wine may be increased where climatic conditions have made this necessary. |
|
(2) |
Commission Regulation (EC) No 1622/2000 (2) lays down certain detailed rules for implementing Regulation (EC) No 1493/1999 as regards the maximum permissible total sulphur dioxide content of wine in particular. Under Article 19(4) thereof, Annex XIIa of that Regulation lists the cases where the Member States may authorise an increase in the maximum total sulphur dioxide content of wine of less than 300 milligrams per litre by a maximum of 40 milligrams per litre because of weather conditions. |
|
(3) |
By letter of 12 January 2007, the German Government requested authorisation to increase the maximum permissible total sulphur dioxide content of wine of less than 300 milligrams per litre by a maximum of 40 milligrams per litre for wine produced in Baden-Württemberg, Bavaria, Hessen and Rhineland-Palatinate from the 2006 grape harvest in the wake of exceptionally unfavourable weather conditions. That request should be acceded to. |
|
(4) |
The scientific reports provided by the competent German authorities show that the quantities of sulphur dioxide needed to ensure the proper vinification and proper preservation of the wines and that they are suitable for placing on the market should be increased above the level normally authorised. This temporary measure is the only available option to allow the grapes affected by these unfavourable weather conditions to be used to produce wine suitable for placing on the market. |
|
(5) |
Regulation (EC) No 1622/2000 should therefore be amended accordingly. |
|
(6) |
The measures provided for in this Regulation are in accordance with the opinion of the Management Committee for Wine, |
HAS ADOPTED THIS REGULATION:
Article 1
Annex XIIa to Regulation (EC) No 1622/2000 is hereby replaced by the text in the Annex to this Regulation.
Article 2
This Regulation shall enter into force on the third day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 11 April 2007.
For the Commission
Mariann FISCHER BOEL
Member of the Commission
(1) OJ L 179, 14.7.1999, p. 1. Regulation as last amended by Regulation (EC) No 1791/2006 (OJ L 363, 20.12.2006, p. 1).
(2) OJ L 194, 31.7.2000, p. 1. Regulation as last amended by Regulation (EC) No 2030/2006 (OJ L 414, 30.12.2006, p. 40).
ANNEX
‘ANNEX XIIa
Increase in the maximum total sulphur-dioxide content where the weather conditions make this necessary
(Article 19 of this Regulation)
|
|
Year |
Member State |
Wine-growing zone(s) |
Wines concerned |
|
1. |
2000 |
Germany |
All wine-growing zones of Germany |
All wines obtained from grapes harvested in 2000 |
|
2. |
2006 |
Germany |
The wine-growing zones in the regions of Baden-Württemberg, Bavaria, Hessen and Rhineland-Palatinate |
All wines obtained from grapes harvested in 2006’ |
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/5 |
COMMISSION REGULATION (EC) No 389/2007
of 11 April 2007
amending Regulation (EC) No 1622/2000 laying down certain detailed rules for implementing Regulation (EC) No 1493/1999 on the common organisation of the market in wine and establishing a Community code of oenological practices and processes
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Regulation (EC) No 1493/1999 of 17 May 1999 on the common organisation of the market in wine (1), and in particular Article 46(1)(b) thereof,
Whereas:
|
(1) |
Commission Regulation (EC) No 1622/2000 (2) lays down certain conditions for the use of substances authorised by Regulation (EC) No 1493/1999. In particular, Annex IXa provides that dimethyldicarbonate must be added prior to bottling. The translation of the term ‘bottling’ and its different meanings in certain languages have led to diverging interpretations of the scope of this provision by operators and control authorities. |
|
(2) |
Article 7 of Commission Regulation (EC) No 753/2002 of 29 April 2002 laying down certain rules for applying Council Regulation (EC) No 1493/1999 as regards the description, designation, presentation and protection of certain wine sector products (3) defines the term ‘bottling’ for its application. |
|
(3) |
To ensure a uniform interpretation of the requirements applicable to the use of dimethyldicarbonate, the definition of the term ‘bottling’ provided in Regulation (EC) No 753/2002 should be used to clarify the requirements contained in Regulation (EC) No 1622/2000. Annex IXa to Regulation (EC) No 1622/2000 should therefore be amended accordingly. |
|
(4) |
The measures provided for in this Regulation are in accordance with the opinion of the Management Committee for Wine, |
HAS ADOPTED THIS REGULATION:
Article 1
The first indent of the second paragraph of Annex IXa to Regulation (EC) No 1622/2000 is replaced by the following:
|
‘— |
Addition must be carried out only a short time prior to bottling, defined as putting the product concerned up for commercial purposes in containers of a capacity not exceeding 60 litres.’ |
Article 2
This Regulation shall enter into force on the third day following its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 11 April 2007.
For the Commission
Mariann FISCHER BOEL
Member of the Commission
(1) OJ L 179, 14.7.1999, p. 1. Regulation as last amended by Regulation (EC) No 1791/2006 (OJ L 363, 20.12.2006, p. 1).
(2) OJ L 194, 31.7.2000, p. 1. Regulation as last amended by Regulation (EC) No 2030/2005 (OJ L 414, 30.12.2006, p. 40).
(3) OJ L 118, 4.5.2002, p. 1. Regulation as last amended by Regulation (EC) No 2016/2006 (OJ L 384, 29.12.2006, p. 38).
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/6 |
COMMISSION REGULATION (EC) No 390/2007
of 11 April 2007
imposing a provisional anti-dumping duty on imports of peroxosulphates (persulphates) originating in the United States of America, the People’s Republic of China and Taiwan
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Regulation (EC) No 384/96 of 22 December 1995 on protection against dumped imports from countries not members of the European Community (1) (the basic Regulation), and in particular Article 7 thereof,
Whereas:
A. PROCEDURE
1. Initiation
|
(1) |
On 31 May 2006, the Commission received a complaint concerning peroxosulphates (persulphates) originating in the United States of America (USA), the People's Republic of China (PRC) and Taiwan lodged pursuant to Article 5 of the basic Regulation by CEFIC (the complainant) on behalf of producers representing 100 % of the total Community production of persulphates. |
|
(2) |
This complaint contained evidence of dumping and of material injury resulting therefrom, which was considered sufficient to justify the opening of a proceeding. |
|
(3) |
On 13 July 2006, the proceeding was initiated by the publication of a notice of initiation in the Official Journal of the European Union (2). |
2. Parties concerned by the proceeding
|
(4) |
The Commission officially advised the complainant Community producers, the exporting producers in the USA, the PRC and Taiwan, importers, traders, users, suppliers and associations known to be concerned, and the representatives of the USA, the PRC and Taiwan of the initiation of the proceeding. Interested parties were given the opportunity to make their views known in writing and to request a hearing within the time limit set in the notice of initiation. |
|
(5) |
In order to allow exporting producers in the PRC to submit a claim for market economy treatment (MET) or individual treatment (IT), if they so wished, the Commission sent claim forms to the Chinese exporting producers known to be concerned and to other Chinese exporting producers that made themselves known within the deadlines set out in the notice of initiation. Six exporting producers together with their related sales companies, where appropriate, requested MET pursuant to Article 2(7) of the basic Regulation, or IT should the investigation establish that they did not meet the conditions for MET. |
|
(6) |
In view of the apparent high number of exporting producers in the PRC the Commission indicated in the notice of initiation that sampling might be applied in this investigation for the determination of dumping and injury in accordance with Article 17 of the basic Regulation. |
|
(7) |
In order to enable the Commission to decide whether sampling would be necessary and, if so, to select a sample, all exporting producers in the PRC were asked to make themselves known to the Commission and to provide, as specified in the notice of initiation, basic information on their activities related to the product concerned during the calendar year 2005. |
|
(8) |
Given that only six exporting producers cooperated in the investigation, it was decided that sampling was not required. |
|
(9) |
Questionnaires were sent to all parties known to be concerned and to all other companies that made themselves known within the deadlines set out in the notice of initiation. Replies were received from six exporting producers in the PRC, two in the USA and one in Taiwan, and one producer in the analogue country, Turkey. Full questionnaire replies were also received from two Community producers and two importers cooperated by submitting a questionnaire reply. Furthermore, none of the users replied to the questionnaire and no other users supplied the Commission with any information or made themselves known in the course of this investigation. |
|
(10) |
The Commission sought and verified all the information deemed necessary for a provisional determination of dumping, resulting injury and Community interest and carried out verifications at the premises of the following companies:
|
|
(11) |
In view of the need to establish a normal value for exporting producers to which MET might not be granted, a verification to establish normal value on the basis of data from an analogue country, Turkey in this case, took place at the premises of the following company: Producer in Turkey
|
3. Investigation period and period considered
|
(12) |
The investigation of dumping and injury covered the period from 1 July 2005 to 30 June 2006 (investigation period or IP). The examination of the trends relevant for the assessment of injury covered the period from 1 January 2003 to the end of the investigation period (period considered). |
B. PRODUCT CONCERNED AND LIKE PRODUCT
1. Product concerned
|
(13) |
The product concerned is peroxosulphates (persulphates) originating in the USA, the PRC and Taiwan (the product concerned). The product concerned is normally declared within CN codes 2833 40 00 and ex 2842 90 80 (CN codes since 1 January 2007). |
|
(14) |
Persulphates are white, crystalline, odourless salts, which comprise four main types: ammonium persulphate (NH4)2S2O8, sodium persulphate (Na2S2O8), potassium persulphate (K2S2O8) and potassium monopersulphate (2KHSO5 * KHSO4 * K2SO4). |
|
(15) |
The product concerned is used as an initiator or as an oxidising agent in a number of applications. Some examples include its use as a polymerisation initiator in the production of polymers, as an etching agent in the production of printed circuit boards, in hair cosmetics, in textile desizing, in paper manufacturing, as a denture cleanser and as a disinfectant. |
|
(16) |
One US exporting producer claimed that potassium monopersulphate (KMPS) should not be considered part of the same product on the grounds that it has a different chemical composition and structure, different end-uses and different customers. It was also claimed that KMPS would be sold at different price levels from the other product types. |
|
(17) |
The investigation has however shown that, despite differences in chemical formula, and partially distinct uses, the different types of the product concerned all share the same basic chemical and technical characteristics and can be used for the same basic purposes. It is acknowledged that not all types are used for all applications, but they were all found to be interchangeable for at least some significant applications. As far as the different price levels are concerned, it is recalled that this alone is not determinant as to whether several product types constitute one single product. As it has been found that all four types had similar characteristics and common end-uses this argument had to be rejected. Consequently, all four types are provisionally considered to constitute a single product for the purpose of this proceeding. |
2. Like product
|
(18) |
The investigation showed that the basic physical and technical characteristics of the persulphates produced and sold by the Community industry in the Community, persulphates produced and sold on the domestic markets of the USA, the PRC and Taiwan and persulphates imported into the Community from these countries, as well as those produced and sold in Turkey, the analogue country, are the same and that these products have the same uses. |
|
(19) |
It is therefore provisionally concluded that these products are alike within the meaning of Article 1(4) of the basic Regulation. |
C. DUMPING
1. General methodology
|
(20) |
The general methodology set out below has been applied to all cooperating exporting producers in the USA and Taiwan, as well as for the cooperating Chinese exporting producers for which MET was granted. The presentation of the findings on dumping for each of the countries concerned therefore only describes matters specific to each exporting country. |
1.1. Normal value
|
(21) |
In accordance with Article 2(2) of the basic Regulation, the Commission first examined for each exporting producer whether the domestic sales of the product concerned to independent customers were representative, i.e. whether the total volume of such sales was equal to or greater than 5 % of the total volume of the corresponding export sales to the Community. |
|
(22) |
The Commission subsequently identified those product types sold domestically by the companies having overall representative domestic sales, which were identical or directly comparable with the types sold for export to the Community. |
|
(23) |
Domestic sales of a particular product type were considered as sufficiently representative when the volume of that product type sold on the domestic market to independent customers during the investigation period represented 5 % or more of the total volume of the comparable product type sold for export to the Community. |
|
(24) |
The Commission subsequently examined whether the domestic sales of each type of persulphates sold domestically in representative quantities by each company in each exporting country could be considered as being made in the ordinary course of trade pursuant to Article 2(4) of the basic Regulation. This was done by establishing the proportion of profitable domestic sales to independent customers, of each exported product type, on the domestic market during the investigation period, as follows: |
|
(25) |
Where the sales volume of a product type, sold at a net sales price equal to or above the calculated cost of production, represented more than 80 % of the total sales volume of that type, and where the weighted average price of that type was equal to or above the cost of production, normal value was based on the actual domestic price. This price was calculated as a weighted average of the prices of all domestic sales of that type made during the IP, irrespective of whether these sales were profitable or not. |
|
(26) |
Where the volume of profitable sales of a product type represented 80 % or less of the total sales volume of that type, or where the weighted average price of that type was below the cost of production, normal value was based on the actual domestic price, calculated as a weighted average of profitable sales of that type only, provided that these sales represented 10 % or more of the total sales volume of that type. |
|
(27) |
Where the volume of profitable sales of any product type represented less than 10 % of the total sales volume of that type, it was considered that this particular type was sold in insufficient quantities for the domestic price to provide an appropriate basis for the establishment of the normal value. |
|
(28) |
Wherever domestic prices of a particular product type sold by an exporting producer could not be used in order to establish normal value, another method had to be applied. In accordance with Article 2(3) of the basic Regulation the Commission instead calculated a constructed normal value, as follows. |
|
(29) |
Normal value was constructed by adding to each exporter’s manufacturing costs of the exported types, adjusted where necessary, a reasonable amount for selling, general and administrative expenses (SG&A) and a reasonable margin of profit. |
|
(30) |
In all cases SG&A and profit were established pursuant to the methods set out in Article 2(6) of the basic Regulation. To this end, the Commission examined whether the SG&A incurred and the profit realised by each of the exporting producers concerned on the domestic market constituted reliable data. |
1.2. Export price
|
(31) |
In all cases where the product concerned was exported to independent customers in the Community, the export price was established in accordance with Article 2(8) of the basic Regulation, namely on the basis of export prices actually paid or payable. |
|
(32) |
Where the export sale was made via related importers based in the Community, the export price was constructed, pursuant to Article 2(9) of the basic Regulation, on the basis of the price at which the imported products were first resold to an independent buyer, duly adjusted for all costs incurred between importation and resale, as well as a reasonable margin for SG&A and profits. In this regard, the related importers’ own SG&A costs were used. The profit margin was established on the basis of the information available from cooperating unrelated importers. |
|
(33) |
Where the export sale was made via a related trader located outside the Community, the export price was established on the basis of the first resale price to independent customers in the Community. |
1.3. Comparison
|
(34) |
The comparison between normal value and export price was made on an ex-factory basis. |
|
(35) |
For the purpose of ensuring a fair comparison between the normal value and the export price, due allowance in the form of adjustments was made for differences affecting prices and price comparability in accordance with Article 2(10) of the basic Regulation. |
1.4. Dumping margins
|
(36) |
Pursuant to Articles 2(11) and (12) of the basic Regulation dumping margins were established on the basis of a comparison of a weighted average normal value by product type with a weighted average export price by product type as established above. |
|
(37) |
In order to determine the dumping margin for non-cooperating exporting producers, the level of non-cooperation was first established. To this end, the volume of exports to the Community reported by the cooperating exporting producers was compared with the equivalent Eurostat import statistics. |
|
(38) |
As the level of cooperation in the USA and Taiwan was high (effectively 100 %), and since there was no reason to believe that any exporting producer in these countries deliberately abstained from cooperating, it was considered appropriate to set the residual dumping margin for any non-cooperating exporting producers in each of the countries concerned at the level of the highest margin imposed on a cooperating exporter. |
|
(39) |
As regards the PRC, the level of cooperation assessed at the level of the country was also very high (above 85 %). The specific methodology applied to determine the countrywide dumping margin for the PRC is explained below. |
2. USA
2.1. Normal value
|
(40) |
For both cooperating exporting producers, the total volume of domestic sales of the like product was representative as defined in recital 21 above. For all product types normal value was thus based on prices paid or payable, in the ordinary course of trade, by independent customers in the USA, as explained in recitals 25 and 26, since these sales represented in all cases 10 % or more of the total sales volume of that type. |
2.2. Export price
|
(41) |
The exports of the one of the cooperating exporting producers were made directly to independent customers in the Community. The export price was therefore based, for that exporter, on the prices actually paid or payable for the product concerned in accordance with Article 2(8) of the basic Regulation. |
|
(42) |
For the other exporter, all its sales to the Community are through a related trading company in Switzerland. The export price was therefore established as described in recital 33. |
|
(43) |
Furthermore, a significant part of the export sales of this exporting producer in the USA to the Community via its related company in Switzerland were to a related company which used the product concerned as a raw material to produce another product which is not considered to be a like product within the meaning of Article 1(4) of the basic Regulation. |
|
(44) |
Since the prices set for the transactions between the exporting producer and its related company in the Community via their related trading company in Switzerland was found to have been affected by the relationship between the three companies, these prices could not be relied upon to determine an export price for those transactions. |
|
(45) |
Since furthermore no export price could be constructed based on the resale price of the related company to independent customers, as the product concerned goes under substantial transformation to get the final product manufactured by the related company, all those transactions corresponding to captive use were ignored in the determination of the export price. |
2.3. Comparison
|
(46) |
The normal value and export prices were compared on an ex-works basis, as described above, with adjustments, where appropriate, in accordance with Article 2(10) of the basic Regulation. Adjustments were made for discounts, rebates, transport, insurance, handling, loading and ancillary costs, packing, credit and import duties. |
|
(47) |
For the sales channelled by one exporting producer through its related trader in Switzerland, an adjustment was made in accordance with Article 2(10)(i) of the basic Regulation. The adjustment was based on the markup received by the related trader in Switzerland, but in this calculation the actual profit of the related trader could not be used since the relationship between the exporting producer and the related trader significantly affected transfer prices. The markup was thus calculated as the sum of the SG&A expenses borne by the related trading company during the IP and a reasonable margin for profit, which was set at 5 % at this stage, in the absence of any meaningful information from cooperating unrelated companies assuming comparable functions. |
|
(48) |
One exporting producer in the USA claimed a level of trade adjustment under Article 2(10)(d) of the basic Regulation on the grounds that certain of its domestic sales were allegedly not comparable to its export sales due to the existence of categories of customers on the domestic market for which different functions are assumed by the exporting producer. However, the investigation established that this claim was unwarranted, insofar as the company did not provide evidence in support of the alleged differences in functions. Moreover, the alleged differences in prices between the categories claimed were not found consistent across product types. Therefore, the claim was rejected. |
2.4. Dumping margins
|
(49) |
Since the level of cooperation was high, and there was no reason to believe that any exporting producer deliberately abstained from cooperation, the residual margin applicable to all other exporters in the USA was set at the same level as the one established for the cooperating exporting producer with the highest dumping margin. |
|
(50) |
The dumping margins, expressed as a percentage of the cif import price at the Community border, duty unpaid, are provisionally the following:
|
3. China
3.1. Market economy treatment (MET)
|
(51) |
Pursuant to Article 2(7)(b) of the basic Regulation, in anti-dumping investigations concerning imports originating in the PRC, normal value shall be determined in accordance with paragraphs 1 to 6 of the said Article for those producers which are found to meet the criteria laid down in Article 2(7)(c) of the basic Regulation. |
|
(52) |
Briefly, and for ease of reference only, the MET criteria are set out in summarised form below:
|
|
(53) |
Six exporting producers requested MET pursuant to Article 2(7)(b) of the basic Regulation and replied to the MET claim form for exporting producers within the given deadline. The Commission sought and verified at the premises of these companies all information submitted in the MET applications as deemed necessary. The investigation revealed that the MET could only be granted to three exporting producers, whereas the claim had to be rejected for the other three exporting producers. It is noted that for one of the three exporting producers granted MET, this decision is subject to further examination of late information that could not be fully investigated at this stage, as described below. This information, if confirmed by the further investigation, could lead to a significant change in the factual situation on the basis of which MET was granted to that company, insofar as it may affect the fulfilment of criterion 1. The following table summarises the determination for the three companies for which MET was not granted against each of the five above-mentioned criteria:
|
|||||||||||||||||||||||||||||
|
(54) |
The investigation showed for companies 1, 2 and 3 above that they did not meet the requirements of the aforementioned criteria 1, 2 and 3. |
|
(55) |
Namely, all three companies could not demonstrate that their business decisions were taken in response to market signals, without significant State interference. |
|
(56) |
Indeed for company 1 it was found that the majority of the Directors on the Board, including the Chairman, who owns a significant share in the company, were the same as before privatisation and had been appointed by the State. They were also found to be members of the Communist Party. Moreover, the company was unable to prove payment for the shares during the privatisation process. In company 2, which was founded as a State-owned enterprise and privatised in 2000, the investigation showed that three members of the management staff that were in post prior to the privatisation conducted the privatisation and still had control over the main decision-making bodies of the company. Those three persons were found to be members of the Communist Party. Furthermore, company 2 was found to have provided false information with regard to the ownership of its shares and the privatisation process, thereby hiding significant State interference. As to company 3, there were strong indications that capital used to start the company was obtained from the village-owned and collectively-owned enterprises managed by the current Chairman of the company and the company was not able to explain and demonstrate the origin of the capital. |
|
(57) |
Moreover, all three companies’ accounts were not kept in line with IAS, and signs of serious negligence in the audit of those accounts were found. |
|
(58) |
Finally, distortions carried over from the non-market economy system were found to affect the costs for both companies, in particular with regard to cost of the land-use rights acquired (companies 1 and 3) or assets transferred during the privatisation (company 2). |
|
(59) |
With regard to the three other cooperating exporting producers it was initially concluded that the companies met all five criteria. |
|
(60) |
However, after the disclosure to the interested parties, which were given an opportunity to comment on the above findings, the Community industry claimed that two of the three exporting producers for which it was proposed to grant MET should be denied MET (hereunder referred to as company 4 and company 5). |
|
(61) |
They alleged for company 4 that there was State interference in the management as well as in the financing of the company. |
|
(62) |
For company 5 the Community industry stated that they had understated their number of employees, and queried the payment of the original capital. They also queried the losses made by a related trading company. |
|
(63) |
With regard to company 4, it was not possible to conclude at this stage whether the allegations constituted sufficient grounds to deny MET. Whereas the allegations are considered to be serious, there is at present uncertainty about the facts underlying these allegations. Further analyses of the information submitted by the company as well as additional investigations are necessary before a final decision is reached. In these particular circumstances, in order not to impede the rights of defence of interested parties, it was therefore considered appropriate to grant MET to company 4 at this stage and to continue the investigation regarding its MET claim. |
|
(64) |
As regards the allegations made against company 5, these were merely assumptions. The Commission verified the information submitted by this exporting producer and concluded that the allegations were not substantiated. The Commission's findings as regards this company are thus maintained. |
|
(65) |
Following disclosure of the Commission's findings, all three companies denied MET submitted that the determination was incorrect, that they met all five MET criteria and that they should be granted MET as a consequence. |
|
(66) |
In particular, company 1 contended that it had submitted the proof of the payment in cash for the shares to the Commission and denied any State influence on its decision-making process. It also argued that its accounts were kept in line with IAS and that the cost of the land-use right was in line with market values. |
|
(67) |
Company 2 contested that membership in the Communist Party of its top management should lead to the conclusion that there is State interference in the company's decision process, and said that it submitted supporting evidence for the payment of the shares during its privatisation process. The company further argued that, despite breaching some of the IAS, its accounts were in line with the Chinese Accounting Standards. |
|
(68) |
Company 3 stated that the capital used to start the company was sourced from other companies owned by the same shareholder, that the accounts were in line with IAS, and that their land-use right prices were similar to other companies in the same area and at market prices. |
|
(69) |
Those comments were taken into consideration by the Commission. However they did not change the finding that these three companies should be denied MET. |
|
(70) |
Indeed, company 1 merely alleged that bank statements were not commonly used in China, but it could not give any supporting evidence that the shares for its privatisation were effectively paid for. The company did not either contest the fact that the management structure of the company was the same as prior to privatisation nor that the Chairman was a member of the Communist Party. The fact that the main shareholders were not represented in the Board of Directors was also not contested. Therefore it was found that the company had not sufficiently demonstrated that it was not subject to significant State interference. |
|
(71) |
Furthermore, no new elements were provided to reject the findings and support the claim that accounts complied with IAS and that land-use rights costs were within market conditions. |
|
(72) |
Company 2 disputed the conclusion reached by the Commission but did not deny the facts on which they were based. Concerning the privatisation process and the payment of the shares, the company argued that it provided supporting evidence but did not address the fact that these documents were false, as admitted by the General Manager during the verification visit. The company also confirmed that the key management positions were occupied by members of the Communist Party. |
|
(73) |
Moreover, no new elements were provided to support the claim that accounts complied with IAS, the company's statement only being that they were in line with the Chinese Accounting Standards. |
|
(74) |
For company 3, the Commission continued to doubt the origin of the capital of the company. Indeed, company 3 merely stated that the capital was sourced from related companies belonging to the Chairman of company 3 through loans which were repaid within a few months. This new information was not only found to contradict the statements made by representatives of company 3 during the verification at the premises of the company, where no documented evidence was made available for inspection, but is also clearly deficient as it does not provide any indication of the origin of the funds used to repay those loans. |
|
(75) |
The company furthermore restated that its accounts were kept in line with IAS and that the discrepancies identified during the investigation were in fact reconcilable. However, it provided only partial information to document the alleged reconciliation, accounting merely for a minor part of the differences found. In any case, this new information was submitted only after the investigation and at such a late stage that it would be impractical to verify it. Furthermore, the company also did not demonstrate that their low land-use right price was actually a market price. |
|
(76) |
On this basis, MET is granted to three exporting producers:
|
3.2. Individual treatment (‘IT’)
|
(77) |
Pursuant to Article 2(7)(a) of the basic Regulation, a countrywide duty, if any, is to be established for countries falling under that Article, except in those cases where companies are able to demonstrate that they meet all criteria set out in Article 9(5) of the basic Regulation and are therefore granted individual treatment (‘IT’). |
|
(78) |
The exporting producers to which MET could not be granted also claimed IT in the event that they were not granted MET. However, the companies’ claim for individual treatment was also rejected as they failed to meet the criteria set out in Article 9(5)(b), namely that export prices and quantities were freely determined and that State interference would not be such as to permit circumvention of measures if individual exporters are given different rates of duty. |
3.3. Normal value
(a) Determination of normal value for the exporting producers granted MET
|
(79) |
For the three cooperating exporting producers granted MET, the total volume of domestic sales of the like product was representative as defined in recital 21 above. For some of the product types normal value was based on prices paid or payable, in the ordinary course of trade, by independent customers in the PRC, as explained in recitals 25 and 26, and for the product types for which the domestic sales were insufficient to be considered representative or they were not made in the ordinary course of trade, normal value was constructed as described in recitals 27 to 30. |
|
(80) |
In the cases where the normal value had to be constructed, the margins for SG&A expenses and profit referred to in recitals 29 and 30 were based on the actual SG&A and profit made by the exporting producer for its domestic sales, in the ordinary course of trade, of the like product on the domestic market, in accordance with the first sub-paragraph of Article 2(6) of the basic Regulation. |
(b) Determination of normal value for the exporting producers not granted MET
(i) Analogue country
|
(81) |
According to Article 2(7)(a) of the basic Regulation, normal value for the exporting producers not granted MET has to be established on the basis of the domestic prices or constructed normal value in an analogue country. |
|
(82) |
In the notice of initiation, the Commission indicated its intention to use Japan as an appropriate analogue country for the purpose of establishing normal value and interested parties were invited to comment on this. However, no comment was received. |
|
(83) |
All known producers of persulphates in Japan were contacted, but none agreed to cooperate. |
|
(84) |
The Commission then contacted and sent questionnaires to all known producers of persulphates in the other countries where the Commission was aware that such producers existed, namely India and Turkey. No response was received from the Indian companies contacted but one producer in Turkey replied to the questionnaire. |
|
(85) |
The Commission then examined whether Turkey was a reasonable choice of analogue country. It was concluded that Turkey, despite having only one producer of the product concerned, was an open market with a low import duty and significant imports from third countries. Furthermore, the investigation showed no reason, such as excessively high cost of raw materials or energy, to consider that Turkey was not adequate for the purpose of establishing normal value. |
|
(86) |
The data submitted in the cooperating Turkish producer's reply was verified on the spot and was found to be reliable information on which a normal value could be based. |
|
(87) |
It is therefore provisionally concluded that Turkey is an appropriate and reasonable analogue country in accordance with Article 2(7) of the basic Regulation. |
(ii) Normal value
|
(88) |
Pursuant to Article 2(7)(a) of the basic Regulation, normal value for the exporting producers not granted MET was established on the basis of verified information received from the producer in the analogue country, i.e. on the basis of prices paid or payable on the Turkish market for comparable product types, in accordance with the methodology set out above. |
|
(89) |
Since sales on the domestic market to unrelated customers were representative and overall profitable, normal value was established on the basis of all prices paid or payable on the Turkish market for comparable product types, for sales in the ordinary course of trade as described in recitals 25 and 26. |
3.4. Export prices
|
(90) |
All cooperating exporting producers made export sales to the Community either directly to independent customers in the Community; or through related or unrelated companies located in the PRC, in Hong Kong or in the Community. |
|
(91) |
Where the product concerned was directly exported to independent customers in the Community, the export prices were based on the prices actually paid or payable for the product concerned, in accordance with Article 2(8) of the basic Regulation. |
|
(92) |
Where sales were made via a related company in the Community the export price was established in accordance with Article 2(9) of the basic Regulation. Where sales were made via a related company outside the Community the export price was established on the basis of the method set out in recital 33. |
3.5. Comparison
|
(93) |
Adjustments were made, where appropriate, in accordance with Article 2(10) of the basic Regulation, in respect of transport, insurance, handling and ancillary costs, packing, credit, bank charges and commissions were granted in all cases where they were found to be reasonable, accurate and supported by verified evidence. |
|
(94) |
For the sales channelled through related trading companies, an adjustment was applied in accordance with Article 2(10)(i) of the basic Regulation, where these companies have been shown to perform functions similar to those of an agent working on a commission basis. In this respect, the allocation of SG&A expenditure provided by the related company was found to be reliable and this adjustment was based on that amount of SG&A expenses plus a profit margin of 5 %, being a reasonable profit margin in the absence of any meaningful data from cooperating unrelated importers or traders in the Community. |
3.6. Dumping margins
(a) For the cooperating exporting producers granted MET
|
(95) |
The comparison between the normal value and the export price showed that for two of the three exporters concerned the dumping margin was below 2 %, i.e. de minimis. The dumping margins expressed as a percentage of the cif import price at the Community border, duty unpaid, are thus provisionally the following:
|
(b) For all other exporting producers
|
(96) |
In order to calculate the countrywide dumping margin applicable to all other exporters in the PRC, since the level of cooperation found was high as explained above a comparison was made, on an ex-works basis, between the weighted average export price of the three cooperating exporters that were not granted MET and the normal value calculated based on the data in the analogue country. |
|
(97) |
On this basis the countrywide level of dumping was provisionally established at 102,7 % of the Cif Community frontier price, duty unpaid. |
4. Taiwan
4.1. Normal value
|
(98) |
For the only cooperating exporting producer, the total volume of domestic sales of the like product was representative as defined in recital 21 above. For all product types normal value was thus based on prices paid or payable, in the ordinary course of trade, by independent customers in Taiwan, as explained in recitals 25 and 26, since these sales represented in all cases 10 % or more of the total sales volume of that type. |
4.2. Export price
|
(99) |
The exports of the only cooperating exporting producer were made directly to independent customers in the Community. The export price was therefore based on the prices actually paid or payable for the product concerned in accordance with Article 2(8) of the basic Regulation. |
4.3. Comparison
|
(100) |
The normal value and export prices were compared on an ex-works basis, as described above, with adjustments, where appropriate, in accordance with Article 2(10) of the basic Regulation. Adjustments were made for rebates, transport, insurance, handling, loading and ancillary costs, packing and credit costs. |
|
(101) |
The cooperating exporting producer claimed a level of trade adjustment on the grounds that certain of its domestic sales were allegedly made at a level of trade not comparable with its export sales. In the course of the investigation, it was found that the level of trade declared for one of the main domestic customers was erroneous, which cast serious doubts on the reliability of the classification of customers submitted to support this level of trade adjustment. In any case, the alleged differences in prices between the categories claimed were not found consistent across product types during the IP and the claim had therefore to be rejected. |
|
(102) |
The cooperating exporting producer claimed an adjustment for differences in commissions paid, referring in particular to commissions paid to an agent in Taiwan for sales on the Taiwanese market. However, it was found during the investigation that, based on the information submitted by the exporting producer, the agent was performing similar functions to the ones exerted by the sales department of the exporting producer for export sales. Whereas fees were found to have been effectively paid to that agent, they were thus not found to affect the price comparability of domestic prices and export prices. The claim was therefore rejected, since the conditions set out in the first sub-paragraph of Article 2(10) of the basic Regulation were not met. |
|
(103) |
Furthermore, the exporting producer claimed an adjustment for transport costs incurred on its domestic market. However, the company could not properly substantiate its claim and the documents submitted were partially misleading. It was consequently informed that in the light of Article 18 of the basic Regulation the provisions on partial cooperation would apply. In the absence of a satisfying explanation from the company with regard to the submitted documents, recourse was made in the dumping calculation to facts available as regards the transport costs on the domestic market. The adjustment was based on the amounts that could be justified by invoices received from freight forwarding agents. |
4.4. Dumping margins
|
(104) |
Since the level of cooperation was high, and there was no reason to believe that any exporting producer deliberately abstained from cooperation, the residual margin applicable to all other exporters in Taiwan was set at the same level as the one established for the cooperating exporting producer. |
|
(105) |
The dumping margins, expressed as a percentage of the cif import price at the Community border, duty unpaid, are provisionally the following:
|
D. INJURY
1. Community production and Community industry
|
(106) |
Within the Community the like product is manufactured by two producers located in Germany, on behalf of which the complaint was lodged and which cooperated in the investigation. During the IP, their output which was in the range 24 000 and 29 000 tonnes represented 100 % of the Community production. Moreover, both producers are deemed to constitute the Community industry within the meaning of Articles 4(1) and 5(4) of the basic Regulation. One exporting producer claimed that one of the Community producers imported the product concerned form China during the IP and should therefore be excluded from the definition of the Community industry. However, and as also mentioned below in recital 151, the import levels were low and were only made to maintain global customers. Since, in addition, the average resale price levels were significantly higher than the Chinese import prices, it was considered that imports of this Community producer were rather an act of self-defence against the dumped imports than self-inflicted injury. Therefore, the two Community producers were considered as being the Community industry within the meaning of Article 4(1)(a) of the basic Regulation. |
2. Community consumption
|
(107) |
Community consumption was established on the basis of the sales volumes on the Community market by the two Community producers, the imports from the countries concerned and from other third countries under the relevant CN codes according to Eurostat and in the case of Taiwan and USA on the basis of actual verified data. As far as the PRC is concerned, the reported import volume by the cooperating Chinese exporting producers did not represent the total imports from the PRC. Therefore, as regards imports from the PRC are concerned, it was considered that in the purpose of determining total Community consumption Eurostat data were the most reliable source of information. |
|
(108) |
As mentioned in recital 13 the product concerned is presently declared within CN codes 2833 40 00 and ex 2842 90 80. The Eurostat data concerning CN code ex 2842 90 80 comprises one particular type of persulphates (monopersulphate) mainly imported from the USA and other products such as salts of inorganic acids or peroxoacids, which are not product concerned. As it was not possible to retrieve from this broader category of products the data for persulphates only, it was considered that import data from Eurostat concerning this particular CN code would not reflect a reliable picture of the situation and should therefore not be used. In any case, and as mentioned above in recital 107, as regards the USA and Taiwan actual verified import data were used. |
|
(109) |
On the basis of these data, it was found that over the period considered, consumption increased by 7 %. Table 1 Consumption in the EU (volume)
|
3. Cumulative assessment of the effects of the imports concerned
|
(110) |
The Commission examined whether imports of persulphates originating in the PRC, Taiwan and the USA should be assessed cumulatively in accordance with Article 3(4) of the basic Regulation. |
|
(111) |
The dumping margin established in relation to the imports of each of the countries concerned was above the de minimis threshold as defined in Article 9(3) of the basic Regulation and the volume of imports from each of these countries was not negligible in the sense of Article 5(7) of the basic Regulation, i.e. their market shares attaining 14,9 %, 5,9 % and 9,3 % respectively in the IP As mentioned in recital 95 for two exporting producers in the PRC the dumping margin established was below the de minimis threshold. Therefore, imports of these companies were not considered. |
|
(112) |
As regards the conditions of competition, the investigation showed, as outlined in recital 18 above that persulphates imported from the countries concerned and that of the Community industry were alike in all their basic physical and technical characteristics. Also, persulphates originating in these countries on the one hand and persulphates produced and sold in the Community on the other hand were sold through comparable sales channels and under similar commercial conditions; thus competing with each other. It was also found that export prices from the PRC, Taiwan and the USA showed a similar trend during the period considered and were significantly undercutting the Community prices. |
|
(113) |
In the light of the above, it is provisionally considered that all the criteria set out in Article 3(4) of the basic Regulation were met and that imports from the countries concerned should be examined cumulatively. |
4. Imports from the countries concerned
4.1. Volume and market share of the imports concerned
|
(114) |
Imports from the countries concerned increased by 43 % between 2003 and the IP. While these imports amounted to 8 778 tonnes in 2003 they reached a level of 12 593 tonnes during the IP. The increase of imports was particularly marked between 2003 and 2004 since they rose by 31 %. Table 2 Imports from the countries concerned
|
|
(115) |
The market share held by the countries concerned increased between 2003 and the IP from 22,6 % to 30,2 % i.e. by 7,6 percentage points. The increase was particularly marked between 2003 and 2004 when it went up by 4,8 percentage points. Table 3 Market share of the countries concerned
|
4.2. Prices
|
(116) |
From 2003 to the IP, prices of the imports from the countries concerned decreased by 12 %. Thus, they decreased from EUR 946/tonne in 2003 to EUR 828/tonne in the IP. Table 4 Prices of the imports concerned
|
4.3. Price undercutting
|
(117) |
For the determination of price undercutting the price data referring to the IP was analysed. The relevant sales prices of the Community industry were net prices after deduction of discounts and rebates. Where necessary, these prices were adjusted to an ex-works level, i.e. excluding freight cost in the Community. The import prices of the countries concerned were also net of discounts and rebates and were adjusted where necessary to Cif Community frontier. |
|
(118) |
The Community industry's sales prices and the import prices of the countries concerned were compared at the same level of trade, namely to independent customers within the Community market. |
|
(119) |
During the IP, the weighted average price undercutting margins, expressed as a percentage of the Community industry's sales prices, was 30,2 % for the Taiwanese exporter, 30,3 % for the PRC, and 7,4 % for the USA. The total weighted average undercutting margin for all countries concerned was 22,7 % during the IP. |
5. Situation of the Community industry
|
(120) |
In accordance with Article 3(5) of the basic Regulation, the examination of the impact of the dumped imports on the Community industry included an evaluation of all economic factors having a bearing on the state of the Community industry during the period considered. For confidentiality reasons, given that the analysis concerns only two companies, most indicators are presented in indexed form or ranges are given. |
5.1. Production, capacity and capacity utilisation
Table 5
Production, capacity and capacity utilisation
|
|
2003 |
2004 |
2005 |
IP |
|
Production in tonnes (ranges) |
29 000-34 000 |
29 000-34 000 |
26 000-31 000 |
24 000-29 000 |
|
Production (index) |
100 |
100 |
90 |
86 |
|
Production capacity in tonnes (ranges) |
37 000-42 000 |
37 000-42 000 |
37 000-42 000 |
37 000-42 000 |
|
Production capacity (index) |
100 |
100 |
100 |
100 |
|
Capacity utilisation |
83 % |
83 % |
75 % |
71 % |
|
(121) |
The production volume of the Community industry showed a clear negative trend between 2003 and the IP. Whereas between 2003 and 2004 the production volume was stable, it suddenly diminished by 10 % in 2005 and this trend continued during the IP. Although the capacity utilisation was above 70 % during the IP, the Community industry's production overall decreased by 14 % during the period considered. |
|
(122) |
The production capacity remained stable between 2003 and the IP. |
5.2. Sales volume, market shares, growth and average unit price in the EC
|
(123) |
The table below shows the Community industry’s performances in relation to its sales to independent customers in the Community. Table 6 Sales volume, market share, prices and average unit prices in the Community
|
|
(124) |
The Community industry’s sales volume decreased gradually by 10 % during the period considered. This has to be seen in the light of an increasing consumption in the EC. |
|
(125) |
Overall, the market share of the Community industry continuously decreased by 11 percentage points between 2003 and the IP. The decrease was particularly pronounced between 2003 and 2004 where 7,6 percentage points were lost. Both the decrease in sales volume and market shares should be seen in the light of the evolution of the Community consumption which rose by 7 % and the increasing imports from the countries concerned during the period considered. During the same period, unit costs of the Community industry increased by 5 %. Indeed, the decrease of production volume when capacities remained stable and resulted in a higher allocation of overhead costs by unit. |
|
(126) |
The Community industry’s unit sales prices also continuously decreased by 8 % during the period considered. This decrease in prices shows that the Community industry was not able to pass on the overall cost increase to their customers. On the contrary, the Community industry had to decrease prices in order not to lose more clients or orders. |
5.3. Stocks
|
(127) |
The figures below represent the volume of stocks at the end of each period. Table 7 Stocks
|
|
(128) |
The investigation revealed that stocks cannot be considered as a meaningful injury factor since the vast majority of production is made in response to orders. Therefore, the trends on stocks are given for information. In any case, the level of stocks remained rather stable overall. It decreased by 17 % between 2003 and 2004, then increased by 41 % until the end of 2005 and then decreased again by 21 % to reach almost the same level as in 2003. |
5.4. Investments and ability to raise capital
Table 8
Investments
|
|
2003 |
2004 |
2005 |
IP |
|
Investments (index) |
100 |
21 |
28 |
55 |
|
(129) |
Between 2003 and the IP, investments for the production of the like product diminished by 45 %. Following a sharp decline of 79 % between 2003 and 2004, it remained at a low level in 2004. During the IP, the value of the investments increased by 27 % but in comparison to 2003, it remained at a low level. During the investigation it was found that investments in buildings, plants and machinery were mainly made to maintain the production capacity. In view of the low capacity utilisation mentioned before, investments were in any case not made with the purpose to increase the overall production capacity. |
|
(130) |
The investigation showed that the financial performances of the Community industry deteriorated but it did not reveal that its ability to raise capital was seriously affected yet during the period considered. |
5.5. Profitability, return on investment and cash flow
Table 9
Profitability, return on investment and cash flow
|
|
2003 |
2004 |
2005 |
IP |
|
Profitability on EC sales (range) |
15-25 % |
10-20 % |
2-11 % |
1-10 % |
|
Profitability on EC sales (index) |
100 |
79 |
26 |
20 |
|
Return on total investments (range) |
30-40 % |
20-30 % |
5-15 % |
1-10 % |
|
Return on total investments (index) |
100 |
77 |
26 |
19 |
|
Cash flow (index) |
100 |
88 |
41 |
28 |
|
(131) |
The decline in sales volume combined with decreasing sales prices between 2003 and the IP, significantly affected the profitability achieved by the Community industry, i.e. it declined by 15,5 percentage points between 2003 and the IP. The negative trend was in particular pronounced between 2004 and 2005, when profitability dropped by more than 10 % points. The return on total investments was calculated by expressing the pre-tax net profit of the like product as a percentage of the net book value of fixed assets allocated to the like product. This indicator followed a similar trend as profitability, decreasing significantly over the period considered, particularly pronounced between 2004 and 2005, where the return on investment dropped by 17 percentage points. With regard to the cash flow generated by the Community industry, a similar negative trend was found, resulting in a dramatic overall deterioration of the Community industry's financial situation in the IP. |
5.6. Employment, productivity and wages
Table 10
Employment, productivity and wages
|
|
2003 |
2004 |
2005 |
IP |
|
Number of employees (index) |
100 |
95 |
89 |
87 |
|
Employment cost (index) |
100 |
93 |
88 |
86 |
|
Average labour costs |
100 |
98 |
99 |
99 |
|
Productivity (index) |
100 |
105 |
101 |
99 |
|
(132) |
The number of personnel employed by the Community industry decreased overall by 13 % partly due to the restructuring process at the beginning of the period considered. Thus, although overall employment costs decreased considerably, average wages remained stable. The decrease in the number of employment was similar to the decrease in production. As a consequence the Community industry was able to maintain the same level of productivity as in 2003. |
5.7. Magnitude of the dumping margin
|
(133) |
Given the volume and the price of the dumped imports, the impact of the actual margins of dumping cannot be considered negligible. |
5.8. Recovery from past dumping
|
(134) |
In December 1995, the Council imposed a definitive anti-dumping duty on peroxodisulphates (persulphates) originating in the PRC (3). These measures were terminated in April 2002 (4). The figures collected during the present investigation suggest that although having recovered from past dumping practices, the situation of the Community industry deteriorated significantly after 2002 when anti-dumping duties were repealed and dumped imports re-entered the Community market. |
5.9. Growth
|
(135) |
The investigation showed that despite an increase in consumption by 7 %, the Community industry lost sales volume (– 10 %) and market share (– 11 percentage points) during the period considered. It thus did not benefit from any growth during the period considered. |
6. Conclusion on injury
|
(136) |
During the period considered, the volume of dumped imports of persulphates from the countries concerned increased significantly, i.e. by 43 % and likewise their market shares increased by 7,6 percentage points to reach 30,2 % of the Community market in the IP. At the same time, prices of these imports decreased significantly, undercutting the Community industry's sales prices on average by 22,7 %. |
|
(137) |
The analysis of the injury indicators revealed that the situation of the Community industry deteriorated significantly over the period considered. All injury indicators follow a negative trend over the period considered. In particular, in order not to lose more market share and to keep the production at a reasonable level, the Community industry had no other option than to follow the price levels set by the dumped imports, i.e. to decrease its prices by 8 % between 2003 and the IP. This resulted in the significant drop in profitability over the period considered. Moreover, the Community industry was not able to pass on any cost increase to their customers and their financial situation deteriorated significantly during the period considered. |
|
(138) |
The decrease of sales volume also implied that the Community industry could not benefit from the increase of demand in the persulphates market. |
|
(139) |
In light of the foregoing, it is provisionally concluded that the Community industry has suffered material injury within the meaning of Article 3(5) of the basic Regulation. |
E. CAUSATION
1. Introduction
|
(140) |
In accordance with Article 3(6) and 3(7) of the basic Regulation, the Commission has examined whether the dumped imports of the product concerned originating in the United States of America, PRC and Taiwan have caused injury to the Community industry to a degree that may be considered as material. Known factors other than the dumped imports, which could at the same time have injured the Community industry, were also examined to ensure that possible injury caused by these other factors was not attributed to the dumped imports. |
2. Effect of the dumped imports
|
(141) |
Imports from the countries concerned increased significantly by 43 % in terms of volume, and by 7,6 percentage points in terms of market share. At the same time the market share of the Community industry decreased by roughly 11 percentage points. The average unit selling price per tonne of the imports from the countries concerned diminished by 12 %, undercutting the average Community industry prices by 22,7 % on average in the IP. The substantial increase in the volume of imports from the countries concerned and their gain in market share during the period considered, at prices which were significantly lower than those of the Community industry, coincided with the evident deterioration of the overall financial situation of the Community industry during the same period. This deterioration is seen, in particular, in terms of sales prices, profitability, return on investment, cash flow and employment. |
|
(142) |
In the analysis of the effect of the dumped imports, it was found that price is an important element of competition because quality issues do not play a significant role. It should be noted that the prices of dumped imports were considerably below both, those of the Community industry as well as those of other third country exporters. |
|
(143) |
It is therefore provisionally concluded that the pressure exerted by the imports concerned, which increased their volume and market share from 2003 onwards, and which were made at very low and dumped prices, played a determining role in the deterioration of the Community industry’s financial situation. |
3. Effect of other factors
(a) Imports originating in third countries other than the PRC, US and Taiwan
Table 11
Imports originating in other third countries (quantity)
|
Imports (tonnes) |
2003 |
2004 |
2005 |
IP |
|
Turkey |
2 161 |
2 327 |
1 198 |
1 247 |
|
Index |
100 |
108 |
55 |
58 |
|
Japan |
146 |
0 |
24 |
10 |
|
Index |
100 |
0 |
16 |
7 |
|
Others |
158 |
260 |
976 |
1 005 |
|
Index |
100 |
165 |
618 |
636 |
|
Total other countries |
2 466 |
2 587 |
2 198 |
2 262 |
|
Index |
100 |
105 |
89 |
92 |
Table 12
Imports originating in other third countries (average price)
|
Average prices (EUR) |
2003 |
2004 |
2005 |
IP |
|
Turkey |
1 022 |
974 |
977 |
900 |
|
Index |
100 |
95 |
96 |
88 |
|
Japan |
856 |
0 |
827 |
1 635 |
|
Index |
100 |
0 |
97 |
191 |
|
Others |
2 202 |
1 277 |
805 |
839 |
|
Index |
100 |
58 |
37 |
38 |
|
Total other countries |
1 088 |
1 004 |
899 |
876 |
|
Index |
100 |
92 |
83 |
80 |
Table 13
Market shares
|
Market shares (%) |
2003 |
2004 |
2005 |
IP |
|
Turkey |
5,6 |
5,5 |
2,9 |
3,0 |
|
Japan |
0,4 |
0,0 |
0,1 |
0,0 |
|
Others |
0,4 |
0,6 |
2,4 |
2,4 |
|
Total other countries |
6,3 |
6,2 |
5,4 |
5,4 |
|
(144) |
According to Eurostat data and to the information collected during the investigation, the main third country from which persulphates are imported is Turkey with a market share of 3 % during the IP. Another, however minor exporting country is Japan although it represents import volumes close to 0 %. |
|
(145) |
Imports originating in third countries other than the PRC, US and Taiwan decreased by 8 %, from 2 466 tonnes in 2003 to 2 262 tonnes during the IP. Consequently, their market share decreased overall from 6,3 % in 2003 to 5,4 % in the IP. |
|
(146) |
Imports from Turkey amounted to 2 161 tonnes in 2003 and decreased over the period considered by 42 % to 1 247 tonnes in the IP, attaining a market share of 3 %. Although Turkish imports were made at lower prices than those of the Community industry sales price, their limited and even gradually shrinking market share were not considered as having had a negative effect on the situation of the Community industry. |
|
(147) |
As to imports from the remaining third countries Eurostat statistics show very low import levels; i.e. 304 tonnes in 2003 which augmented to 1 015 tonnes in the IP. In spite of this growth, the level of these imports remains close to de minimis, representing only 2,4 % of the Community consumption in the IP. Furthermore, prices of these imports were significantly higher than prices from the countries concerned and Turkey. Thus, it is concluded that these imports have not had a significant impact on the state of the Community industry. |
|
(148) |
It can thus provisionally be concluded that imports other than from the PRC, the US and Taiwan did not contribute to the material injury suffered by the Community industry. |
(b) Exports by the Community industry
|
(149) |
Exports of persulphates by the Community industry outside the Community were decreasing during the period considered (by 13 %). Likewise, export prices of the Community industry decreased during the period considered by 9 %. However, these exports represented only 6 % of the Community industry’s total sales to unrelated parties in the IP and thus it was concluded that they did not have a significant impact on the material injury suffered by the Community industry. |
(c) Non-dumped imports originating in the PRC
|
(150) |
As mentioned in recital 95 for two exporting producers the dumping margin established was below the de minimis threshold. Therefore, imports of these companies were not considered in the injury analysis above. Instead it was examined whether these imports could have caused the material injury suffered by the Community industry. However, given the limited volume of these imports, attaining a market share of 6,9 % during the IP, it was considered that the non-dumped imports could not break the causal link between the dumped imports and the material injury suffered by the Community industry. |
(d) Imports of the Community industry
|
(151) |
One Community producer imported the product concerned from its related company in the PRC and resold it on the Community market. Although the resale prices were indeed undercutting the Community industry prices, it should be noted that the volume of the Chinese imports only represented a minor part of the total imports from the PRC (less than 4 %). Furthermore, these imports were only made to maintain global customers who would have otherwise purchased the product concerned from the Chinese suppliers at dumped prices. Moreover, the resale price on the Community market was on average significantly higher than the import prices of the other Chinese exporting producers. Therefore, it was concluded that the imports of the Community industry of the product concerned from the PRC did not break the causal link between the dumped imports and the material injury suffered by the Community industry. |
4. Conclusion on causation
|
(152) |
The coincidence in time between, on the one hand, the increase in dumped imports from the PRC, the US and Taiwan, their increase in market shares and the undercutting found and, on the other hand, the evident deterioration in the situation of the Community industry, leads to the conclusion that the dumped imports caused the material injury suffered by the Community industry within the meaning of Article 3(6) of the basic Regulation. In particular, the Community industry had to lower its selling prices on the Community market due to the price pressure of the dumped imports. Thus, the overall cost increase could not be passed on to the customers and profit margins decreased significantly with a drastic impact on the Community industry's overall financial situation. The possible effect of other factors mainly imports from other third countries, non-dumped imports originating from the PRC exports of the Community industry and the development of costs were analysed but found not to be a determining reason for the injury suffered by the Community industry. |
|
(153) |
Based on the above analysis, which has properly distinguished and separated the effects of all known factors having an effect on the situation of the Community industry from the injurious effect of the dumped imports, it is provisionally concluded that the imports of persulphates from the PRC, the US and Taiwan have caused material injury to the Community industry within the meaning of Article 3(6) of the basic Regulation. |
F. COMMUNITY INTEREST
|
(154) |
In accordance with Article 21 of the basic Regulation, it was examined whether, despite the conclusion on injurious dumping, compelling reasons exist for concluding that it is not in the Community interest to adopt measures in this particular case. The likely impact of possible measures on all parties involved in the proceeding and also the consequences of not taking measures have to be considered in this respect. |
|
(155) |
In order to assess the likely impact of the imposition or non-imposition of measures information was requested from all interested parties which were either known to be concerned or which made themselves known. On this basis, the Commission sent questionnaires to the Community industry, 12 unrelated importers and 11 users. |
|
(156) |
As explained in recital 9, the two complainant Community industry producers, two unrelated importers replied to the questionnaire. |
1. Interests of the Community industry
|
(157) |
The injurious situation of the Community industry resulted from its difficulty to compete with the low-priced, dumped imports. |
|
(158) |
The imposition of measures is expected to prevent further distortions of the market, suppression of prices and restore fair competition. The Community industry should then be able to increase the volume of its sales and to regain market share and thereby generating better economies of scale, thus achieving the necessary profit level to improve the industry’s financial situation and to allow it continued investments in its production facilities, thus guaranteeing the Community industry’s survival. |
|
(159) |
Otherwise, should anti-dumping measures not be imposed, the deterioration of the situation of the Community industry would continue. The Community industry is particularly marked by a loss of revenue due to depressed prices, falling market share and significant decrease in profit. Indeed, in view of the decreasing revenue and the significantly worsening trend in the IP, it is most likely that the financial situation of the Community industry will deteriorate further in the absence of any measures. This would ultimately lead to cuts in production, which would therefore threaten employment and investments in the Community. This is particularly true since the European market is now one of the few export markets left to the countries concerned after the imposition of anti-dumping duties on persulphates from China in the United States of America. With the closure of the Community production, the persulphates users would become more dependent on suppliers outside the Community. |
|
(160) |
Accordingly, it is provisionally concluded that the imposition of anti-dumping measures would allow the Community industry to recover from the injurious dumping suffered and would thus be in the interest of the Community industry. |
2. Interest of unrelated importers
|
(161) |
The Commission sent questionnaires to all known importers/traders. As far as importers are concerned, only two importers submitted a questionnaire reply. However, the volumes of the product concerned imported by these two importers represented 18,9 % of the total imports in the Community and 6,7 % of the Community consumption. |
|
(162) |
It should be noted that these importers’ only source of supply during the IP were the countries concerned (US and Taiwan) and these importers thus claimed that the imposition of an anti-dumping duty would have a significant impact on their financial situation. |
|
(163) |
However, on the basis of information submitted by the importers concerned, it was established that the proportion of persulphates imports in the company's total turnover represented only a negligible part (i.e. between 0,03 % and 1,3 %) during the IP. Therefore, although it is not denied that the imposition of an anti-dumping duty may have a certain impact on these companies; this impact would be overall negligible. |
|
(164) |
Furthermore, it can be reasonably expected that any price increase could at least be partly passed on to the customers due to the fact that, as mentioned below in recital 166, persulphates constitute in most of the cases only a fraction of these customers total cost. Finally, it should be noted that other sources of supply are available such as Turkey, Japan as well as those parts of Chinese exports which are not subject to any anti-dumping duty. |
3. Interest of users
|
(165) |
The Commission sent questionnaires to all eleven known users in the Community. None of these users replied to the questionnaire. No other users supplied the Commission with any information or made themselves known in the course of this investigation. |
|
(166) |
There was no indication that users’ interests will be significantly affected. Indeed, on the basis of the information available, persulphates, in most of the cases, only constitutes a minor part of the total production cost and the effect of antidumping duties would be negligible. |
4. Conclusion on Community interest
|
(167) |
Taking into account all of the above factors, it is concluded that the imposition of measures would not have a significantly negative effect, if any at all, on the situation of the users and importers of the product concerned. On this basis, it is provisionally concluded that there are no compelling reasons not to impose anti-dumping measures. |
G. PROVISIONAL ANTI-DUMPING MEASURES
1. Injury elimination level
|
(168) |
In view of the conclusions reached with regard to dumping, resulting injury, causation and Community interest, provisional measures should be imposed in order to prevent further injury being caused to the Community industry by the dumped imports. |
|
(169) |
The measures should be imposed at a level sufficient to eliminate the injury caused by these imports without exceeding the dumping margin found. When calculating the amount of duty necessary to remove the effects of the injurious dumping, it was considered that any measures should allow the Community industry to cover its costs of production and to obtain overall a profit before tax that could be reasonably achieved by an industry of this type in the sector under normal conditions of competition, i.e. in the absence of dumped imports, on the sales of the like product in the Community. The pre-tax profit margin for producers used for this calculation was 12 %, based on the complaint and confirmed during this investigation. The actual profits made by the Community industry in 2003 and 2004, as well as during the years prior to the period under consideration, were never below this reference level. |
|
(170) |
The necessary price increase was then determined on the basis of a comparison of the weighted average import price, as established for the price undercutting calculations (see recitals 117 to 119 above), with the non-injurious price of products sold by the Community industry on the Community market. The non-injurious price has been obtained by adjusting the sales price of the Community industry by the actual loss/profit made during the IP and by adding the above mentioned profit margin. Any difference resulting from this comparison was then expressed as a percentage of the total cif import value. |
|
(171) |
These differences were for the Chinese cooperating exporting producers to whom MET was granted above the dumping margins found. In order to calculate the countrywide injury elimination level for all other exporters in the PRC, it should be recalled that the level of cooperation was high. Therefore, the injury margin was calculated as the difference of the weighted average cif import prices of the companies to which MET was not granted and the non-injurious price as calculated above. In the case of USA, the injury elimination level was below the dumping margin found for the exporting producers, whereas for Taiwan the injury elimination level was above the dumping margin found. |
2. Provisional measures
|
(172) |
In light of the foregoing, it is considered that a provisional anti-dumping duty should be imposed at the level of the dumping margin found, but should not, in accordance with Article 7(2) of the basic Regulation, be higher than the injury margin calculated above. |
|
(173) |
The individual company anti-dumping duty rates specified in this Regulation were established on the basis of the findings of the present investigation. Therefore, they reflect the situation found during this investigation with respect to these companies. These duty rates (as opposed to residual duties applicable to ‘all other companies’ in the USA and in Taiwan and the countrywide duty applicable to ‘all other companies’ in the PRC) are thus exclusively applicable to imports of products originating in the country concerned and produced by the companies and thus by the specific legal entities mentioned. Imported products produced by any other company not specifically mentioned in the operative part of this document with its name and address, including entities related to those specifically mentioned, cannot benefit from these rates and shall be subject to the duty rate applicable to ‘all other companies’. |
|
(174) |
Any claim regarding the application of these individual company anti-dumping duty rates (e.g. following a change in the name of the entity or following the setting up of new production or sales entities) should be addressed to the Commission (5) forthwith with all relevant information, in particular any modification in the company's activities linked to production, domestic and export sales associated with, for example, that name change or that change in the production and sales entities. The Commission, if appropriate, will, after consultation of the Advisory Committee, amend the Regulation accordingly by updating the list of companies benefiting from individual duty rates. |
|
(175) |
On the basis of the above, the provisional duty rates are:
|
3. Special monitoring
|
(176) |
In order to minimise the risks of circumvention due to the high difference in the duty rates as regards exporting producers in the PRC, it is considered that special measures are needed in this case to ensure the proper application of the anti-dumping duties. Only the imports of the product concerned manufactured by the respective exporting producer in the PRC can benefit from the specific dumping margin calculated for the producer concerned. These special measures, which only apply to companies in the PRC for which an individual duty rate is introduced, include the following: |
|
(177) |
The presentation to the customs authorities of the Member States of a valid commercial invoice which must conform to the requirements set out in the Annex to this Regulation. Imports not accompanied by such an invoice must be made subject to the residual anti-dumping duty applicable to all other companies in the PRC. |
|
(178) |
Should the exports by the companies in the PRC benefiting from lower individual duty rates increase significantly in volume after the imposition of the anti-dumping measures, this increase in volume could be considered as constituting, in itself, a change in the pattern of trade due to the imposition of measures within the meaning of Article 13(1) of the basic Regulation. In such circumstances, and provided the conditions are met, an anti-circumvention investigation may be initiated. This investigation may, inter alia, examine the need for the removal of individual duty rates and the consequent imposition of a countrywide or residual duty. |
H. FINAL PROVISION
|
(179) |
In the interest of sound administration, a period should be fixed within which the interested parties which made themselves known within the time limit specified in the notice of initiation may make their views known in writing and request a hearing. Furthermore, it should be stated that the findings concerning the imposition of anti-dumping duties made for the purposes of this Regulation are provisional and may have to be reconsidered for the purpose of any definitive duty, |
HAS ADOPTED THIS REGULATION:
Article 1
1. A provisional anti-dumping duty is hereby imposed on imports of peroxosulphates (persulphates), including potassium peroxymonosulphate sulphate, falling within CN codes 2833 40 00 and ex 2842 90 80 (TARIC code 2842908020) and originating in the United States of America, the People’s Republic of China and Taiwan.
2. The rate of the provisional anti-dumping duty applicable to the net free-at-Community-frontier price, before duty, of the products manufactured by the companies below shall be:
|
Country |
Company |
Anti-dumping duty |
TARIC Additional code |
|
The United States of America |
E.I. DuPont De Nemours, Wilmington, Delaware |
10,6 % |
A818 |
|
FMC Corporation, Tonawanda, New York |
39,0 % |
A819 |
|
|
All other companies |
39,0 % |
A999 |
|
|
The People’s Republic of China |
ABC Chemicals (Shanghai) Co., Ltd, Shanghai |
0 % |
A820 |
|
Degussa-AJ (Shanghai) Initiators Co., Ltd, Shanghai |
14,4 % |
A821 |
|
|
Hebei Yatai Electrochemistry Co., Ltd, Wang Jia Jing |
0 % |
A822 |
|
|
All other companies |
67,4 % |
A999 |
|
|
Taiwan |
San Yuan Chemical Co., Ltd, Chiayi |
22,6 % |
A823 |
|
All other companies |
22,6 % |
A999 |
3. The application of the individual duty rates specified for the companies in the People's Republic of China mentioned in paragraph 2 shall be conditional upon presentation to the customs authorities of the Member States of a valid commercial invoice, which shall conform to the requirements set out in the Annex. If no such invoice is presented, the duty rate applicable to all other companies shall apply.
4. The release for free circulation in the Community of the product referred to in paragraph 1 shall be subject to the provision of a security, equivalent to the amount of the provisional duty.
5. Unless otherwise specified, the provisions in force concerning customs duties shall apply.
Article 2
Without prejudice to Article 20 of Regulation (EC) No 384/96, interested parties may request disclosure of the essential facts and considerations on the basis of which this Regulation was adopted, make their views known in writing and apply to be heard orally by the Commission within one month of the date of entry into force of this Regulation.
Pursuant to Article 21(4) of Regulation (EC) No 384/96, the parties concerned may comment on the application of this Regulation within one month of the date of its entry into force.
Article 3
This Regulation shall enter into force on the day following that of its publication in the Official Journal of the European Union.
Article 1 of this Regulation shall apply for a period of six months.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 11 April 2007.
For the Commission
Peter MANDELSON
Member of the Commission
(1) OJ L 56, 6.3.1996, p. 1. Regulation as last amended by Regulation (EC) No 2117/2005 (OJ L 340, 23.12.2005, p. 17).
(2) OJ C 162, 13.7.2006, p. 5.
(3) Council Regulation (EC) No 2961/95 (OJ L 308, 21.12.1995, p. 61).
(4) Council Regulation (EC) No 695/2002 (OJ L 109, 25.4.2002, p. 1).
(5) European Commission, Directorate-General for Trade, Direction B-1049 Brussels, Belgium.
ANNEX
The valid commercial invoice referred to in Article 1(3) of this Regulation must include a declaration signed by an official of the company and bearing the company's official stamp, in the following format:
|
(1) |
The name and function of the official of the company which has issued the commercial invoice. |
|
(2) |
The following declaration: ‘I, the undersigned, certify that the [volume] of persulphates sold for export to the European Community covered by this invoice was manufactured by [company name and address] [TARIC additional code] in the People's Republic of China. I declare that the information provided in this invoice is complete and correct.’ |
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/30 |
COMMISSION REGULATION (EC) No 391/2007
of 11 April 2007
laying down detailed rules for the implementation of Council Regulation (EC) No 861/2006 as regards the expenditure incurred by Member States in implementing the monitoring and control systems applicable to the Common Fisheries Policy
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Regulation (EC) No 861/2006 of 22 May 2006 establishing Community financial measures for the implementation of the common fisheries policy and in the area of the Law of the Sea (1), and in particular Article 31 thereof,
Whereas:
|
(1) |
The Community has been financing Member States actions in the field of fisheries control and enforcement since 1990, in line with the objectives established under Council Regulation (EC) No 2371/2002 (2). |
|
(2) |
Improvements towards an efficient control system throughout the Community will be difficult to achieve without incentives, in particular when new technologies are to be tested and introduced where appropriate. |
|
(3) |
Evidence shows that the resources of Member States are still inadequate to fulfil their obligations under Regulation (EC) No 2371/2002. In particular, Community aid to assist Member States is requested in order to overcome existing differences in their fisheries control and surveillance capabilities. |
|
(4) |
Regulation (EC) No 861/2006 provides, amongst other actions, for Community financial measures for expenditure on fisheries control, inspection and surveillance for the period 2007 to 2013. |
|
(5) |
Article 8(a) of Regulation (EC) No 861/2006 provides for a list of actions undertaken by Member States in the area of fisheries control and enforcement which shall be considered eligible for Community financial assistance. |
|
(6) |
In view of the principle of sound financial management, Member States must have clear indications on the rules to be followed in order to benefit from Community financial assistance when incurring on expenditure in the area of fisheries control and enforcement. |
|
(7) |
It is necessary to ensure that Community funds available for such actions are allocated efficiently with a view to reducing identified weaknesses in such a way that controls are carried out to a high standard. |
|
(8) |
Member States should assess their programmes and the impact of their expenditure on control, inspection and surveillance, each year and over the whole period 2007 to 2013. |
|
(9) |
With the view of simplifying procedures, as of 1 January 2007 claims for reimbursement relating to expenditure approved on the basis of Council Decisions 95/527/EC (3), 2001/431/EC (4) and 2004/465/EC (5), shall be submitted in accordance with Annexes VI and VII to this Regulation. |
|
(10) |
The measures provided for in this Regulation are in accordance with the opinion of the Committee for Fisheries and Aquaculture, |
HAS ADOPTED THIS REGULATION:
Article 1
Subject-matter
This Regulation establishes detailed rules for the implementation of Regulation (EC) No 861/2006 as regards the Community financial contribution for expenditure incurred by Member States in implementing monitoring and control systems applicable to the Common Fisheries Policy in the period 2007 to 2013.
Article 2
Definitions
For the purpose of this Regulation the following definitions shall apply:
|
(a) |
‘Annual fisheries control programme’ means an annual programme drawn up by a Member State in accordance with Article 20 of Regulation (EC) No 861/2006; |
|
(b) |
‘Budgetary commitment’ means the operation reserving the appropriation necessary to cover subsequent payments to honour a legal commitment; |
|
(c) |
‘Legal commitment’ means the act whereby the authorising authority of a Member State enters into or establishes an obligation which results in a charge. |
Article 3
Annual fisheries control programmes
1. Member States wishing to receive a financial contribution for expenditure incurred under Article 8(a) of Regulation (EC) No 861/2006 shall notify to the Commission an annual fisheries control programme by 31 January of each year.
2. In addition to the information required by Article 20 of Regulation (EC) No 861/2006, Member States shall state in their fisheries control programme, for each project:
|
(a) |
a yearly forecast of reimbursement requests; |
|
(b) |
the measures foreseen to make known to the public that the project received financial support from the Community; |
|
(c) |
when the project concerns the purchase and modernisation of vessels and aircraft: the specification of the type of vessel or aircraft; |
|
(d) |
a description of all means available by the administration for fisheries monitoring and control, drawn up in accordance with Annex I. |
3. Detailed rules as to the eligibility of certain actions are set out in Annexes II, III and IV.
Article 4
Commitment of expenditure
Member States shall enter into legal and budgetary commitments for actions considered eligible for a financial contribution under the decision provided for in Article 21 of Regulation (EC) No 861/2006 within 12 months of the end of the year in which they were notified of such decision.
Article 5
Eligible expenditure
In order to be eligible for reimbursement, expenditure shall:
|
(a) |
be foreseen in the fisheries control programme; and |
|
(b) |
relate to any of the actions referred to in Article 8(a) of Regulation (EC) No 861/2006; |
|
(c) |
concern projects with a cost exceeding EUR 40 000, VAT excluded, except where the project concerns an action referred to in Article 8(a)(ii) or (v) of Regulation (EC) No 861/2006 or where duly justified; |
|
(d) |
arise from legal and budgetary commitments entered into by Member States in accordance with Article 4 of this Regulation; |
|
(e) |
concern projects implemented in accordance with Article 8 of this Regulation; |
|
(f) |
comply with specific Community rules, where those rules apply. |
Article 6
Eligible expenditure related to certain actions
1. Expenditure incurred on new control technologies shall be eligible to the extent that it complies with Annex II and it is used for monitoring and control of fishing activities, as declared by the Member State concerned.
2. Expenditure incurred on the purchase and modernisation of aircraft and vessels shall be eligible to the extent that it complies with Annex III and it is used for monitoring and control of fishing activities, as declared by the Member State concerned, for at least 25 % of their activity.
3. Expenditure incurred on training and exchange programmes and on seminars and media tools shall be eligible to the extent that it complies with Annex IV. Such expenditure may cover, inter alia:
|
(a) |
fisheries surveillance methodology; |
|
(b) |
Community legislation governing the Common Fisheries Policy and, in particular, control; |
|
(c) |
the use of fisheries control techniques; |
|
(d) |
the implementation by Member States of the applicable control system, in accordance with the Common Fisheries Policy rules. |
Article 7
Non-eligible expenditure
1. Expenditure shall not be eligible if incurred before January 1 of the year in which the annual fisheries control programme is submitted to the Commission.
2. Value added tax (VAT) shall not be eligible for reimbursement.
3. An indicative list of non-eligible expenditure items is set out in Annex V.
Article 8
Implementation of projects
1. Projects shall be started and completed in accordance with the schedule laid down in the annual fisheries control programme.
2. The schedule shall state the foreseen date of commencement and termination of projects.
Article 9
Non-implementation and delay of projects
When a Member State decides not to implement all or part of the projects for which a financial contribution has been granted, or a delay occurs, it shall immediately inform the Commission in writing, stating:
|
(a) |
the implications for its annual fisheries control programme, including those of a financial nature; |
|
(b) |
the reasons for the delay or non-implementation; |
|
(c) |
the foreseen new implementation time frame. |
Article 10
Advances
1. At the reasoned request of a Member State, the Commission may grant an advance for each project of up to 50 % of the financial contribution granted in the decision provided for in Article 21 of Regulation (EC) No 861/2006. The amount of the advance shall be deducted from any interim payment as well as from the final payment of the financial contribution for that project to the concerned Member State.
2. The request by the Member State shall be accompanied by a certified copy of the contract between the relevant administration and the supplier.
3. If a binding legal commitment is not made by the competent authority of a Member State within the period laid down in Article 4 of this Regulation, any advance granted shall be repaid forthwith.
Article 11
Claims for reimbursement
1. Member States shall submit to the Commission their claims for reimbursement within nine months of the date on which expenditure was incurred.
2. Claims for reimbursement shall include the items listed in Annex VI and shall be drafted in accordance with the form set out in Annex VII.
3. When submitting claims for reimbursement, Member States shall verify and certify that the expenditure has been incurred in compliance with the conditions laid down in Regulation (EC) No 861/2006, this Regulation and in the decision provided for in Article 21 of Regulation (EC) No 861/2006, and with Community legislation on the award of public contracts. The claim shall include a statement concerning the accuracy and veracity of the transmitted accounts, in accordance with the form set out in Annex VII.
4. Claims for an amount of less than EUR 20 000 shall not be processed, unless duly justified. Claims may be regrouped.
5. Claims for projects which were not completed in accordance with the schedule referred to in Article 8 of this Regulation may be accepted only where the delay is duly justified. Where such claims are not accepted, the Community appropriations shall be de-committed.
6. If the Commission considers that the claim does not comply with the conditions laid down in Regulation (EC) No 861/2006, in this Regulation, in the decision provided for in Article 21 of Regulation (EC) No 861/2006, or with Community legislation on the award of public contracts, it shall request the Member State to submit its observations on the matter within a time limit. If the examination confirms non-compliance, the Commission shall refuse to reimburse all or part of the expenditure at issue and, where appropriate, request reimbursement of undue payments.
Article 12
Currency
1. Fisheries control programmes, claims for reimbursement of expenditure and claims for payment of advances shall be expressed in euro.
2. Reimbursement shall be made in euro on the basis of the exchange rate published in the C series of the Official Journal of the European Union of the day on which the payment order or recovery order is drawn up by the authorising department of the Commission.
3. Member States not participating in the third stage of Economic and Monetary Union shall specify the exchange rate used.
Article 13
Audits and financial corrections
Member States shall provide the Commission and the Court of Auditors with any information those institutions may request for the audits and financial corrections referred to Article 28 of Regulation (EC) No 861/2006.
Article 14
Reports from the Member States
1. Member States shall send the Commission information enabling it to verify the use made of the financial contribution and to assess the impact on control, inspection and surveillance activities of the measures provided for in this Regulation.
2. Member States shall also:
|
(a) |
By 31 March each year, submit to the Commission an intermediate assessment report on their fisheries control programme for the previous year covering the following:
|
|
(b) |
By 31 March 2014, submit to the Commission a final assessment report covering the following:
|
Article 15
Transitional provisions
As of 1 January 2007, claims for reimbursement relating to the financial contribution for expenditure approved on the basis of Decisions 95/527/EC, 2001/431/EC and 2004/465/EC shall be submitted in accordance with Annexes VI and VII to this Regulation.
Article 16
Entry into force
This Regulation shall enter into force on the seventh day following its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 11 April 2007.
For the Commission
Joe BORG
Member of the Commission
(1) OJ L 160, 14.6.2006, p. 1.
(2) OJ L 358, 31.12.2002, p. 59.
(3) OJ L 301, 14.12.1995, p. 30.
(4) OJ L 154, 9.6.2001, p. 22.
(5) OJ L 157, 30.4.2004, p. 114, corrected by OJ L 195, 2.6.2004, p. 36. Decision as amended by Decision 2006/2/EC (OJ L 2, 5.1.2006, p. 4).
ANNEX I
Description of means available to the Member State for fisheries monitoring and control
The description of means available to the Member State for fisheries control, as referred to in Article 3(2)(d) of this Regulation, shall include the following:
|
(a) |
a brief description of the administrative bodies in charge of fisheries control at national, regional and local level; |
|
(b) |
a brief description of human resources, equipments (in particular the number of vessels, aircraft and helicopters available) and main actions undergone the year before to fulfil the duties under the rules of the Common Fisheries Policy; |
|
(c) |
the yearly budget allocated to fisheries control, expressed in euro with details on investments and on running costs of means used for fisheries control (data by category, including human resources). |
ANNEX II
Indicative list of eligible expenditure items relating to the implementation of control technologies
The following expenditure is considered eligible:
|
(a) |
Purchase of, installation and technical assistance for, computer technology and setting up of IT networks, including remote sensing capability, in order to allow efficient and secure data exchange in connection with monitoring, control and surveillance of fisheries activities. Expenditure incurred on technical assistance is covered during two years as from the installation. |
|
(b) |
Purchase and installation of:
Devices must be in conformity with the requirements laid down by the relevant Community rules. |
|
(c) |
Purchase of personal computers, tablet PCs and Personal digital assistants (PDAs) designed to store and process data related to fishing activities. |
|
(d) |
Pilot projects relating to new technologies on the control of fisheries activities and their implementation. |
ANNEX III
Indicative list of eligible expenditure items relating to the purchase and modernisation of aircraft and vessels used for fisheries control purposes
The following expenditure is considered eligible:
|
(a) |
Fixed wing aircraft, unmanned aerial vehicles and helicopters and their equipment designed for fisheries control purposes. In particular, detection, communication and navigation equipment and software which is installed on board and which is a part of vessels or aircraft used for inspection and surveillance of fisheries activities and that enable data exchange between the vessels or aircraft and the fisheries control authorities; |
|
(b) |
Equipment replacing obsolete equipment designed to improve efficiency for fisheries control. The cost incurred in modernizing the engine room, wheelhouse boarding and launching facilities is also eligible; |
|
(c) |
Boarding boats (such as seariders and RIBs), including installed equipment, engines, launching davits and cranes (inclusive of hydraulic systems and installation), changes to the main vessel in order to adapt to the boarding boats (such as reinforcement of the deck and superstructure); |
|
(d) |
Major items for the propulsion system of the vessel, such as propeller systems, gear boxes, new main engines and auxiliary engines; |
|
(e) |
Equipment securing the confidentiality of communications such as encryption equipment and scramblers; |
|
(f) |
Water-proofed Personal Computers installed on board. |
ANNEX IV
Indicative list of eligible expenditure items relating to training and exchange programmes, seminars and media tools
|
(a) |
The following expenditure is considered eligible:
|
|
(b) |
Expenditure is eligible to the extent that it is eligible for reimbursement in conformity with relevant national rules. |
ANNEX V
Indicative list of non-eligible expenditure items
The following expenditure is considered non-eligible:
|
(a) |
Renting and leasing contracts; |
|
(b) |
Equipment which is not used exclusively for fisheries control purposes, such as personal computers, laptops, scanners, printers, mobile phones, telephone standards, walkie-talkies, measure tapes, rules and other similar equipment, videos and cameras; |
|
(c) |
Articles of clothing and footwear, such as uniforms, protecting suits, etc. and general personal equipment; |
|
(d) |
Running and maintenance costs, such as telecommunication costs, financial interests, insurance premiums, fuel; |
|
(e) |
Spare parts needed to keep in service any eligible item; |
|
(f) |
Vehicles and motorbikes; |
|
(g) |
Buildings and sites; |
|
(h) |
Salaries and indemnities. |
ANNEX VI
Content of claims for reimbursement
Claims for reimbursement shall include the following:
|
(a) |
A letter stating the total amount claimed for reimbursement. This letter shall clearly state:
|
|
(b) |
A statement of expenditure, using the form established in Annex VII (one per Commission Decision). |
|
(c) |
A list including the following information:
|
|
(d) |
With regard to each project for which reimbursement is claimed:
|
ANNEX VII
Statement of expenditure
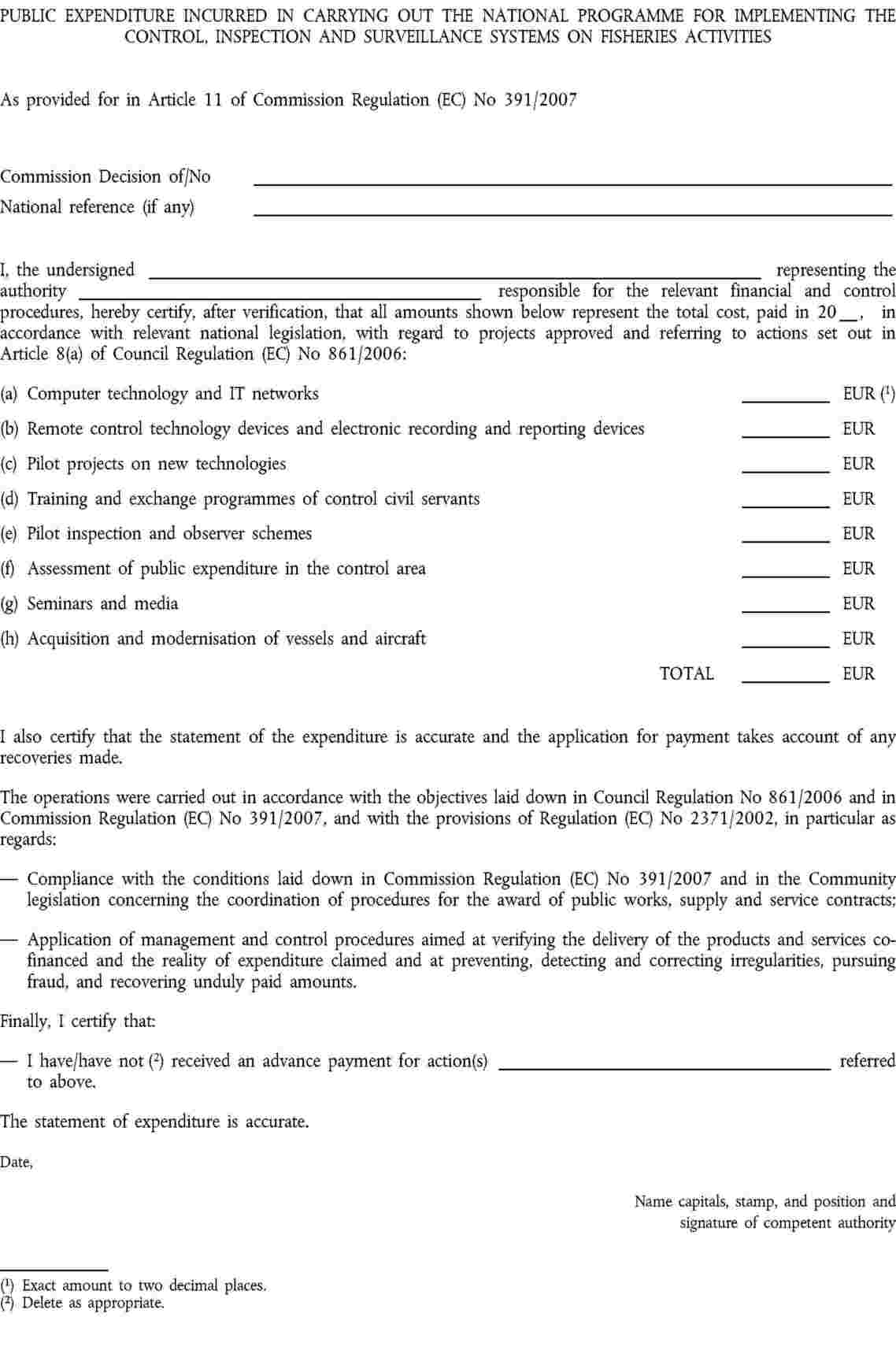
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/39 |
COMMISSION REGULATION (EC) No 392/2007
of 11 April 2007
setting the allocation coefficient for issuing of licences applied for from 2 to 6 April 2007 to import sugar products under tariff quotas and preferential agreements
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Regulation (EC) No 318/2006 of 20 February 2006 on the common organisation of the markets in the sugar sector (1),
Having regard to Commission Regulation (EC) No 950/2006 of 28 June 2006 laying down detailed rules for the 2006/07, 2007/08 and 2008/09 marketing years for importing and refining of sugar products under certain tariff quotas and preferential agreements (2), and in particular Article 5(3) thereof,
Whereas:
|
(1) |
Applications for import licences were submitted to the competent authority during the week of 2 to 6 April 2007, in accordance with Regulation (EC) No 950/2006 or Commission Regulation (EC) No 1832/2006 of 13 December 2006 laying down transitional measures in the sugar sector by reason of the accession of Bulgaria and Romania (3) for a total quantity equal to or exceeding the quantity available for serial number 09.4318. |
|
(2) |
In these circumstances, the Commission should fix an allocation coefficient in order to issue licences in proportion to the quantity available and inform the Member States that the set limit has been reached, |
HAS ADOPTED THIS REGULATION:
Article 1
Licences shall be issued within the quantitative limits set in the Annex to this Regulation in respect of applications for import licences submitted from 2 to 6 April 2007, in accordance with Article 4(2) of Regulation (EC) No 950/2006 or Article 5 of Regulation (EC) No 1832/2006.
Article 2
This Regulation shall enter into force on the day of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
Done at Brussels, 11 April 2007.
For the Commission
Jean-Luc DEMARTY
Director-General for Agriculture and Rural Development
(2) OJ L 178, 1.7.2006, p. 1. Regulation as amended by Regulation (EC) No 2006/2006 (OJ L 379, 28.12.2006, p. 95).
(3) OJ L 354, 14.12.2006, p. 8.
ANNEX
ACP-India Preferential Sugar
Title IV of Regulation (EC) No 950/2006
2006/07 marketing year
|
Serial No |
Country |
Week of 2 to 6 April 2007: % of requested quantity to be granted |
Limit |
|
09.4331 |
Barbados |
100 |
|
|
09.4332 |
Belize |
0 |
Reached |
|
09.4333 |
Côte d’Ivoire |
100 |
|
|
09.4334 |
Republic of the Congo |
100 |
|
|
09.4335 |
Fiji |
100 |
|
|
09.4336 |
Guyana |
100 |
|
|
09.4337 |
India |
0 |
Reached |
|
09.4338 |
Jamaica |
100 |
|
|
09.4339 |
Kenya |
100 |
|
|
09.4340 |
Madagascar |
100 |
|
|
09.4341 |
Malawi |
100 |
|
|
09.4342 |
Mauritius |
100 |
|
|
09.4343 |
Mozambique |
0 |
Reached |
|
09.4344 |
Saint Kitts and Nevis |
— |
|
|
09.4345 |
Suriname |
— |
|
|
09.4346 |
Swaziland |
100 |
|
|
09.4347 |
Tanzania |
0 |
Reached |
|
09.4348 |
Trinidad and Tobago |
100 |
|
|
09.4349 |
Uganda |
— |
|
|
09.4350 |
Zambia |
100 |
|
|
09.4351 |
Zimbabwe |
100 |
|
Complementary Sugar
Title V of Regulation (EC) No 950/2006
2006/07 marketing year
|
Serial No |
Country |
Week of 2 to 6 April 2007: % of requested quantity to be granted |
Limit |
|
09.4315 |
India |
100 |
|
|
09.4316 |
ACP Protocol signatory countries |
100 |
|
CXL Concessions Sugar
Title VI of Regulation (EC) No 950/2006
2006/07 marketing year
|
Serial No |
Country |
Week of 2 to 6 April 2007: % of requested quantity to be granted |
Limit |
|
09.4317 |
Australia |
0 |
Reached |
|
09.4318 |
Brazil |
14,2857 |
Reached |
|
09.4319 |
Cuba |
0 |
Reached |
|
09.4320 |
Other third countries |
0 |
Reached |
Balkans sugar
Title VII of Regulation (EC) No 950/2006
2006/07 marketing year
|
Serial No |
Country |
Week of 2 to 6 April 2007: % of requested quantity to be granted |
Limit |
|
09.4324 |
Albania |
100 |
|
|
09.4325 |
Bosnia and Herzegovina |
0 |
Reached |
|
09.4326 |
Serbia, Montenegro and Kosovo |
100 |
|
|
09.4327 |
Former Yugoslav Republic of Macedonia |
100 |
|
|
09.4328 |
Croatia |
100 |
|
Exceptional import sugar and industrial import sugar
Title VIII of Regulation (EC) No 950/2006
2006/07 Marketing year
|
Serial No |
Type |
Week of 2 to 6 April 2007: % of requested quantity to be granted |
Limit |
|
09.4380 |
Exceptional |
— |
|
|
09.4390 |
Industrial |
100 |
|
Import of sugar under the transitional tariff quotas opened for Bulgaria and Romania
Chapter 1 Section 2 of Regulation (EC) No 1832/2006
2006/07 marketing year
|
Order No |
Type |
Week of 2 to 6 April 2007: % of requested quantity to be granted |
Limit |
|
09.4365 |
Bulgaria |
0 |
Reached |
|
09.4366 |
Romania |
100 |
|
DIRECTIVES
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/42 |
COMMISSION DIRECTIVE 2007/21/EC
of 10 April 2007
amending Council Directive 91/414/EEC as regards the expiry dates for inclusion in Annex I of the active substances azoxystrobin, imazalil, kresoxim-methyl, spiroxamin, azimsulfuron, prohexadion-calcium and fluroxypyr
(Text with EEA relevance)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market (1), and in particular Article 5(5) thereof,
Whereas:
|
(1) |
By Commission Directive 98/47/EC (2) azoxystrobin was included until 1 July 2008, by Commission Directive 97/73/EC (3) imazalil was included until 31 December 2008, by Commission Directive 1999/1/EC (4) kresoxim-methyl was included until 31 January 2009, by Commission Directive 1999/73/EC (5) spiroxamin was included until 1 September 2009, by Commission Directive 1999/80/EC (6) azimsulfuron was included until 1 October 2009, by Commission Directive 2000/50/EC (7) prohexadion-calcium was included until 20 October 2010 and by Commission Directive 2000/10/EC (8) fluroxypyr was included until 30 November 2010 as active substances in Annex I to Directive 91/414/EEC. |
|
(2) |
On request the inclusion of an active substance can be renewed provided an application is made at the latest two years before the entry is due to lapse. The Commission has received requests regarding renewals of inclusions for all the substances above referred to. |
|
(3) |
The Commission will have to lay down detailed rules concerning the submission and evaluation of further information necessary for the renewal of Annex I inclusion. Therefore it is justified to renew the inclusion of above mention active substances in Annex I for a period necessary to enable the notifiers to prepare their applications and to enable the Commission to organise their evaluation and to take a decision. |
|
(4) |
It is therefore appropriate to amend Directive 91/414/EEC accordingly. |
|
(5) |
The measures provided for in this Directive are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS DIRECTIVE:
Article 1
Annex I to Directive 91/414/EEC is amended as set out in the Annex to this Directive.
Article 2
Member States shall adopt and publish by 12 December 2007 at the latest the laws, regulations and administrative provisions necessary to comply with this Directive. They shall forthwith communicate to the Commission the text of those provisions and a correlation table between those provisions and this Directive.
They shall apply those provisions from 13 December 2007.
When Member States adopt those provisions, they shall contain a reference to this Directive or shall be accompanied by such a reference on the occasion of their official publication. Member States shall determine how such reference is to be made.
Article 3
This Directive shall enter into force on the 20th day following that of its publication in the Official Journal of the European Union.
Article 4
This Directive is addressed to the Member States.
Done at Brussels, 10 April 2007.
For the Commission
Markos KYPRIANOU
Member of the Commission
(1) OJ L 230, 19.8.1991, p. 1. Directive as last amended by Commission Directive 2007/6/EC (OJ L 43, 15.2.2007, p. 13).
(2) OJ L 191, 7.7.1998, p. 50.
(3) OJ L 353, 24.12.1997, p. 26.
(4) OJ L 21, 28.1.1999, p. 21.
(5) OJ L 206, 5.8.1999, p. 16. Corrected by OJ L 221, 21.8.1999, p. 19.
(6) OJ L 210, 10.8.1999, p. 13.
(7) OJ L 198, 4.8.2000, p. 39.
ANNEX
In Annex I to Directive 91/414/EEC, rows 1, 2, 3, 4, 5, 6, 8 are replaced by the following:
|
‘1. |
Imazalil CAS No 73790-28-0, 35554-44-0 CIPAC No 335 |
(+)-1-(β-allyloxy-2,4-dichlorophenylethyl)imidazole or (+)-allyl 1-(2,4-dichlorophenyl)-2-imidazol-1-ylethyl ether |
975 g/kg |
1 January 1999 |
31 December 2011 |
Only uses as fungicide may be authorized. For the following uses the following particular conditions apply:
Date of Standing Committee on Plant Health at which the review report was finalised: 11 July 1997. |
||||||
|
2. |
Azoxystrobin CAS No 131860-33-8 CIPAC No 571 |
Methyl (E)-2-{2[6-(2-cyanophenoxy)pyrimidin-4-yloxy] phenyl}-3-methoxyacrylate |
930 g/kg (Z isomer max. 25 g/kg) |
1 July 1998 |
31 December 2011 |
Only uses as fungicide may be authorised. In the decision making according to the Uniform Principles particular attention should be given to the impact on aquatic organisms. Authorisation conditions should include appropriate risk mitigation measures. Date of Standing Committee on Plant Health at which the review report was finalised: 22 April 1998. |
||||||
|
3. |
Kresoxim-Methyl CAS No 143390-89-0 CIPAC No 568 |
Methyl (E)-2-methoxyimino-2-[2-(o-tolyloxymethyl) phenyl]acetate |
910 g/kg |
1 February 1999 |
31 December 2011 |
Only uses as fungicide may be authorised. In their decision making according to the Uniform Principles Member States shall pay particular attention to the protection of groundwater under vulnerable conditions. Date of Standing Committee on Plant Health at which the review report was finalised: 16 October 1998. |
||||||
|
4. |
Spiroxamine CAS No 1181134-30-8 CIPAC No 572 |
(8-tert-Butyl-1,4-dioxa-spiro [4.5] decan-2-ylmethyl)-ethyl-propyl-amine |
940 g/kg (diastereomers A and B combined) |
1 September 1999 |
31 December 2011 |
Only uses as a fungicide may be authorised. In their decision making according to the Uniform Principles Member States:
Date of Standing Committee on Plant Health at which the review report was finalised: 12 May 1999. |
||||||
|
5. |
Azimsulfuron CAS No 120162-55-2 CIPAC No 584 |
1-(4,6-dimethoxypyrimidin-2-yl)-3-[1-methyl-4-(2-methyl-2H-tetrazol-5-yl)-pyrazol-5-ylsulfonyl]-urea |
980 g/kg |
1 October 1999 |
31 December 2011 |
Only uses as herbicide may be authorised. Aerial applications may not be authorised. In their decision making according to the Uniform Principles Member States must pay particular attention to the impact on aquatic organisms and terrestrial non-target plants and must ensure that the conditions of authorisation include, where appropriate, risk mitigation measures (e.g. in rice cultivation minimum holding periods for water prior to discharge). Date of Standing Committee on Plant Health at which the review report was finalised: 2 July 1999. |
||||||
|
6. |
Fluroxypyr CAS No 69377-81-7 CIPAC No 431 |
4-amino-3,5-dichloro-6-fluoro-2-pyridyloxyacetic acid |
950 g/kg |
1 December 2000 |
31 December 2011 |
Only uses as herbicide may be authorised. In their decision making according to the Uniform Principles Member States:
Member States shall inform the Commission if the requested additional trials and information as outlined in point 7 of the Review Report were not submitted by 1 December 2000. Date of Standing Committee on Plant Health at which the review report was finalised: 30 November 1999. |
||||||
|
8. |
Prohexadione Calcium CAS No 127277-53-6 CIPAC No 567 |
Calcium 3,5-dioxo-4-propionylcyclohexanecarboxylate |
890 g/kg |
1 October 2000 |
31 December 2011 |
Only uses as plant growth regulator may be authorised. Date of Standing Committee on Plant Health at which the review report was finalised: 16 June 2000.’ |
II Acts adopted under the EC Treaty/Euratom Treaty whose publication is not obligatory
DECISIONS
Commission
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/47 |
COMMISSION DECISION
of 11 April 2007
concerning the extension of the deadline for placing on the market of biocidal products containing certain active substances not examined during the 10-year work programme referred to in Article 16(2) of Directive 98/8/EC of the European Parliament and of the Council
(notified under document number C(2007) 1545)
(Only the French and Polish texts are authentic)
(2007/226/EC)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market (1), and in particular Article 16(2) thereof,
Whereas:
|
(1) |
Article 16(2) second subparagraph, and (3) of Directive 98/8/EC (hereinafter referred to as the Directive) provide that, where the requisite information and data for the evaluation of an active substance have not been submitted within the prescribed period, it may be decided not to include the active substance in Annexes I, IA or IB of the Directive. Following such a decision, Member States should withdraw all authorisations for biocidal products containing the active substance. |
|
(2) |
Commission Regulations (EC) No 1896/2000 (2) and (EC) No 2032/2003 (3) lay down the detailed rules for the implementation of the first and second phase of the 10-year work programme referred to in Article 16(2) of the Directive. Article 4(2) of Regulation (EC) No 2032/2003 specifies 1 September 2006 as the date with effect from which Member States shall cancel authorisations for biocidal products containing identified existing active substances in respect of which there has been neither an accepted notification nor an expression of interest by a Member State. |
|
(3) |
Article 4a of Regulation (EC) No 2032/2003, as amended by Commission Regulation (EC) No 1048/2005 (4), lays down the conditions under which Member States may apply to the Commission for an extension of the phase-out period laid down in its Article 4(2) and the conditions for granting such an extension. |
|
(4) |
For some of the active substances for which use in biocidal products shall be prohibited after 1 September 2006, applications for extension of this phase-out period have been submitted by individual Member States to the Commission together with information demonstrating a need for further use of the substances concerned. |
|
(5) |
Poland has submitted information demonstrating the temporary absence of suitable alternatives to cyfluthrin with regard to its use as an insecticide for the protection of building timber in historical and other constructions. A brief extension of the phase-out period for this substance seems appropriate, to allow for efficacy data to be submitted for other alternative substances and their placing on the Polish market to become possible according to national legislation. |
|
(6) |
France has submitted information demonstrating the need to provide for as wide a spectrum as possible of available larvicides to combat mosquitoes that are vectors of serious diseases affecting the population of the Member State's overseas departments, and requested to maintain temephos on the market of these regions. An extension of the phase-out period for this substance seems appropriate to allow for its replacement by other suitable substances. |
|
(7) |
France has submitted information demonstrating the need for temporary continuation of the use of ammonia as a veterinary hygiene biocidal product to prevent infections by coccidia, cryptosporidium and nematodes in livestock. An extension of the phase-out period for this substance seems appropriate to permit its gradual replacement by other available substances that are notified for evaluation under the Directive's review programme. |
|
(8) |
The measures provided for in this Decision are in accordance with the opinion of the Standing Committee on Biocidal Products, |
HAS ADOPTED THIS DECISION:
Article 1
By derogation from Article 4(2) of Regulation (EC) No 2032/2003, the Member States listed in column B of the Annex to this Decision may grant or maintain an existing approval for placing on the market of biocidal products containing substances listed in column A of the Annex, for the essential uses listed in column D and until the dates stated in column C of that Annex.
Article 2
1. Member States making use of the derogation provided for in Article 1 of this Decision shall ensure that the following conditions are complied with:
|
(a) |
continued use is only possible under the conditions that products containing the substance are approved for the intended essential use; |
|
(b) |
the continued use is only accepted so far as it has no unacceptable effect on human or animal health or on the environment; |
|
(c) |
all appropriate risk reduction measures are imposed when granting approval; |
|
(d) |
such biocidal products remaining on the market after 1 September 2006 are relabelled in order to match the restricted use conditions; |
|
(e) |
where appropriate, Member States shall ensure that alternatives for such uses are being sought by the holders of the approvals or by the Member States concerned, or that a dossier is being prepared for submission in accordance with the procedure laid down in Article 11 of Directive 98/8/EC by 14 May 2008 at the latest. |
2. Where appropriate, the Member States concerned shall inform the Commission annually on the application of paragraph 1 and in particular on the actions taken pursuant to point (e).
Article 3
This Decision is addressed to the French Republic and the Republic of Poland.
Done at Brussels, 11 April 2007.
For the Commission
Stavros DIMAS
Member of the Commission
(1) OJ L 123, 24.4.1998, p. 1. Directive as last amended by Commission Directive 2006/140/EC (OJ L 414, 30.12.2006, p. 78).
(2) OJ L 228, 8.9.2000, p. 6. Regulation as amended by Regulation (EC) No 2032/2003 (OJ L 307, 24.11.2003, p. 1).
(3) Regulation as last amended by Regulation (EC) No 1849/2006 (OJ L 355, 15.12.2006, p. 63).
ANNEX
List of authorisations referred to in Article 1
|
Column A |
Column B |
Column C |
Column D |
|
Active substance |
Member State |
Dates |
Use |
|
Cyfluthrin EC No 269-855-7 CAS No 68359-37-5 |
Poland |
1.9.2007 |
For the protection of construction wood against insects; for professional use only. |
|
Temephos EC No 222-191-1 CAS No 3383-96-8 |
France |
14.5.2009 |
For vector mosquito control; in the Overseas Departments of France only. |
|
Ammonia EC No 231-635-3 CAS No 7664-41-7 |
France |
14.5.2008 |
Veterinary hygiene biocidal product for the prevention of infections by coccidia, cryptosporidia and nematodes in livestock; only when no other means with similar effect can be used. |
Corrigenda
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/50 |
Corrigendum to Commission Directive 2007/19/EC of 30 March 2007 amending Directive 2002/72/EC relating to plastic materials and articles intended to come into contact with food and Council Directive 85/572/EEC laying down the list of simulants to be used for testing migration of constituents of plastic materials and articles intended to come into contact with foodstuffs
( Official Journal of the European Union L 91 of 31 March 2007 )
Directive 2007/19/EC should read as follows:
COMMISSION DIRECTIVE 2007/19/EC
of 2 April 2007
amending Directive 2002/72/EC relating to plastic materials and articles intended to come into contact with food and Council Directive 85/572/EEC laying down the list of simulants to be used for testing migration of constituents of plastic materials and articles intended to come into contact with foodstuffs
(Text with EEA relevance)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC (1), and in particular Article 5(2) thereof,
After consulting the European Food Safety Authority (the Authority),
Whereas:
|
(1) |
Commission Directive 2002/72/EC (2) is a specific Directive within the meaning of the framework Regulation (EC) No 1935/2004, harmonising the rules for the plastics materials and articles intended to come into contact with food. |
|
(2) |
Directive 2002/72/EC establishes a list of authorised substances for the manufacture of those materials and articles, in particular additives and monomers, the restrictions on their use, rules on labelling as well as the information to be given to consumers or to food business operators concerning correct use of those materials and articles. |
|
(3) |
Information provided to the Commission demonstrates that the plasticizers used e.g. in polyvinyl chloride (PVC) gaskets in lids may migrate into fatty foods in quantities that could endanger human health or bring about an unacceptable change in the composition of the foods. It should therefore be made clear that, even if they are part of e.g. metal lids, gaskets fall under the scope of Directive 2002/72/EC. At the same time, specific rules should be laid down as regard the use of additives for the manufacture of those gaskets. It is appropriate to take account of the need of lid manufacturers to have sufficient time to adapt to some of the provisions of Directive 2002/72/EC. In particular, taking into account the time needed to prepare an application for the evaluation of specific additives used for the manufacture of gaskets of lids, it is not yet possible to establish a timetable for their evaluation. Therefore, in a first stage, the positive list of authorised additives that will be adopted in the future for plastic materials and articles should not apply for the manufacture of gaskets in lids, so that the use of other additives will remain possible, subject to national law. This situation should be reassessed at a later stage. |
|
(4) |
On the basis of new information related to the risk assessment of substances evaluated by the Authority and the need to adapt to technical progress the existing rules for calculating migration, Directive 2002/72/EC should be updated. For reasons of clarity definitions of technical terms used should be introduced. |
|
(5) |
The rules for overall migration and specific migration should be based on the same principle and should therefore, be aligned. |
|
(6) |
Specific rules should be introduced to improve the protection of infants, since infants ingest more food in proportion to their body weight than adults. |
|
(7) |
The verification of compliance with the specific migration limits (SML) in simulant D for additives listed in Annex III, Section B, to Directive 2002/72/EC should be applied at the same time as the other provisions for calculating migration introduced in this Directive for better estimation of the real exposure of the consumer to those additives. Therefore, the deadline for application of the abovementioned verification of compliance should be extended. |
|
(8) |
The status of additives acting as polymerisation production aids (PPA) should be clarified. The PPA which also function as additives are to be evaluated and included in the future positive list of additives. Some of them are already included in the current incomplete list of additives. As regards additives which exclusively act as PPA and are therefore not intended to remain in the finished article, it should be made clear that their use will remain possible, subject to national law, even after the adoption of the future positive list of additives. That situation should be reassessed at a later stage. |
|
(9) |
Studies have shown that azodicarbonamide decomposes into semicarbazide during high temperature processing. In 2003 the Authority was asked to gather data and to assess the possible risks posed by semicarbazide in food. Until that information was obtained and in accordance to Article 7 of Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety (3), the use of azodicarbonamide in plastic materials and articles was suspended by Commission Directive 2004/1/EC (4). In its opinion of 21 June 2005, the Authority (5) concluded that carcinogenicity of semicarbazide is not of concern for human health at the concentrations encountered in food, if the source of semicarbazide related to azodicarbonamide is eliminated. Therefore it is appropriate to maintain the prohibition of use of azodicarbonamide in plastic materials and article. |
|
(10) |
The concept of the plastic functional barrier, that is a barrier within plastic materials or articles preventing or reducing the migration from behind this barrier into the food should be introduced. Only glass and some metals may ensure complete blockage of migration. Plastics may be partial functional barriers with properties and effectiveness to be assessed and may help reducing the migration of a substance below a SML or a limit of detection. Behind a plastic functional barrier, non-authorised substances may be used, provided they fulfil certain criteria and their migration remains below a given detection limit. Taking into account foods for infants and other particularly susceptible persons as well as the difficulties of this type of analysis affected by a large analytical tolerance, a maximum level of 0,01 mg/kg in food or a food simulant should be established for the migration of a non-authorised substance through a plastic functional barrier. |
|
(11) |
Article 9 of Directive 2002/72/EC provides that materials and articles must be accompanied by a written declaration of compliance attesting that they comply with the rules applicable to them. In accordance with Article 5(1)(h) and (i) of Regulation (EC) No 1935/2004, to strengthen the co-ordination and responsibility of the suppliers at each stage of manufacture, including that of the starting substances, the responsible persons should document the compliance with the relevant rules in a declaration of compliance which is made available to his customer. Further, at each stage of manufacture, supporting documentation, substantiating the declaration of compliance, should be kept available for the enforcement authorities. |
|
(12) |
Article 17(1) of Regulation (EC) No 178/2002 requires the food business operator to verify that foods are compliant with the rules applicable to them. To this end subject to the requirement of confidentiality, food business operators should be given access to the relevant information to enable them to ensure that the migration from the materials and articles to food complies with the specifications and restrictions laid down in food legislation. |
|
(13) |
Compliance with Article 3 of Regulation (EC) No 1935/2004 for substances non-listed in Annexes II and III of Directive 2002/72/EC such as impurities or reaction products referred to in point 3 of Annex II and point 3 of Annex III to Directive 2002/72/EC should be assessed by the relevant business operator in accordance with internationally recognised scientific principles. |
|
(14) |
For a more adequate estimation of exposure of the consumer, a new reduction factor should be introduced in migration testing, called Fat Reduction Factor (FRF). Until now, the exposure to substances migrating predominantly into fatty food (lipophilic substances) was based on the general assumption that a person ingests daily 1 kg of food. However, a person ingests at most 200 g of fat on a daily basis. This should be taken into consideration through the correction of the specific migration by the FRF applicable to lipophilic substances in accordance with the opinion of the Scientific Committee on Food (SCF) (6) and the opinion of the Authority (7). |
|
(15) |
On the basis of new information related to the risk assessment of monomers and other starting substances evaluated by the Authority (8), certain monomers provisionally admitted at national level as well as new monomers should be included in the Community list of authorised substances. For others, the restrictions and/or specifications already established at Community level should be amended on the basis of the new information available. |
|
(16) |
The incomplete list of additives which may be used in the manufacture of plastic materials and articles should be amended so as to include other additives evaluated by the Authority. For certain additives, the restrictions and/or specifications already established at Community level should be amended on the basis of those new evaluations available. |
|
(17) |
Commission Directive 2005/79/EC (9) introduced the changes in the restrictions and/or specifications for substance Ref. No 35760 in section A instead of section B of Annex III to Directive 2002/72/EC and for substance Ref. No 67180 the changes were introduced in section B instead of section A of that Annex. In addition, for substances Ref. No 43480, 45200, 81760 and 88640 the indication to the restrictions and/or specifications in Annex III to Directive 2002/72/EC is ambiguous. Therefore, for legal certainty, there is a need to place substances Ref. No 35760 and 67180 in the appropriate section of the list of additives and re-introduce the restrictions and specifications for substances Ref. No 43480, 45200, 81760 and 88640. |
|
(18) |
It has been shown that distilled water, which is used at present is not an adequate simulant for some milk products. It should be replaced by 50 % ethanol, which better simulates their fatty character. |
|
(19) |
Epoxidised soybean oil (ESBO) is used as plasticizer in gaskets. Taking into account the opinion of the Authority adopted on 16 March 2006 (10) concerning exposure of adults to ESBO used in food contact materials, it is appropriate to set a shorter deadline for the compliance of gaskets of lids with the restrictions of ESBO and its substitutes set out in Directive 2002/72/EC. The same deadline should apply as regards the prohibition of use of azodicarbonamide. |
|
(20) |
Certain phthalates are used as plasticizers in gaskets and in other plastic applications. In its opinions on certain phthalates (11) published in September 2005 the Authority set tolerable daily intakes (TDI) for certain phthalates and estimated that the exposure of humans to certain phthalates is in the same range as the TDI. Therefore, it is appropriate to set a shorter deadline for the compliance of plastic materials and articles with the restrictions set in Directive 2002/72/EC for those substances. |
|
(21) |
Council Directive 85/572/EEC (12) and Directive 2002/72/EC should therefore be amended accordingly. |
|
(22) |
The measures provided for in this Directive are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS DIRECTIVE:
Article 1
Directive 2002/72/EC is amended as follows:
|
1. |
Article 1 is amended as follows:
|
|
2. |
The following Article 1a is inserted: ‘Article 1a For the purpose of this Directive the following definitions shall apply:
|
|
3. |
Article 2 is replaced by the following: ‘Article 2 1. Plastic materials and articles shall not transfer their constituents to foodstuffs in quantities exceeding 60 milligrams of the constituents released per kilogram of foodstuff or food simulant (mg/kg) (overall migration limit). However, this limit shall be 10 milligrams per square decimetre of surface area of material or article (mg/dm2) in the case of the following:
2. For plastic materials and articles intended to be brought into contact with or already in contact with food intended for infants and young children, as defined by Commission Directives 91/321/EEC (14) and 96/5/EC (15), the overall migration limit shall always be 60 mg/kg. |
|
4. |
In Article 4(2), the date of ‘1 July 2006’ is replaced by ‘1 May 2008’. |
|
5. |
The following Articles 4c, 4d and 4e are inserted: ‘Article 4c For the use of additives for the manufacture of plastic layers or plastic coatings in lids referred to in Article 1(2)(c), the following rules shall apply:
Article 4d For the use of additives exclusively acting as polymerisation production aids which are not intended to remain in the finished article (hereinafter PPAs), for the manufacture of plastic materials and articles, the following rules shall apply:
Article 4e The use of azodicarbonamide, Ref. No 36640 (CAS No 000123-77-3) in the manufacture of plastic materials and articles is prohibited.’ |
|
6. |
In Article 5a paragraph 2 is replaced by the following: ‘2. At the marketing stages other than the retail stages, plastic materials and articles which are intended to be placed in contact with foodstuffs and which contain additives referred to in paragraph 1 shall be accompanied by a written declaration containing the information referred to in Article 9.’ |
|
7. |
In Article 7 the following paragraph is added: ‘For plastic materials and articles intended to be brought into contact with or already in contact with food for infants and young children, as defined by Directives 91/321/EEC and 96/5/EC, the SMLs shall always be applied as mg/kg.’ |
|
8. |
The following Article 7a is inserted: ‘Article 7a 1. In a plastic multi-layer material or article, the composition of each plastic layer shall comply with this Directive. 2. By way of derogation from paragraph 1, a layer which is not in direct contact with food and is separated from the food by a plastic functional barrier, may, provided that the finished material or article complies with the specific and overall migration limits specified in this Directive:
3. The migration of the substances referred to in paragraph 2(b) into food or simulant shall not exceed 0,01 mg/kg, measured with statistical certainty by a method of analysis in accordance with Article 11 of Regulation (EC) No 882/2004 of the European Parliament and of the Council (16). This limit shall always be expressed as concentration in foods or simulants. It shall apply to a group of compounds, if they are structurally and toxicologically related, in particular isomers or compounds with the same relevant functional group, and shall include possible set-off transfer. 4. The substances referred to in paragraph 2(b) shall not belong to either of the following categories:
|
|
9. |
In Article 8 the following paragraph 5 is added: ‘5. Notwithstanding paragraph 1, for phthalates (Ref. No 74640, 74880, 74560, 75100, 75105) referred to in Annex III Section B, the verification of the SML shall only be performed in food simulants. However, verification of the SML may be performed in food where the food has not already been in contact with the material or article and is pre-tested for the phthalate and the level is not statistically significant or greater than or equal to the limit of quantification.’ |
|
10. |
Article 9 is replaced by the following: ‘Article 9 1. At the marketing stages other than the retail stage, plastic materials and articles as well as the substances intended for the manufacturing of those materials and articles, shall be accompanied by a written declaration in accordance with Article 16 of Regulation (EC) No 1935/2004. 2. The declaration referred to in paragraph 1 shall be issued by the business operator and shall contain the information laid down in Annex VIa. 3. Appropriate documentation to demonstrate that the materials and articles as well as the substances intended for the manufacturing of those materials and articles comply with the requirements of this Directive shall be made available by the business operator to the national competent authorities on request. That documentation shall contain the conditions and results of testing, calculations, other analysis, and evidence on the safety or reasoning demonstrating compliance.’ |
|
11. |
Annexes I, II and III are amended in accordance with Annexes I, II and III to this Directive. |
|
12. |
The text in Annex IV to this Directive is inserted as Annex IVa. |
|
13. |
Annexes V and VI are amended in accordance with Annexes V and VI to this Directive. |
|
14. |
The text in Annex VII to this Directive is inserted as Annex VIa. |
Article 2
The Annex to Directive 85/572/EEC is amended in accordance with Annex VIII to this Directive.
Article 3
1. Member States shall adopt and publish, by 1 May 2008 at the latest, the laws, regulations and administrative provisions necessary to comply with this Directive. They shall forthwith communicate to the Commission the text of those provisions and a correlation table between those provisions and this Directive.
When Member States adopt those provisions, they shall contain a reference to this Directive or be accompanied by such a reference on the occasion of their official publication. Member States shall determine how such reference is to be made.
They shall apply those provisions in such a way as to:
|
(a) |
permit the trade in and use of plastic materials and articles intended to come into contact with food and complying with Directive 2002/72/EC, as amended by this Directive, from 1 May 2008; |
|
(b) |
prohibit the manufacture and importation into the Community of lids containing a gasket which do not comply with restrictions and specifications for Ref. No 30340; 30401; 36640; 56800; 76815; 76866; 88640 and 93760 laid down in Directive 2002/72/EC as amended by this Directive from 1 July 2008; |
|
(c) |
prohibit the manufacture and importation into the Community of plastic materials and articles intended to come into contact with food which do not comply with restrictions and specifications for phthalates Ref. No 74560; 74640; 74880; 75100; 75105 laid down in Directive 2002/72/EC as amended by this Directive from 1 July 2008; |
|
(d) |
without prejudice to point (b) and (c), prohibit the manufacture and importation into the Community of plastic materials and articles intended to come into contact with food which do not comply with Directive 2002/72/EC as amended by this Directive from 1 May 2009. |
2. Member States shall communicate to the Commission the text of the main provisions of national law which they adopt in the field covered by this Directive.
Article 4
This Directive shall enter into force on the 20th day following its publication in the Official Journal of the European Union.
Article 5
This Directive is addressed to the Member States.
Done at Brussels, 2 April 2007.
For the Commission
Markos KYPRIANOU
Member of the Commission
ANNEX I
Annex I to Directive 2002/72/EC is amended as follows:
|
(1) |
The following points 2a and 2b are inserted: 2a. Correction of specific migration in foods containing more than 20 % fat by the Fat Reduction Factor (FRF): “Fat Reduction Factor” (FRF) is a factor between 1 and 5 by which measured migration of lipophilic substances into a fatty food or simulant D and its substitutes shall be divided before comparison with the specific migration limits. General rules Substances considered “lipophilic” for the application of the FRF are listed in Annex IVa. The specific migration of lipophilic substances in mg/kg (M) shall be corrected by the FRF variable between 1 and 5 (MFRF). The following equations shall be applied before comparison with the legal limit: MFRF = M/FRF and FRF = (g fat in food/kg of food)/200 = (% fat × 5)/100 This correction by the FRF is not applicable in the following cases:
This correction by the FRF is applicable under certain conditions in the following case: For containers and other fillable articles with a capacity of less than 500 millilitres or more than 10 litres and for sheets and films in contact with foods containing more than 20 % fat, either the migration is calculated as concentration in the food or food simulant (mg/kg) and corrected by the FRF, or it is re-calculated as mg/dm2 without applying the FRF. If one of the two values is below the SML, the material or article shall be considered in compliance. The application of the FRF shall not lead to a specific migration exceeding the overall migration limit. 2b. Correction of specific migration in food simulant D: The specific migration of lipophilic substances into simulant D and its substitutes shall be corrected by the following factors:
|
|
(2) |
The following point 5a is inserted: 5a. Caps, lids, gaskets, stoppers and similar sealing articles:
|
ANNEX II
Annex II to Directive 2002/72/EC is amended as follows:
|
(1) |
Section A is amended as follows:
|
|
(2) |
In section B, the following monomers and other starting substances are deleted:
|
ANNEX III
Annex III to Directive 2002/72/EC is amended as follows:
|
(1) |
Section A is amended as follows:
|
|
(2) |
Section B is amended as follows:
|
ANNEX IV
‘ANNEX IVa
LIPOPHILIC SUBSTANCES FOR WHICH THE FRF APPLIES
|
Ref. No |
CAS No |
Name |
|
31520 |
061167-58-6 |
Acrylic acid, 2-tert-butyl-6-(3-tert-butyl-2-hydroxy-5-methylbenzyl)-4-methylphenyl ester |
|
31530 |
123968-25-2 |
Acrylic acid, 2,4-di-tert-pentyl-6-[1-(3,5-di-tert-pentyl-2-hydroxyphenyl)ethyl]phenyl ester |
|
31920 |
000103-23-1 |
Adipic acid, bis(2-ethylhexyl) ester |
|
38240 |
000119-61-9 |
Benzophenone |
|
38515 |
001533-45-5 |
4,4′-Bis(2-benzoxazolyl)stilbene |
|
38560 |
007128-64-5 |
2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene |
|
38700 |
063397-60-4 |
Bis(2-carbobutoxyethyl)tin-bis(isooctyl mercaptoacetate) |
|
38800 |
032687-78-8 |
N,N′-Bis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl)hydrazide |
|
38810 |
080693-00-1 |
Bis(2,6-di-tert-butyl-4-methylphenyl)pentaerythritol diphosphite |
|
38820 |
026741-53-7 |
Bis(2,4-di-tert-butylphenyl)pentaerythritol diphosphite |
|
38840 |
154862-43-8 |
Bis(2,4-dicumylphenyl)pentaerythritoldiphosphite |
|
39060 |
035958-30-6 |
1,1-Bis(2-hydroxy-3,5-di-tert-butylphenyl)ethane |
|
39925 |
129228-21-3 |
3,3-Bis(methoxymethyl)-2,5-dimethylhexane |
|
40000 |
000991-84-4 |
2,4-Bis(octylmercapto)-6-(4-hydroxy-3,5-di-tert-butylanilino)-1,3,5-triazine |
|
40020 |
110553-27-0 |
2,4-Bis(octylthiomethyl)-6-methylphenol |
|
40800 |
013003-12-8 |
4,4′-Butylidene-bis(6-tert-butyl-3-methylphenyl-ditridecyl phosphite) |
|
42000 |
063438-80-2 |
(2-Carbobutoxyethyl)tin-tris(isooctyl mercaptoacetate) |
|
45450 |
068610-51-5 |
p-Cresol-dicyclopentadiene-isobutylene, copolymer |
|
45705 |
166412-78-8 |
1,2-cyclohexanedicarboxylic acid, diisononyl ester |
|
46720 |
004130-42-1 |
2,6-Di-tert-butyl-4-ethylphenol |
|
47540 |
027458-90-8 |
Di-tert-dodecyl disulphide |
|
47600 |
084030-61-5 |
Di-n-dodecyltin bis(isooctyl mercaptoacetate) |
|
48800 |
000097-23-4 |
2,2′-Dihydroxy-5,5′-dichlorodiphenylmethane |
|
48880 |
000131-53-3 |
2,2′-Dihydroxy-4-methoxybenzophenone |
|
49485 |
134701-20-5 |
2,4-Dimethyl-6-(1-methylpentadecyl)-phenol |
|
49840 |
002500-88-1 |
Dioctadecyl disulphide |
|
51680 |
000102-08-9 |
N,N′-Diphenylthiourea |
|
52320 |
052047-59-3 |
2-(4-Dodecylphenyl)indole |
|
53200 |
023949-66-8 |
2-Ethoxy-2′-ethyloxanilide |
|
54300 |
118337-09-0 |
2,2′-Ethylidenebis(4,6-di-tert-butyl phenyl) fluorophosphonite |
|
59120 |
023128-74-7 |
1,6-Hexamethylene-bis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionamide) |
|
59200 |
035074-77-2 |
1,6-Hexamethylene-bis(3-(3,5-di-tert-butyl–4-hydroxyphenyl)propionate) |
|
60320 |
070321-86-7 |
2-[2-Hydroxy-3,5-bis(1,1-dimethylbenzyl)phenyl]benzotriazole |
|
60400 |
003896-11-5 |
2-(2′-Hydroxy-3′-tert-butyl-5′-methylphenyl)-5-chlorobenzotriazole |
|
60480 |
003864-99-1 |
2-(2′-Hydroxy-3,5′-di-tert-butylphenyl)-5-chlorobenzotriazole |
|
61280 |
003293-97-8 |
2-Hydroxy-4-n-hexyloxybenzophenone |
|
61360 |
000131-57-7 |
2-Hydroxy-4-methoxybenzophenone |
|
61600 |
001843-05-6 |
2-Hydroxy-4-n-octyloxybenzophenone |
|
66360 |
085209-91-2 |
2,2′-Methylene bis(4,6-di-tert-butylphenyl) sodium phosphate |
|
66400 |
000088-24-4 |
2,2′-Methylene bis(4-ethyl-6-tert-butylphenol) |
|
66480 |
000119-47-1 |
2,2′-Methylene bis(4-methyl-6-tert-butylphenol) |
|
66560 |
004066-02-8 |
2,2′-Methylene bis(4-methyl-6-cyclohexylphenol) |
|
66580 |
000077-62-3 |
2,2′-Methylene bis(4-methyl-6-(1-methyl-cyclohexyl) phenol) |
|
68145 |
080410-33-9 |
2,2′,2′-Nitrilo[triethyl tris(3,3′,5,5′-tetra-tert-butyl-1,1′-bi-phenyl-2,2′-diyl)phosphite] |
|
68320 |
002082-79-3 |
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate |
|
68400 |
010094-45-8 |
Octadecylerucamide |
|
69840 |
016260-09-6 |
Oleylpalmitamide |
|
71670 |
178671-58-4 |
Pentaerythritol tetrakis (2-cyano-3,3-diphenylacrylate) |
|
72081/10 |
— |
Petroleum Hydrocarbon Resins (hydrogenated) |
|
72160 |
000948-65-2 |
2-Phenylindole |
|
72800 |
001241-94-7 |
Phosphoric acid, diphenyl 2-ethylhexyl ester |
|
73160 |
— |
Phosphoric acid, mono- and di-n-alkyl (C16 and C18) esters |
|
74010 |
145650-60-8 |
Phosphorous acid, bis(2,4-di-tert-butyl-6-methylphenyl) ethyl ester |
|
74400 |
— |
Phosphorous acid, tris(nonyl- and/or dinonylphenyl) ester |
|
76866 |
— |
Polyesters of 1,2-propanediol and/or 1,3- and/or 1,4-butanediol and/or polypropyleneglycol with adipic acid, also end-capped with acetic acid or fatty acids C12-C18 or n-octanol and/or n-decanol |
|
77440 |
— |
Polyethyleneglycol diricinoleate |
|
78320 |
009004-97-1 |
Polyethyleneglycol monoricinoleate |
|
81200 |
071878-19-8 |
Poly[6-[(1,1,3,3-tetramethylbutyl)amino]-1,3,5-triazine-2,4-diyl]-[(2,2,6,6-tetramethyl-4-piperidyl)-imino]hexamethylene[(2,2,6,6-tetramethyl-4-piperidyl)imino] |
|
83599 |
068442-12-6 |
Reaction products of oleic acid, 2-mercaptoethyl ester, with dichlorodimethyltin, sodium sulphide and trichloromethyltin |
|
83700 |
000141-22-0 |
Ricinoleic acid |
|
84800 |
000087-18-3 |
Salicylic acid, 4-tert-butylphenyl ester |
|
92320 |
— |
Tetradecyl-polyethyleneglycol(EO=3-8) ether of glycolic acid |
|
92560 |
038613-77-3 |
Tetrakis(2,4-di-tert-butyl-phenyl)-4,4′-biphenylylene diphosphonite |
|
92700 |
078301-43-6 |
2,2,4,4-Tetramethyl-20-(2,3-epoxypropyl)-7-oxa-3,20-diazadispiro[5.1.11.2]-heneicosan-21-one, polymer |
|
92800 |
000096-69-5 |
4,4′-Thiobis(6-tert-butyl-3-methylphenol) |
|
92880 |
041484-35-9 |
Thiodiethanol bis(3-(3,5-di-tert-butyl-4-hydroxy phenyl) propionate) |
|
93120 |
000123-28-4 |
Thiodipropionic acid, didodecyl ester |
|
93280 |
000693-36-7 |
Thiodipropionic acid, dioctadecyl ester |
|
95270 |
161717-32-4 |
2,4,6-Tris(tert-butyl)phenyl-2-butyl-2-ethyl-1,3-propanediol phosphite |
|
95280 |
040601-76-1 |
1,3,5-Tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione |
|
95360 |
027676-62-6 |
1,3,5-Tris(3,5-di-tert-butyl-4-hydroxybenzyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione |
|
95600 |
001843-03-4 |
1,1,3-Tris(2-methyl-4-hydroxy-5-tert-butylphenyl) butane’ |
ANNEX V
Annex V to Directive 2002/72/EC is amended as follows:
|
(1) |
Part A is replaced by the following: ‘Part A: General specifications Plastic material and articles shall not release primary aromatic amines in a detectable quantity (DL = 0,01 mg/kg of food or food simulant). The migration of the primary aromatic amines appearing in the lists in Annex II and III is excluded from this restriction.’ |
|
(2) |
In Part B, the following new specifications are inserted, in the appropriate numerical order:
|
ANNEX VI
Annex VI to Directive 2002/72/EC is amended as follows:
|
(1) |
Note (8) is replaced by the following:
|
|
(2) |
The following notes 41 and 42 are added:
|
ANNEX VII
‘ANNEX VIa
DECLARATION OF COMPLIANCE
The written declaration referred to in Article 9 shall contain the following information:
|
(1) |
the identity and address of the business operator which manufactures or imports the plastic materials or articles or the substances intended for the manufacturing of those materials and articles; |
|
(2) |
the identity of the materials, the articles or the substances intended for the manufacturing of those materials and articles; |
|
(3) |
the date of the declaration; |
|
(4) |
confirmation that the plastic materials or articles meet relevant requirements laid down in this Directive and Regulation (EC) No 1935/2004; |
|
(5) |
adequate information relative to the substances used for which restrictions and/or specifications are in place under this Directive to allow the downstream business operators to ensure compliance with those restrictions; |
|
(6) |
adequate information relative to the substances which are subject to a restriction in food, obtained by experimental data or theoretical calculation about the level of their specific migration and, where appropriate, purity criteria in accordance with Directives 95/31/EC, 95/45/EC and 96/77/EC to enable the user of these materials or articles to comply with the relevant Community provisions or, in their absence, with national provisions applicable to food; |
|
(7) |
specifications on the use of the material or article, such as:
|
|
(8) |
when a plastic functional barrier is used in a plastic multi-layer material or article, the confirmation that the material or article complies with the requirements of Article 7a(2), (3) and 4 of this Directive. |
The written declaration shall permit an easy identification of the materials, articles or substances for which it is issued and shall be renewed when substantial changes in the production bring about changes in the migration or when new scientific data are available.’
ANNEX VIII
The Annex to Directive 85/572/EEC is amended as follows:
|
(1) |
Point 3 is replaced by the following:
|
|
(2) |
The following point 4a is inserted:
|
|
(3) |
In the table, Section 07 is replaced by the following:
|
(1) OJ L 338, 13.11.2004, p. 4.
(2) OJ L 220, 15.8.2002, p. 18. Directive as last amended by Directive 2005/79/EC (OJ L 302, 19.11.2005, p. 35).
(3) OJ L 31, 1.2.2002, p. 1. Regulation as last amended by Commission Regulation (EC) No 575/2006 (OJ L 100, 8.4.2006, p. 3).
(5) The EFSA Journal (2005) 219, 1-36.
(6) SCF opinion of 4 December 2002 on the introduction of a Fat (Consumption) Reduction Factor (FRF) in the estimation of the exposure to a migrant from food contact materials.
http://ec.europa.eu/food/fs/sc/scf/out149_en.pdf
(7) Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to the introduction of a Fat (consumption) Reduction Factor for infants and children, The EFSA Journal (2004) 103, 1-8.
(8) The EFSA Journal (2005) 218, 1-9.
The EFSA Journal (2005) 248, 1-16.
The EFSA Journal (2005) 273, 1-26.
The EFSA Journal (2006) 316 to 318, 1-10.
The EFSA Journal (2006) 395 to 401, 1-21.
(9) OJ L 302, 19.11.2005, p. 35.
(10) The EFSA Journal (2006) 332, 1-9.
(11) The EFSA Journal (2005) 244, 1-18.
The EFSA Journal (2005) 245, 1-14.
The EFSA Journal (2005) 243, 1-20.
The EFSA Journal (2005) 242, 1-17.
The EFSA Journal (2005) 241, 1-14.
(12) OJ L 372, 31.12.1985, p. 14.
(13) OJ L 338, 13.11.2004, p. 4.’
(14) OJ L 175, 4.7.1991, p. 35.
(15) OJ L 49, 28.2.1996, p. 17.’
(16) OJ L 165, 30.4.2004, p. 1, as corrected by OJ L 191, 28.5.2004, p. 1.
(17) OJ 196, 16.8.1967, p. 1.’
(18) OJ L 339, 30.12.1996, p. 1.’
|
12.4.2007 |
EN |
Official Journal of the European Union |
L 97/70 |
Corrigendum to Commission Regulation (EC) No 372/2007 of 2 April 2007 laying down transitional migration limits for plasticisers in gaskets in lids intended to come into contact with foods
( Official Journal of the European Union L 92 of 3 April 2007 )
On page 9, recital 2 is replaced by the following:
|
‘(2) |
Commission Directive 2007/19/EC of 2 April 2007 amending Directive 2002/72/EC relating to plastic materials and articles intended to come into contact with food (3) clarifies that gaskets in lids fall under the scope of Directive 2002/72/EC. It stipulates that Member States have to adopt measures by 1 May 2008 that allow free circulation of gaskets in lids if they comply with SML. Non-compliant gaskets in lids will be prohibited as from 1 July 2008.’ |
On page 9, footnote 3 is replaced by the following:
‘OJ L 91, 31.3.2007, p. 17, corrected by OJ L 97, 12.4.2007, p. 50.’