ISSN 1977-091X
Official Journal
of the European Union
C 305

English edition
Information and Notices
Volume 61
30 August 2018
|
ISSN 1977-091X |
||
|
Official Journal of the European Union |
C 305 |
|

|
||
|
English edition |
Information and Notices |
Volume 61 |
|
Contents |
page |
|
|
|
II Information |
|
|
|
INFORMATION FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES |
|
|
|
European Commission |
|
|
2018/C 305/01 |
Non-opposition to a notified concentration (Case M.8970 — Sumitomo/Parkwind/Northwester2) ( 1 ) |
|
|
V Announcements |
|
|
|
ADMINISTRATIVE PROCEDURES |
|
|
|
European Personnel Selection Office (EPSO) |
|
|
2018/C 305/13 |
||
|
|
PROCEDURES RELATING TO THE IMPLEMENTATION OF COMPETITION POLICY |
|
|
|
European Commission |
|
|
2018/C 305/14 |
Prior notification of a concentration (Case M.9072 — KKR/Altice/SFR Filiale) — Candidate case for simplified procedure ( 1 ) |
|
|
|
|
|
(1) Text with EEA relevance. |
|
EN |
|
II Information
INFORMATION FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES
European Commission
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/1 |
Non-opposition to a notified concentration
(Case M.8970 — Sumitomo/Parkwind/Northwester2)
(Text with EEA relevance)
(2018/C 305/01)
On 30 July 2018, the Commission decided not to oppose the above notified concentration and to declare it compatible with the internal market. This decision is based on Article 6(1)(b) of Council Regulation (EC) No 139/2004 (1). The full text of the decision is available only in English and will be made public after it is cleared of any business secrets it may contain. It will be available:
|
— |
in the merger section of the Competition website of the Commission (http://ec.europa.eu/competition/mergers/cases/). This website provides various facilities to help locate individual merger decisions, including company, case number, date and sectoral indexes, |
|
— |
in electronic form on the EUR-Lex website (http://eur-lex.europa.eu/homepage.html?locale=en) under document number 32018M8970. EUR-Lex is the online access to European law. |
IV Notices
NOTICES FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES
European Commission
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/2 |
Euro exchange rates (1)
29 August 2018
(2018/C 305/03)
1 euro =
|
|
Currency |
Exchange rate |
|
USD |
US dollar |
1,1660 |
|
JPY |
Japanese yen |
129,73 |
|
DKK |
Danish krone |
7,4571 |
|
GBP |
Pound sterling |
0,90500 |
|
SEK |
Swedish krona |
10,6923 |
|
CHF |
Swiss franc |
1,1385 |
|
ISK |
Iceland króna |
124,90 |
|
NOK |
Norwegian krone |
9,7475 |
|
BGN |
Bulgarian lev |
1,9558 |
|
CZK |
Czech koruna |
25,745 |
|
HUF |
Hungarian forint |
324,63 |
|
PLN |
Polish zloty |
4,2838 |
|
RON |
Romanian leu |
4,6417 |
|
TRY |
Turkish lira |
7,5236 |
|
AUD |
Australian dollar |
1,5989 |
|
CAD |
Canadian dollar |
1,5093 |
|
HKD |
Hong Kong dollar |
9,1524 |
|
NZD |
New Zealand dollar |
1,7413 |
|
SGD |
Singapore dollar |
1,5941 |
|
KRW |
South Korean won |
1 299,27 |
|
ZAR |
South African rand |
16,8176 |
|
CNY |
Chinese yuan renminbi |
7,9626 |
|
HRK |
Croatian kuna |
7,4370 |
|
IDR |
Indonesian rupiah |
17 087,73 |
|
MYR |
Malaysian ringgit |
4,8076 |
|
PHP |
Philippine peso |
62,375 |
|
RUB |
Russian rouble |
79,4075 |
|
THB |
Thai baht |
38,140 |
|
BRL |
Brazilian real |
4,8451 |
|
MXN |
Mexican peso |
22,3252 |
|
INR |
Indian rupee |
82,3405 |
(1) Source: reference exchange rate published by the ECB.
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/3 |
Explanatory Notes to the Combined Nomenclature of the European Union
(2018/C 305/04)
Pursuant to Article 9(1)(a) of Council Regulation (EEC) No 2658/87 (1), the Explanatory Notes to the Combined Nomenclature of the European Union (2) are hereby amended as follows:
On page 379
|
9401 |
Seats (other than those of heading 9402), whether or not convertible into beds, and parts thereof |
The following text shall be added after the existing text:
‘For the purposes of this heading, any reference to bamboo applies only to vegetable materials of heading 1401. On the other hand, for the purposes of this heading, any reference to wood or wooden applies also to bamboo plates of heading 4412. (See also note 1(b) and note 6 to Chapter 44).’
|
9403 |
Other furniture and parts thereof |
The following text shall be added after the existing paragraph:
‘The Explanatory Note to heading 9401 regarding the references to “bamboo” and “wood or wooden”, applies mutatis mutandis.’
(1) Council Regulation (EEC) No 2658/87 of 23 July 1987 on the tariff and statistical nomenclature and on the Common Customs Tariff (OJ L 256, 7.9.1987, p. 1).
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/4 |
Explanatory Notes to the Combined Nomenclature of the European Union
(2018/C 305/05)
Pursuant to Article 9(1)(a) of Council Regulation (EEC) No 2658/87 (1), the Explanatory Notes to the Combined Nomenclature of the European Union (2) are hereby amended as follows:
On page 379
|
9403 |
Other furniture and parts thereof |
The following text shall be added after the existing text:
‘This heading does not include “information displays” such as “street boards” and “roll-ups”.
They are to be classified in other headings of the Nomenclature under which they are more specifically included (for example, street boards with writing or drawing surfaces corresponding to products of heading 9610) or according to their constituent material:
|
(a) |
under a heading specifically covering these articles (for example, plates of base metal corresponding to products of heading 8310 are classified under this heading), or |
|
(b) |
under a heading covering various articles of this material (for example, heading 3926 or heading 7616). |
Example of a street board to be classified in heading 9610:

Street board with a blackboard surface.
Example of a street board to be classified in heading 8310:
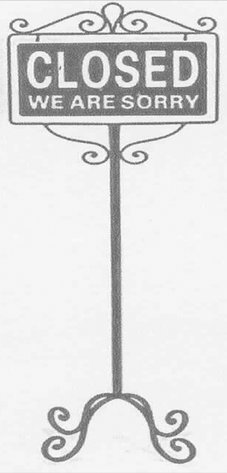
Street board made solely of base metal.
Examples of “information displays” which are to be classified according to their constituent material, under a heading covering various articles of this material:
|
|
|
|
A base of hard plastics, a top of an aluminium frame with a plastic sheet in the middle covered by transparent PVC foils from both sides. |
A base and a frame of aluminium with rubber attachments and transparent PVC foils covering a sheet of paper. |
|
Heading 7616 (the essential character is provided by the aluminium frame). |
Heading 7616 (the essential character is provided by the aluminium frame). |
|
|
|
|
A central plastic plate attached to five plastic rods (bars) of almost equal length, all of which can be tilted in different directions. Four of them have a plastic hook at the end and a plastic cap is mounted on the fifth rod. |
|
|
Heading 3926 (the article is made solely of plastics).’ |
|
(1) Council Regulation (EEC) No 2658/87 of 23 July 1987 on the tariff and statistical nomenclature and on the Common Customs Tariff (OJ L 256, 7.9.1987, p. 1).
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/6 |
New national side of euro coins intended for circulation
(2018/C 305/06)

National side of the new commemorative 2-euro coin intended for circulation and issued by Luxembourg
Euro coins intended for circulation have legal tender status throughout the euro area. For the purpose of informing the public and all parties who handle the coins, the Commission publishes a description of the designs of all new coins (1). In accordance with the Council conclusions of 10 February 2009 (2), euro-area Member States and countries that have concluded a monetary agreement with the European Union providing for the issuing of euro coins are allowed to issue commemorative euro coins intended for circulation, provided that certain conditions are met, particularly that only the 2-euro denomination is used. These coins have the same technical characteristics as other 2-euro coins, but their national face features a commemorative design that is highly symbolic in national or European terms.
Issuing country : Luxembourg
Subject of commemoration : The 175th anniversary of the death of the Grand Duke Guillaume Ist
Description of the design : The design shows on the right hand the effigy of His Royal Highness, the Grand Duke Henri, looking to the left and on the left hand the effigy of HRH the Grand Duke Guillaume Ist. Between both effigies are depicted vertically the year-dates ‘1772-1843’ as well as the name ‘Guillaume Ier’. At the bottom appears the text ‘LUXEMBOURG’ and the year-date ‘2018’.
The coin’s outer ring depicts the 12 stars of the European flag.
Estimated number of coins to be issued :
500 000Date of issue : September 2018
(1) See OJ C 373, 28.12.2001, p. 1 for the national faces of all the coins issued in 2002.
(2) See the conclusions of the Economic and Financial Affairs Council of 10 February 2009 and the Commission Recommendation of 19 December 2008 on common guidelines for the national sides and the issuance of euro coins intended for circulation (OJ L 9, 14.1.2009, p. 52).
European Data Protection Supervisor
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/7 |
Summary of the opinion on the proposal for a recast of the Public Sector Information (PSI) re-use Directive
(The full text of this Opinion can be found in English, French and German on the EDPS website www.edps.europa.eu)
(2018/C 305/07)
The Public Sector Information (PSI) Directive aims to facilitate the re-use of public sector information throughout the European Union by harmonising the basic conditions that make PSI available to re-users, to enhance the development of Community products and services based on PSI and to avoid distortions in competition.
The new provisions include the extension of the scope of the Directive to documents held by public undertakings active in the areas on procurement such as entities operating in the water, energy, transport and postal services sectors. Moreover, it applies to documents held by public undertakings acting as public services operators, as long as such documents are produced as part of the services in the general interest. In addition, the Proposal's scope will also be extended to specific research data such as results of scientific fact-finding processes.
The Opinion focuses on specific recommendations in order to better clarify the relation and coherence of the PSI Directive with the GDPR exceptions and on the reference to applicable data protection law. Additionally it provides for further recommendations on anonymisation and its relation to costs and data protection, also focusing on a data protection impact assessment, while taking into account an ‘acceptable re-use policy’.
The EDPS with this Opinion on PSI re-use builds on the work already done on the ‘Good Big Data’ (the ‘EU values-based data sharing’), and notably on EDPS opinions and formal comments previously issued, consistently with our practice on supervision cases. Moreover, we point out to the issues that need harmonization at EU level to allow the recast of the PSI Directive to rip the expected benefits.
In the context of Article 1(2)(g) of the Proposal, the EDPS recommends to better clarify the relationship and coherence of the PSI with the GDPR by putting forward a drafting suggestion.
Moreover, the EDPS suggests to re-introduce the specific provision currently contained in Article 1(4) of the Directive 2013/37/EU in the main provisions of the Directive and to clealry state in the Proposal that the definition of ‘personal data’ according to Article 4(1) of the GDPR applies. The EDPS also recommends to add the reference to the Supervisory Authority set up by Article 51 of the GDPR under Article 4(4) of the Proposal.
The EDPS also recommends to support the use of anonymisation by making a reference to ‘anonymous information’ in the legal text and extending the scope of the entities entitled to include anonmysation costs within the costs that can be charged to reusers.
As a last recommendation, the EDPS suggests to provide for data protection impact assessments, for specific sectors dealing with sensitive data, such as the health sector, on which the licensor should base its decision and consequently take into account the conditions for re-use.
1. INTRODUCTION AND BACKGROUND
|
1. |
On 25 April 2018, the Commission adopted a Proposal for a Directive amending Directive 2013/37/EU (following a review of Directive 2003/98/EC) on the re-use of public sector information (PSI) (the ‘Proposal’). The Proposal is part of the ‘2018 Data Package’, which also includes other important documents: (i) a Commission Communication entitled ‘Towards a common European data space’ (the ‘Communication’); (ii) Guidance on sharing private sector data, in the form of a Staff Working Document (‘Guidance’); and (iii) an evaluation of the PSI Directive. |
|
2. |
The objective of the Proposal is to update and amend the existing text of Directive 2013/37/EU and Directive 2003/98/EC on re-use of public sector information (the PSI Directive). |
|
3. |
The review of the Directive is one of the three ‘measures’ proposed by the Commission towards a common data space in the EU (see the ‘umbrella’ Communication from the Commission COM (2018) 232, hence ‘the Communication’), together with the Guidance on sharing private sector data […] and the update of the Recommendation on access to and preservation of scientific information […]. |
|
4. |
In proposing to amend the PSI Directive, the European Commission aims to facilitate the re-use of public sector information such as legal, traffic, meteorological, economic and financial data throughout the European Union by harmonising the basic conditions that make PSI available to re-users, to enhance the development of Community products and services based on PSI and to avoid distortions in competition. |
|
5. |
In particular, the Proposal's overall objective is to be in line with the Digital Single Market Strategy's objectives. The Proposal aims to enhance the effect of the Directive by strengthening specific provisions and modifying them accordingly in order to increase the amount of public sector data available for re-use. Specifically, the initiative also aims to strengthen Small and Medium Enterprises' position in the data market by granting fairer competition and an easier access to markets, together with the enhancement of cross-border innovation. |
|
6. |
Relevant new provisions to the Directive include the extension of its scope to documents held by public undertakings active in the areas on procurement by entities operating in the water, energy, transport and postal services sectors. Moreover, it applies to documents held by public undertakings acting as public services operators, as long as such documents are produced as part of the services in the general interest. The proposal's scope will also be extended to specific research data such as results of scientific fact-finding processes (i.e. experiments and surveys). The Proposal in practice ‘(…) lays down a horizontal framework providing minimum harmonisation of reuse conditions across domains and sectors.’ (1) |
|
7. |
The EDPS positively notes that according to the European Commission the recast of the PSI Directive aims to foster the reuse of public sector information, as pointed out in the Communication, by ‘reducing market entry barriers, in particular for small and medium-sized enterprises; minimising the risk of excessive first-mover advantage, which benefits large companies and thereby limits the number of users of the data in question; increasing business opportunities by encouraging the publication of dynamic data and the uptake of application programming interfaces (APIs).’ (2) |
|
8. |
The PSI directive is part of the EU vision on the fostering of ‘Good Big Data’. Public sector information is a key source of ‘the raw material’ of the Big Data of the Digital Single Market. The smart use of data, including its processing via Artificial Intelligence, can have a transformative effect on all sectors of the economy. |
|
9. |
Already in September 2016, the EDPS, with the Opinion on coherent enforcement of fundamental rights in the age of big data (3), has put forward a strategy for shaping an EU cyberspace based on EU values, pointing out to issues such as concentration of market and informational power; and a weak market for Privacy Enhancing Technologies (‘PETs’) as measures for minimising personal data processing without losing the functionality of a product or a service (as inspired by the principle of privacy by design (4) and by default). |
|
10. |
Moreover, the EDPS would like to recall the data protection-relevance of the ‘key principles’ that, according to the European Commission, should be respected in the context of data re-use, namely (i) minimised data lock-in and ensure undistorted competition; (ii) transparency and societal participation on the purpose of the reuse vis-à-vis the citizens/data subjects as well as transparency and clear purpose definition between the licensor and the licensees; (iii) data protection impact assessment and appropriate data protection safeguards for reuse (according to a ‘do no harm’- under the data protection viewpoint- principle). |
|
11. |
While the EDPS has been informally consulted by the European Commission, it has not been formally consulted as required by Article 28(2) of Regulation (EC) No 45/2001. The Opinion is therefore based on Article 41(2) of the same Regulation. The EDPS recommends that a reference to this Opinion be included in the preamble of the adopted instrument. |
7. CONCLUSION
Therefore the EDPS recommends:
|
— |
to modify Article 1(2)(g) of the Proposal and to provide for specific wording on the difference between ‘documents’ and ‘parts of documents’ to which the PSI Directive would not be applicable on data protection grounds. |
|
— |
to add a reference to the Supervisory Autority set up by Article 51 of the GDPR under Article 4(4) of the Proposal, in order to further enhance the link between the re-use of public sector information and the protection of personal data. |
|
— |
to re-introduce the specific provision on applicable data protection law currently contained in Article 1(4) of Directive 2013/37/EU in the substantive part of the Proposal (including the necessary update of references to the legal instruments currently in force). |
|
— |
to further point out to the use of anonymisation in the context of the reuse of public sector information by including a reference to ‘anonymous information’ in the legal text and extending the scope of the entities entitled to include anonmysation costs within the costs that can be charged to reusers. |
|
— |
to clearly state in the Proposal that the definition of ‘personal data’ according to Article 4(1) of the GDPR applies. |
|
— |
to provide for data protection impact assessments, for specific sectors dealing with sensitive data, such as the health sector, on which the licensor should base its decision and consequently take into account the conditions for re-use. |
|
— |
As a last comment, in putting forward these recommendations, the EDPS stresses the data protection-relevance of the following ‘key principles’, that according to the Commission should be respected in the context of data re-use, namely:
|
Brussels, 10 July 2018.
Giovanni BUTTARELLI
European Data Protection Supervisor
(1) Explanatory memorandum to the Proposal for a Directive of the European Parliament and the Council on the re-use of public sector information (recast), p. 3.
(2) Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions ‘Towards a common European data space’, p. 5
(3) https://edps.europa.eu/sites/edp/files/publication/16-09-23_bigdata_opinion_en.pdf, on re-use, p. 9.
(4) European Data Protection Supervisor Opinion 05/2018-Preliminary Opinion on Privacy by Design
NOTICES FROM MEMBER STATES
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/10 |
Commission information notice pursuant to Article 16 (4) of Regulation (EC) 1008/2008 of the European Parliament and of the Council on common rules for the operation of air services in the Community
Imposition of public service obligations in respect of scheduled air services
(Text with EEA relevance)
(2018/C 305/08)
|
Member State |
Portugal |
||||||
|
Route concerned |
Bragança - Vila Real – Viseu – Cascais – Portimão – Cascais – Viseu – Vila Real - Bragança |
||||||
|
Date of entry into force of the public service obligations |
As from 23 December 2018 |
||||||
|
Address where the text and any relevant information and/or documentation relating to the public service obligations can be obtained |
All documents are available at: http://www.saphety.com For more information, please contact:
|
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/11 |
Commission information notice pursuant to Article 16(4) of Regulation (EC) No 1008/2008 of the European Parliament and of the Council on common rules for the operation of air services in the Community
Modification of public service obligations in respect of scheduled air services
(Text with EEA relevance)
(2018/C 305/09)
|
Member State |
United Kingdom |
|||||||||||
|
Route concerned |
Oban – Coll Oban – Colonsay Oban – Tiree Coll – Tiree |
|||||||||||
|
Original date of entry into force of the public service obligations |
2 March 2007 |
|||||||||||
|
Date of entry into force of modifications |
16 May 2019 |
|||||||||||
|
Address where the text and any relevant information and/or documentation relating to the public service obligation can be obtained |
All documents will be available from: http://www.publiccontractsscotland.gov.uk For further information please contact:
|
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/12 |
Commission information notice pursuant to Article 17(5) of Regulation (EC) No 1008/2008 of the European Parliament and of the Council on common rules for the operation of air services in the Community
Invitation to tender in respect of the operation of scheduled air services in accordance with public service obligations
(Text with EEA relevance)
(2018/C 305/10)
|
Member State |
United Kingdom |
|||||||||||
|
Route concerned |
Oban – Coll Oban – Colonsay Oban – Tiree Coll – Tiree |
|||||||||||
|
Period of validity of the contract |
16 May 2019-15 May 2022 |
|||||||||||
|
Deadline for submission of applications and tenders |
19 November 2018 |
|||||||||||
|
Address from which the text of the invitation to tender and any relevant information and/or documentation relating to the public tender and the public service obligation can be obtained |
All documents will be available from: http://www.publiccontractsscotland.gov.uk For further information please contact:
|
NOTICES CONCERNING THE EUROPEAN ECONOMIC AREA
Standing Committee of the EFTA States
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/13 |
Dangerous substances — List of authorisation decisions taken by the EEA EFTA States in accordance with Article 64(8) of Regulation (EC) No 1907/2006 (REACH) in the second half of 2017
(2018/C 305/11)
Subcommittee I on the free movement of goods
To be noted by the EEA Joint Committee
With reference to EEA Joint Committee Decision No 25/2008 of 14 March 2008, the EEA Joint Committee is invited to note the following lists concerning authorisation decisions adopted on the basis of Article 64(8) of Regulation (EC) No 1907/2006 (REACH) for the period 1 July – 31 December 2017, at their meeting on 27 April 2018.
ANNEX
List of authorisation decisions
The following authorisation decisions in accordance with Article 64(8) of Regulation (EC) No 1907/2006 (REACH) have been taken in the EEA EFTA States during the period 1 July – 31 December 2017:
|
Substance name |
Commission decision under Article 64(8) of Regulation (EC) No 1907/2006 |
Country |
Date of decision |
|
Ammonium dichromate |
C(2017) 3237 |
Iceland |
7.7.2017 |
|
Sodium dichromate |
C(2017) 3453 |
Iceland |
7.7.2017 |
|
Sodium dichromate |
C(2017) 3764 |
Iceland |
7.7.2017 |
|
Sodium dichromate |
C(2017) 3765 |
Iceland |
7.7.2017 |
|
Sodium dichromate |
C(2017) 3801 |
Iceland |
7.7.2017 |
|
Sodium dichromate |
C(2017) 3806 |
Iceland |
7.7.2017 |
|
Sodium dichromate |
C(2017) 3816 |
Iceland |
7.7.2017 |
|
1,2-dichloroethane |
C(2017) 3821 |
Iceland |
7.7.2017 |
|
Potassium dichromate |
C(2017) 3910 |
Iceland |
7.7.2017 |
|
Chromium trioxide and dichromium tris(chromate) |
C(2017) 5001 |
Iceland |
5.10.2017 |
|
Chromium trioxide and dichromium tris(chromate) |
C(2017) 5001 |
Liechtenstein |
4.9.2017 |
|
Chromium trioxide and dichromium tris(chromate) |
C(2017) 5001 |
Norway |
18.8.2017 |
|
Bis(2-methoxyethyl)ether (diglyme) |
C(2017) 5025 |
Iceland |
5.10.2017 |
|
Bis(2-methoxyethyl)ether (diglyme) |
C(2017) 5025 |
Liechtenstein |
4.9.2017 |
|
Bis(2-methoxyethyl)ether (diglyme) |
C(2017) 5025 |
Norway |
18.8.2017 |
|
Lead chromate |
C(2017) 5012 |
Iceland |
5.10.2017 |
|
Lead chromate |
C(2017) 5012 |
Liechtenstein |
4.9.2017 |
|
Lead chromate |
C(2017) 5012 |
Norway |
18.8.2017 |
|
Chromium trioxide |
C(2017) 5880 |
Iceland |
5.10.2017 |
|
Chromium trioxide |
C(2017) 5880 |
Liechtenstein |
20.9.2017 |
|
Chromium trioxide |
C(2017) 5880 |
Norway |
25.9.2017 |
|
Chromium trioxide |
C(2017) 6727 |
Iceland |
9.11.2017 |
|
Chromium trioxide |
C(2017) 6727 |
Liechtenstein |
25.10.2017 |
|
Chromium trioxide |
C(2017) 6727 |
Norway |
8.11.2017 |
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/15 |
Medicinal products — List of marketing authorisations granted by the EEA EFTA States for the second half of 2017
(2018/C 305/12)
Subcommittee I on the free movement of goods
To be noted by the EEA Joint Committee
With reference to EEA Joint Committee Decision No 74/1999 of 28 May 1999, the EEA Joint Committee is invited to note the following lists concerning marketing authorisations for medicinal products for the period 1 July-31 December 2017, at their meeting on 23 March 2018:
|
Annex I |
List of new marketing authorisations |
|
Annex II |
List of renewed marketing authorisations |
|
Annex III |
List of extended marketing authorisations |
|
Annex IV |
List of withdrawn marketing authorisations |
|
Annex V |
List of suspended marketing authorisations |
ANNEX I
List of new marketing authorisations
The following marketing authorisations have been granted in the EEA EFTA States during the period 1 July-31 December 2017:
|
EU Number |
Product |
Country |
Date of authorisation |
|
EU/1/02/226 |
InductOs |
Iceland |
18.8.2017 |
|
EU/1/15/999 |
Zykadia (Switch to non-conditional) |
Liechtenstein |
31.8.2017 |
|
EU/1/16/1138 |
Venclyxto |
Liechtenstein |
31.12.2017 |
|
EU/1/16/1155 |
Kyntheum |
Iceland |
16.8.2017 |
|
EU/1/16/1155 |
Kyntheum |
Liechtenstein |
31.8.2017 |
|
EU/1/16/1155 |
Kyntheum |
Norway |
14.8.2017 |
|
EU/1/17/1179 |
Veltassa |
Iceland |
11.8.2017 |
|
EU/1/17/1179 |
Veltassa |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1179 |
Veltassa |
Norway |
9.8.2017 |
|
EU/1/17/1181 |
Spherox |
Iceland |
24.7.2017 |
|
EU/1/17/1181 |
Spherox |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1181 |
Spherox |
Norway |
13.9.2017 |
|
EU/1/17/1184 |
Riximyo |
Iceland |
11.7.2017 |
|
EU/1/17/1185 |
Rixathon |
Iceland |
11.7.2017 |
|
EU/1/17/1191 |
Dinutuximab beta Apeiron |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1194 |
Febuxostat Mylan |
Iceland |
11.7.2017 |
|
EU/1/17/1195 |
Erelzi |
Iceland |
14.7.2017 |
|
EU/1/17/1195 |
Erelzi |
Norway |
7.7.2017 |
|
EU/1/17/1196 |
Kevzara |
Iceland |
14.7.2017 |
|
EU/1/17/1196 |
Kevzara |
Norway |
5.7.2017 |
|
EU/1/17/1197 |
Oxervate |
Iceland |
24.7.2017 |
|
EU/1/17/1197 |
OXERVATE |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1197 |
OXERVATE |
Norway |
17.7.2017 |
|
EU/1/17/1199 |
Cuprior |
Iceland |
13.9.2017 |
|
EU/1/17/1199 |
Cuprior |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1199 |
Cuprior |
Norway |
13.9.2017 |
|
EU/1/17/1200 |
Besponsa |
Iceland |
24.7.2017 |
|
EU/1/17/1200 |
Besponsa |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1200 |
Besponsa |
Norway |
13.7.2017 |
|
EU/1/17/1201 |
Skilarence |
Iceland |
14.7.2017 |
|
EU/1/17/1201 |
Skilarence |
Norway |
7.7.2017 |
|
EU/1/17/1202 |
Ucedane |
Iceland |
12.7.2017 |
|
EU/1/17/1202 |
Ucedane |
Norway |
5.7.2017 |
|
EU/1/17/1203 |
Insulin lispro Sanofi |
Iceland |
11.8.2017 |
|
EU/1/17/1203 |
Insulin Lispro Sanofi |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1203 |
Insulin lispro Sanofi |
Norway |
18.8.2017 |
|
EU/1/17/1205 |
Blitzima |
Iceland |
9.8.2017 |
|
EU/1/17/1205 |
Blitzima |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1205 |
Blitzima |
Norway |
1.8.2017 |
|
EU/1/17/1206 |
Tuxella |
Iceland |
9.8.2017 |
|
EU/1/17/1206 |
Tuxella |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1206 |
Tuxella |
Norway |
1.8.2017 |
|
EU/1/17/1207 |
Ritemvia |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1207 |
Ritemvia |
Iceland |
10.8.2017 |
|
EU/1/17/1207 |
Ritemvia |
Norway |
1.8.2017 |
|
EU/1/17/1208 |
Trimbow |
Iceland |
11.8.2017 |
|
EU/1/17/1208 |
Trimbow |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1208 |
Trimbow |
Norway |
9.8.2017 |
|
EU/1/17/1209 |
Reagila |
Iceland |
9.8.2017 |
|
EU/1/17/1209 |
Reagila |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1209 |
Reaglia |
Norway |
9.8.2017 |
|
EU/1/17/1210 |
Efavirenz/Emtricitabin/Tenofovirdisproksil Zentiva |
Norway |
8.8.2017 |
|
EU/1/17/1210 |
Efavirenz/Emtricitabine/Tenofovir disoproxil Zentiva |
Iceland |
10.8.2017 |
|
EU/1/17/1210 |
Efavirenz/Emtricitabine/Tenofovir disoproxil Zentiva |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1211 |
Entecavir Accord |
Iceland |
5.10.2017 |
|
EU/1/17/1211 |
Entecavir Accord |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1211 |
Entecavir Accord |
Norway |
20.10.2017 |
|
EU/1/17/1212 |
Mavenclad |
Iceland |
13.9.2017 |
|
EU/1/17/1212 |
MAVENCLAD |
Norway |
30.8.2017 |
|
EU/1/17/1212 |
MAVENCLAD |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1213 |
Maviret |
Iceland |
17.8.2017 |
|
EU/1/17/1213 |
Maviret |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1213 |
Maviret |
Norway |
7.8.2017 |
|
EU/1/17/1214 |
Bavencio |
Iceland |
4.10.2017 |
|
EU/1/17/1214 |
Bavencio |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1214 |
Bavencio |
Norway |
25.9.2017 |
|
EU/1/17/1215 |
Fotivda |
Iceland |
13.9.2017 |
|
EU/1/17/1215 |
Fotivda |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1215 |
Fotivda |
Norway |
31.8.2017 |
|
EU/1/17/1216 |
Imraldi |
Iceland |
12.9.2017 |
|
EU/1/17/1216 |
Imraldi |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1216 |
Imraldi |
Norway |
11.9.2017 |
|
EU/1/17/1217 |
Nitisinone MendeliKABS |
Iceland |
19.9.2017 |
|
EU/1/17/1217 |
Nitisinone MendeliKABS |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1217 |
Nitisinone MendeliKABS |
Norway |
1.9.2017 |
|
EU/1/17/1218 |
Rydapt |
Iceland |
4.10.2017 |
|
EU/1/17/1218 |
Rydapt |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1218 |
Rydapt |
Norway |
25.9.2017 |
|
EU/1/17/1220 |
Tecentriq |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1220 |
Tecentriq |
Norway |
27.9.2017 |
|
EU/1/17/1220 |
Tecentriq |
Iceland |
5.10.2017 |
|
EU/1/17/1221 |
Kisqali |
Iceland |
12.9.2017 |
|
EU/1/17/1221 |
Kisqali |
Norway |
30.8.2017 |
|
EU/1/17/1221 |
Kisqali |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1222 |
Efavirenz/Emtricitabine/Tenofovir disoproxil Mylan |
Iceland |
8.11.2017 |
|
EU/1/17/1222 |
Efavirenz/Emtricitabine/Tenofovir disoproxil Mylan |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1222 |
Efavirenz/Emtricitabine/Tenofovir disoproxil Mylan |
Norway |
27.9.2017 |
|
EU/1/17/1223 |
Vosevi |
Iceland |
16.8.2017 |
|
EU/1/17/1223 |
Vosevi |
Liechtenstein |
31.8.2017 |
|
EU/1/17/1223 |
Vosevi |
Norway |
8.8.2017 |
|
EU/1/17/1224 |
Xermelo |
Iceland |
4.10.2017 |
|
EU/1/17/1224 |
Xermelo |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1224 |
Xermelo |
Norway |
3.10.2017 |
|
EU/1/17/1225 |
Symtuza |
Iceland |
5.10.2017 |
|
EU/1/17/1225 |
Symtuza |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1225 |
Symtuza |
Norway |
11.10.2017 |
|
EU/1/17/1226 |
Lutathera |
Iceland |
10.10.2017 |
|
EU/1/17/1226 |
Lutathera |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1226 |
Lutathera |
Norway |
3.10.2017 |
|
EU/1/17/1227 |
Entecavir Mylan |
Iceland |
4.10.2017 |
|
EU/1/17/1227 |
Entecavir Mylan |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1227 |
Entecavir Mylan |
Norway |
27.9.2017 |
|
EU/1/17/1228 |
Tookad |
Iceland |
29.11.2017 |
|
EU/1/17/1228 |
TOOKAD |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1228 |
TOOKAD |
Norway |
22.11.2017 |
|
EU/1/17/1229 |
Dupixent |
Iceland |
10.10.2017 |
|
EU/1/17/1229 |
Dupixent |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1229 |
Dupixent |
Norway |
10.10.2017 |
|
EU/1/17/1230 |
Lacosamide Accord |
Iceland |
5.10.2017 |
|
EU/1/17/1230 |
Lacosamide Accord |
Liechtenstein |
31.10.2017 |
|
EU/1/17/1230 |
Lacosamide Accord |
Norway |
27.9.2017 |
|
EU/1/17/1232 |
Miglustat Gen.Orph |
Iceland |
30.11.2017 |
|
EU/1/17/1232 |
Miglustat Gen.Orph |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1232 |
Miglustat Gen.Orph |
Norway |
27.11.2017 |
|
EU/1/17/1233 |
Zubsolv |
Iceland |
29.11.2017 |
|
EU/1/17/1233 |
Zubsolv |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1233 |
Zubsolv |
Norway |
22.11.2017 |
|
EU/1/17/1234 |
Tremfya |
Iceland |
4.12.2017 |
|
EU/1/17/1235 |
Zejula |
Iceland |
4.12.2017 |
|
EU/1/17/1235 |
Zejula |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1235 |
Zejula |
Norway |
27.11.2017 |
|
EU/1/17/1236 |
Trelegy Ellipta |
Iceland |
30.11.2017 |
|
EU/1/17/1236 |
Trelegy Ellipta |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1236 |
Trelegy Ellipta |
Norway |
22.11.2017 |
|
EU/1/17/1237 |
Elebrato Ellipta |
Iceland |
30.11.2017 |
|
EU/1/17/1237 |
Elebrato Ellipta |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1237 |
Elebrato Ellipta |
Norway |
27.11.2017 |
|
EU/1/17/1238 |
Nyxoid |
Iceland |
29.11.2017 |
|
EU/1/17/1238 |
Nyxoid |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1238 |
Nyxoid |
Norway |
27.11.2017 |
|
EU/1/17/1239 |
VeraSeal |
Iceland |
30.11.2017 |
|
EU/1/17/1239 |
VeraSeal |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1239 |
VeraSeal |
Norway |
1.12.2017 |
|
EU/1/17/1240 |
Cyltezo |
Iceland |
29.11.2017 |
|
EU/1/17/1240 |
Cyltezo |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1240 |
Cyltezo |
Norway |
1.12.2017 |
|
EU/1/17/1241 |
Ontruzant |
Iceland |
30.11.2017 |
|
EU/1/17/1241 |
Ontruzant |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1241 |
Ontruzant |
Norway |
27.11.2017 |
|
EU/1/17/1242 |
Ritonavir Mylan |
Iceland |
29.11.2017 |
|
EU/1/17/1242 |
Ritonavir Mylan |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1242 |
Ritonavir Mylan |
Norway |
22.11.2017 |
|
EU/1/17/1243 |
Imatinib Teva B.V. |
Iceland |
30.11.2017 |
|
EU/1/17/1243 |
Imatinib Teva B.V. |
Liechtenstein |
31.12.2017 |
|
EU/1/17/1243 |
Imatinib Teva B.V. |
Norway |
22.11.2017 |
|
EU/1/17/1244 |
Tacforius |
Liechtenstein |
31.12.2017 |
|
EU/2/16/197 |
CLYNAV |
Iceland |
13.7.2017 |
|
EU/2/16/197 |
CLYNAV |
Liechtenstein |
31.8.2017 |
|
EU/2/16/197 |
CLYNAV |
Norway |
18.7.2017 |
|
EU/2/17/211 |
Prevomax |
Iceland |
13.7.2017 |
|
EU/2/17/211 |
Prevomax |
Norway |
6.7.2017 |
|
EU/2/17/212 |
Exzolt |
Iceland |
30.8.2017 |
|
EU/2/17/212 |
Exzolt |
Liechtenstein |
31.10.2017 |
|
EU/2/17/212 |
Exzolt |
Norway |
15.9.2017 |
|
EU/2/17/213 |
Innovax-ND-IBD |
Iceland |
11.9.2017 |
|
EU/2/17/213 |
Innovax-ND-IBD |
Norway |
18.9.2017 |
|
EU/2/17/214 |
VEPURED |
Iceland |
12.9.2017 |
|
EU/2/17/214 |
VEPURED |
Norway |
15.9.2017 |
|
EU/2/17/215 |
Suvaxyn PRRS MLV |
Iceland |
11.9.2017 |
|
EU/2/17/215 |
Suvaxyn PRRS MLV |
Liechtenstein |
31.10.2017 |
|
EU/2/17/215 |
Suvaxyn PRRS MLV |
Norway |
18.9.2017 |
|
EU/2/17/217 |
Nobivac LeuFel |
Iceland |
9.11.2017 |
|
EU/2/17/217 |
Nobivac LeuFel |
Liechtenstein |
31.12.2017 |
|
EU/2/17/217 |
Nobivac LeuFel |
Norway |
14.11.2017 |
|
EU/2/17/218 |
Bovilis Blue-8 |
Iceland |
1.12.2017 |
|
EU/2/17/218 |
Bovilis Blue-8 |
Liechtenstein |
31.12.2017 |
|
EU/2/17/218 |
Bovilis Blue-8 |
Norway |
19.12.2017 |
ANNEX II
List of renewed marketing authorisations
The following marketing authorisations have been renewed in the EEA EFTA States during the period 1 July-31 December 2017:
|
EU Number |
Product |
Country |
Date of authorisation |
|
EU/1/07/401 |
alli |
Iceland |
14.7.2017 |
|
EU/1/07/401 |
alli |
Liechtenstein |
31.8.2017 |
|
EU/1/07/401 |
alli |
Norway |
5.7.2017 |
|
EU/1/07/402 |
Increlex |
Iceland |
12.7.2017 |
|
EU/1/07/402 |
INCRELEX |
Norway |
5.7.2017 |
|
EU/1/07/403 |
Atriance |
Iceland |
11.7.2017 |
|
EU/1/07/416 |
Ecalta |
Iceland |
13.9.2017 |
|
EU/1/07/416 |
Ecalta |
Liechtenstein |
31.10.2017 |
|
EU/1/07/416 |
Ecalta |
Norway |
22.9.2017 |
|
EU/1/07/421 |
Glubrava |
Iceland |
30.11.2017 |
|
EU/1/07/421 |
Glubrava |
Liechtenstein |
31.12.2017 |
|
EU/1/07/421 |
Glubrava |
Norway |
22.11.2017 |
|
EU/1/07/424 |
Torisel |
Iceland |
24.7.2017 |
|
EU/1/07/424 |
Torisel |
Liechtenstein |
31.8.2017 |
|
EU/1/07/424 |
Torisel |
Norway |
1.8.2017 |
|
EU/1/08/446 |
Privigen |
Iceland |
4.12.2017 |
|
EU/1/08/446 |
Privigen |
Liechtenstein |
31.12.2017 |
|
EU/1/08/446 |
Privigen |
Norway |
5.12.2017 |
|
EU/1/08/453 |
Prepandrix |
Iceland |
4.12.2017 |
|
EU/1/08/453 |
Prepandrix |
Liechtenstein |
31.12.2017 |
|
EU/1/08/453 |
Prepandrix |
Norway |
5.12.2017 |
|
EU/1/12/784 |
Cuprymina |
Iceland |
11.8.2017 |
|
EU/1/12/784 |
Cuprymina |
Liechtenstein |
31.8.2017 |
|
EU/1/12/784 |
Cuprymina |
Norway |
9.8.2017 |
|
EU/1/12/787 |
Revestive |
Iceland |
13.7.2017 |
|
EU/1/12/787 |
Revestive |
Norway |
5.7.2017 |
|
EU/1/12/788 |
Seebri Breezhaler |
Iceland |
11.8.2017 |
|
EU/1/12/788 |
Seebri Breezhaler |
Liechtenstein |
31.8.2017 |
|
EU/1/12/788 |
Seebri Breezhaler |
Norway |
9.8.2017 |
|
EU/1/12/789 |
Enurev Breezhaler |
Iceland |
24.7.2017 |
|
EU/1/12/789 |
Enurev Breezhaler |
Liechtenstein |
31.8.2017 |
|
EU/1/12/789 |
Enurev Breezhaler |
Norway |
9.8.2017 |
|
EU/1/12/790 |
Tovanor Breezhaler |
Iceland |
14.8.2017 |
|
EU/1/12/790 |
Tovanor Breezhaler |
Liechtenstein |
31.8.2017 |
|
EU/1/12/790 |
Tovanor Breezhaler |
Norway |
9.8.2017 |
|
EU/1/12/794 |
Adcetris |
Iceland |
4.12.2017 |
|
EU/1/12/794 |
ADCETRIS |
Liechtenstein |
31.12.2017 |
|
EU/1/12/794 |
ADCETRIS |
Norway |
27.11.2017 |
|
EU/1/12/795 |
Forxiga |
Iceland |
13.9.2017 |
|
EU/1/12/795 |
Forxiga |
Liechtenstein |
31.10.2017 |
|
EU/1/12/795 |
Forxiga |
Norway |
27.9.2017 |
|
EU/1/12/796 |
Picato |
Iceland |
24.7.2017 |
|
EU/1/12/796 |
Picato |
Liechtenstein |
31.8.2017 |
|
EU/1/12/796 |
Picato |
Norway |
8.8.2017 |
|
EU/1/12/797 |
Eylea |
Iceland |
10.8.2017 |
|
EU/1/12/797 |
Eylea |
Liechtenstein |
31.8.2017 |
|
EU/1/12/797 |
Eylea |
Norway |
18.8.2017 |
|
EU/1/12/798 |
Ibandronic acid Accord |
Iceland |
6.10.2017 |
|
EU/1/12/798 |
Ibandronic acid Accord |
Liechtenstein |
31.10.2017 |
|
EU/1/12/798 |
Ibandronic acid Accord |
Norway |
27.9.2017 |
|
EU/1/12/799 |
Memantine Merz |
Iceland |
24.7.2017 |
|
EU/1/12/799 |
Memantine Merz |
Liechtenstein |
31.8.2017 |
|
EU/1/12/799 |
Memantine Merz |
Norway |
9.8.2017 |
|
EU/1/12/800 |
Zoledronic Acid Hospira |
Iceland |
11.9.2017 |
|
EU/1/12/800 |
Zoledronic acid Hospira |
Liechtenstein |
31.8.2017 |
|
EU/1/12/800 |
Zoledronsyre Hospira |
Norway |
4.9.2017 |
|
EU/1/12/801 |
Constella |
Iceland |
14.9.2017 |
|
EU/1/12/801 |
Constella |
Liechtenstein |
31.10.2017 |
|
EU/1/12/801 |
Constella |
Norway |
22.9.2017 |
|
EU/1/12/802 |
Capecitabine medac |
Iceland |
12.7.2017 |
|
EU/1/12/803 |
NexoBrid |
Iceland |
30.11.2017 |
|
EU/1/12/803 |
NexoBrid |
Liechtenstein |
31.12.2017 |
|
EU/1/12/803 |
NexoBrid |
Norway |
11.12.2017 |
|
EU/1/12/805 |
AMYViD |
Iceland |
9.10.2017 |
|
EU/1/12/805 |
Amyvid |
Liechtenstein |
31.10.2017 |
|
EU/1/12/805 |
Amyvid |
Norway |
3.10.2017 |
|
EU/1/12/806 |
Ryzodeg |
Iceland |
4.10.2017 |
|
EU/1/12/806 |
Ryzodeg |
Liechtenstein |
31.10.2017 |
|
EU/1/12/806 |
Ryzodeg |
Norway |
10.10.2017 |
|
EU/1/12/807 |
Tresiba |
Iceland |
9.10.2017 |
|
EU/1/12/807 |
Tresiba |
Liechtenstein |
31.10.2017 |
|
EU/1/12/807 |
Tresiba |
Norway |
10.10.2017 |
|
EU/1/12/808 |
Imatinib Teva |
Iceland |
9.10.2017 |
|
EU/1/12/808 |
Imatinib Teva |
Liechtenstein |
31.10.2017 |
|
EU/1/12/808 |
Imatinib Teva |
Norway |
27.9.2017 |
|
EU/1/12/809 |
Betmiga |
Iceland |
6.10.2017 |
|
EU/1/12/809 |
Betmiga |
Liechtenstein |
31.10.2017 |
|
EU/1/12/809 |
Betmiga |
Norway |
3.10.2017 |
|
EU/1/12/811 |
Lyxumia |
Iceland |
6.10.2017 |
|
EU/1/12/811 |
Lyxumia |
Liechtenstein |
31.10.2017 |
|
EU/1/12/811 |
Lyxumia |
Norway |
9.10.2017 |
|
EU/1/12/812 |
Bexsero |
Iceland |
9.10.2017 |
|
EU/1/12/812 |
Bexsero |
Liechtenstein |
31.10.2017 |
|
EU/1/12/812 |
Bexsero |
Norway |
3.10.2017 |
|
EU/1/12/814 |
Zaltrap |
Iceland |
9.10.2017 |
|
EU/1/12/814 |
ZALTRAP |
Liechtenstein |
31.10.2017 |
|
EU/1/12/814 |
ZALTRAP |
Norway |
27.9.2017 |
|
EU/1/12/815 |
Selincro |
Iceland |
30.11.2017 |
|
EU/1/12/815 |
Selincro |
Liechtenstein |
31.12.2017 |
|
EU/1/12/815 |
Selincro |
Norway |
1.12.2017 |
|
EU/1/13/813 |
Perjeta |
Iceland |
19.12.2017 |
|
EU/1/13/813 |
Perjeta |
Liechtenstein |
31.12.2017 |
|
EU/1/13/817 |
Actelsar HCT |
Liechtenstein |
31.12.2017 |
|
EU/1/13/819 |
JETREA |
Iceland |
15.12.2017 |
|
EU/1/13/819 |
JETREA |
Liechtenstein |
31.12.2017 |
|
EU/1/13/819 |
JETREA |
Norway |
19.12.2017 |
|
EU/1/13/902 |
Translarna |
Iceland |
12.7.2017 |
|
EU/1/13/902 |
Translarna |
Norway |
5.7.2017 |
|
EU/1/14/987 |
Holoclar |
Iceland |
19.12.2017 |
|
EU/1/16/1094 |
Ninlaro |
Iceland |
6.10.2017 |
|
EU/1/16/1094 |
Ninlaro |
Liechtenstein |
31.10.2017 |
|
EU/1/16/1094 |
Ninlaro |
Norway |
26.9.2017 |
|
EU/1/16/1121 |
Zalmoxis |
Iceland |
24.7.2017 |
|
EU/1/16/1121 |
Zalmoxis |
Liechtenstein |
31.8.2017 |
|
EU/1/16/1121 |
Zalmoxis |
Norway |
4.8.2017 |
|
EU/1/16/1138 |
Venclyxto |
Iceland |
6.11.2017 |
|
EU/1/16/1138 |
Venclyxto |
Norway |
14.11.2017 |
|
EU/1/16/1139 |
Ocaliva |
Iceland |
8.12.2017 |
|
EU/1/16/1139 |
OCALIVA |
Liechtenstein |
31.12.2017 |
|
EU/1/16/1139 |
OCALIVA |
Norway |
18.12.2017 |
|
EU/1/16/1143 |
Lartruvo |
Iceland |
6.10.2017 |
|
EU/1/16/1143 |
Lartruvo |
Liechtenstein |
31.10.2017 |
|
EU/1/16/1143 |
Lartruvo |
Norway |
27.9.2017 |
|
EU/1/16/1169 |
Alecensa |
Iceland |
8.12.2017 |
|
EU/1/16/1169 |
Alecensa |
Liechtenstein |
31.12.2017 |
|
EU/1/16/1169 |
Alecensa |
Norway |
15.12.2017 |
|
EU/1/43/890 |
Cometriq |
Norway |
3.8.2017 |
|
EU/2/12/142 |
Cardalis |
Iceland |
4.7.2017 |
|
EU/2/12/144 |
Contacera |
Iceland |
5.12.2017 |
|
EU/2/12/144 |
Contacera |
Liechtenstein |
31.12.2017 |
|
EU/2/12/144 |
Contacera |
Norway |
15.12.2017 |
|
EU/2/12/145 |
Kexxtone |
Liechtenstein |
31.12.2017 |
|
EU/2/12/145 |
Kexxtone |
Norway |
28.12.2017 |
|
EU/2/12/147 |
Pexion |
Iceland |
5.12.2017 |
|
EU/2/12/147 |
Pexion |
Liechtenstein |
31.12.2017 |
|
EU/2/12/147 |
Pexion |
Norway |
6.12.2017 |
ANNEX III
List of extended marketing authorisations
The following marketing authorisations have been extended in the EEA EFTA States during the period 1 July-31 December 2017:
|
EU Number |
Product |
Country |
Date of authorisation |
|
EU/1/01/177/002 |
SonoVue |
Norway |
4.9.2017 |
|
EU/1/03/256/022 |
Humira |
Iceland |
19.12.2017 |
|
EU/1/03/256/022 |
Humira |
Norway |
10.11.2017 |
|
EU/1/04/292/013-015 |
Mimpara |
Iceland |
15.9.2017 |
|
EU/1/04/292/013-015 |
Mimpara |
Norway |
28.8.2017 |
|
EU/1/06/356/020-022 |
Exjade |
Norway |
28.11.2017 |
|
EU/1/06/356/020-022 |
Exjade |
Iceland |
1.12.2017 |
|
EU/1/07/418/011-013 |
Celsentri |
Norway |
3.8.2017 |
|
EU/1/07/418/011-013 |
Celsentri |
Iceland |
13.7.2017 |
|
EU/1/07/422/015 |
Tasigna |
Iceland |
4.12.2017 |
|
EU/1/07/422/015 |
Tasigna |
Norway |
28.11.2017 |
|
EU/1/07/436/006 |
Isentress |
Iceland |
9.8.2017 |
|
EU/1/07/436/006 |
Isentress |
Norway |
7.8.2017 |
|
EU/1/08/481/004-005 |
Kuvan |
Iceland |
10.8.2017 |
|
EU/1/08/481/004-005 |
Kuvan |
Norway |
13.7.2017 |
|
EU/1/09/522/003 |
ellaOne |
Iceland |
1.12.2017 |
|
EU/1/09/522/003 |
ellaOne |
Norway |
10.11.2017 |
|
EU/1/09/531/022-033 |
Instanyl |
Iceland |
6.11.2017 |
|
EU/1/09/539/005-006 |
Samsca |
Iceland |
5.10.2017 |
|
EU/1/09/539/005-006 |
Samsca |
Norway |
18.9.2017 |
|
EU/1/10/618 |
Prolia |
Norway |
27.9.2017 |
|
EU/1/11/703 |
Xgeva |
Norway |
27.9.2017 |
|
EU/1/12/753/018-019 |
Signifor |
Iceland |
4.10.2017 |
|
EU/1/12/753/018-019 |
Signifor |
Norway |
18.9.2017 |
|
EU/1/12/787/003 |
Revestive |
Norway |
5.7.2017 |
|
EU/1/12/787/003 |
Revestive |
Iceland |
13.7.2017 |
|
EU/1/13/846/002-003 |
Xtandi |
Norway |
3.10.2017 |
|
EU/1/13/846/002-003 |
Xtandi |
Iceland |
6.10.2017 |
|
EU/1/13/860/003 |
Nexium Control |
Iceland |
11.7.2017 |
|
EU/1/13/860/003 |
Nexium Control |
Norway |
5.7.2017 |
|
EU/1/15/1070/002 |
Oncaspar |
Iceland |
19.12.2017 |
ANNEX IV
List of withdrawn marketing authorisations
The following marketing authorisations have been withdrawn in the EEA EFTA States during the period 1 July-31 December 2017:
|
EU Number |
Product |
Country |
Date of withdrawal |
|
EU/1/00/167 |
Prevenar |
Liechtenstein |
31.12.2017 |
|
EU/1/00/167 |
Prevenar |
Norway |
11.12.2017 |
|
EU/1/00/167 |
Prevenar |
Iceland |
1.12.2017 |
|
EU/1/07/394 |
Optaflu |
Norway |
18.10.2017 |
|
EU/1/07/398 |
Optimark |
Iceland |
10.10.2017 |
|
EU/1/09/561 |
Clopidogrel Teva Pharma |
Iceland |
11.7.2017 |
|
EU/1/11/674 |
Repso |
Liechtenstein |
31.8.2017 |
|
EU/1/11/674 |
Repso |
Norway |
8.8.2017 |
|
EU/1/11/674 |
Repso |
Iceland |
10.8.2017 |
|
EU/1/13/868 |
EVARREST |
Liechtenstein |
31.12.2017 |
|
EU/1/13/868 |
EVARREST |
Norway |
28.11.2017 |
|
EU/1/13/868 |
EVARREST |
Iceland |
4.12.2017 |
|
EU/1/14/976 |
Zontivity |
Liechtenstein |
31.8.2017 |
|
EU/1/15/996 |
Ristempa |
Liechtenstein |
31.10.2017 |
|
EU/1/15/996 |
Ristempa |
Norway |
9.10.2017 |
|
EU/1/15/996 |
Ristempa |
Iceland |
9.10.2017 |
|
EU/1/16/1113 |
Enzepi |
Iceland |
11.8.2017 |
|
EU/1/16/1113 |
Enzepi |
Liechtenstein |
31.8.2017 |
|
EU/1/16/1113 |
Enzepi |
Norway |
8.8.2017 |
ANNEX V
List of suspended marketing authorisations
The following marketing authorisations have been suspended in the EEA EFTA States during the period 1 July-31 December 2017:
|
EU Number |
Product |
Country |
Date of suspension |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
V Announcements
ADMINISTRATIVE PROCEDURES
European Personnel Selection Office (EPSO)
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/34 |
NOTICE OF OPEN COMPETITION
(2018/C 305/13)
The European Personnel Selection Office (EPSO) is organising the following open competition:
|
|
EPSO/AST-SC/07/18 — ARMED SECURITY AND PROTECTION OFFICERS (SC 1/SC 2) |
The competition notice is published in 24 languages in Official Journal of the European Union C 305 A of 30 August 2018.
Further information can be found on the EPSO website: https://epso.europa.eu/
PROCEDURES RELATING TO THE IMPLEMENTATION OF COMPETITION POLICY
European Commission
|
30.8.2018 |
EN |
Official Journal of the European Union |
C 305/35 |
Prior notification of a concentration
(Case M.9072 — KKR/Altice/SFR Filiale)
Candidate case for simplified procedure
(Text with EEA relevance)
(2018/C 305/14)
1.
On 24 August 2018, the Commission received notification of a proposed concentration pursuant to Article 4 of Council Regulation (EC) No 139/2004 (1).This notification concerns the following undertakings:
|
— |
KKR & Co. Inc (‘KKR’) (USA), |
|
— |
Altice France S.A. (‘Altice’) (France), belonging to the Altice group, |
|
— |
SFR Filiale SAS (‘SFR Filiale’), controlled by Altice. |
KKR and Altice acquire within the meaning of Article 3(1)(b) of the Merger Regulation joint control of the whole of SFR Filiale.
The concentration is accomplished by way of purchase of shares.
2.
The business activities of the undertakings concerned are:— for KKR: investment firm that provides asset management services and capital markets solutions,
— for Altice: telecoms, content, media, entertainment and advertising services.
— for SFR Filiale: telecommunication towers business of Altice’s subsidiary in France, SFR S.A.
3.
On preliminary examination, the Commission finds that the notified transaction could fall within the scope of the Merger Regulation. However, the final decision on this point is reserved.Pursuant to the Commission Notice on a simplified procedure for treatment of certain concentrations under the Council Regulation (EC) No 139/2004 (2) it should be noted that this case is a candidate for treatment under the procedure set out in the Notice.
4.
The Commission invites interested third parties to submit their possible observations on the proposed operation to the Commission.Observations must reach the Commission not later than 10 days following the date of this publication. The following reference should always be specified:
M.9072 — KKR/Altice/SFR Filiale
Observations can be sent to the Commission by email, by fax, or by post. Please use the contact details below:
|
E-mail: COMP-MERGER-REGISTRY@ec.europa.eu |
|
Fax +32 22964301 |
|
Postal address: |
|
European Commission |
|
Directorate-General for Competition |
|
Merger Registry |
|
1049 Bruxelles/Brussel |
|
BELGIQUE/BELGIË |
(1) OJ L 24, 29.1.2004, p. 1 (the ‘Merger Regulation’).