ANNEX
PART 1
|
CAS No 110-85-0 |
Einecs No 203-808-3 |
|
Structural formula: |
|
|
Einecs name: |
Piperazine |
|
IUPAC name: |
Piperazine |
|
Rapporteur: |
Sweden |
|
Classification (1): |
C; R34 |
|
R42/43 |
|
|
R52/53 |
|
|
Proposed classification (to replace C & L from 22nd ATP after 30th ATP is published) (2) |
|
|
Repr. Cat. 3; R62-63 |
|
|
C; R34 |
|
|
R42/43 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the risk assessment forwarded to the Commission by the Member State Rapporteur (3).
The risk assessment has, based on the available information, determined that in the European Community, piperazine as such or as salts, is mainly used as an intermediate in the chemical industry including the production of pharmaceuticals. Piperazine as such or as salts, is also used for human and veterinary medicinal drugs, as formulation in gas-washing (scrubbers), and as a catalyst in urethane production. The described scenarios represent the main use of piperazine.
|
Note |
: |
The use of piperazine in veterinary medicines is not addressed under this legislation, it is covered by Council Regulation (EEC) No 2377/90 (4) (Maximum residue limits in foodstuff of animal origin). |
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for skin sensitisation as a consequence of dermal exposure arising in the scenarios handling piperazine salts (i.e. final handling during production and loading activities during formulation); |
|
— |
concerns for asthma as a consequence of inhalation exposure arising from all occupational scenarios; |
|
— |
concerns for neurotoxicity and reproductive toxicity as a consequence of repeated exposure to piperazine salts in the scenarios final handling during production and loading activities during formulation. |
The conclusion of the assessment of the risks to
CONSUMERS
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ATMOSPHERE
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to the
AQUATIC ECOSYSTEM
is that there is a need for specific measures to limit the risks. The conclusion is reached because of:
|
— |
concerns for aquatic ecosystem as a consequence of exposure arising at one production site and one formulation site and for industrial use of gas washer formulations with piperazine at 21 sites. |
The conclusion of the assessment of the risks to the
TERRESTRIAL ECOSYSTEM
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
is that there is a need for specific measures to limit the risks. The conclusion is reached because of:
|
— |
concerns for micro-organisms in sewage treatment plants as a consequence of exposure arising from the majority of local gaswasher scenarios. |
STRATEGY FOR LIMITING RISKS
For WORKERS
|
— |
to consider at Community level a harmonised classification under Council Directive 67/548/EEC (5) of the salts of piperazine. |
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed and shall apply.
Within this framework it is recommended:
|
— |
to establish at Community level occupational exposure limit values for the salts of piperazine according to Council Directive 98/24/EC (6). |
PART 2
|
CAS No 110-82-7 |
Einecs No 203-806-2 |
|
Structural formula: |
|
|
Einecs name: |
Cyclohexane |
|
IUPAC name: |
|
|
Rapporteur: |
France |
|
Classification (7): |
F; R11 |
|
Xn; R65 |
|
|
Xi; R38 |
|
|
R67 |
|
|
N; R50/53 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the risk assessment forwarded to the Commission by the Member State Rapporteur.
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as intermediate in chemical industry. Other uses reported are as a solvent in chemical production process and in adhesives and coatings.
The risk assessment has identified other sources of exposure to the substance, relevant for man and the environment, in particular from crude oil and plants, combustion products (tobacco smoke, volcanic emissions) and petroleum derived fuels (gasoline vapours), which do not result from the life-cycle of the substance produced in or imported into the European Community. The assessment of the risks arising from these exposures are not part of this risk assessment. The comprehensive Risk Assessment Report (8), as forwarded to the Commission by the Member State Rapporteur, does however provide information which could be used to assess these risks.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for acute toxicity (neurobehavioural effects) and general systemic toxicity (hepatic effects) as a consequence of inhalation exposure arising from formulation and industrial use of products containing the substance as well as from use of products containing the substance in craft industries. |
The conclusion of the assessment of the risks to
CONSUMERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for acute toxicity (neurobehavioral effects) as a consequence of exposure arising from use of products containing the substance. |
The conclusion of the assessment of the risks to
HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ATMOSPHERE, AQUATIC ECOSYSTEM and TERRESTRIAL ECOSYSTEM
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
STRATEGY FOR LIMITING RISKS
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed and shall apply.
For CONSUMERS
To consider at Community level marketing and use restrictions in Council Directive 76/769/EEC (9) (marketing and use Directive) for the use of cyclohexane in neoprene based adhesives.
PART 3
|
CAS No 26447-40-5 |
Einecs No 247-714-0 |
|
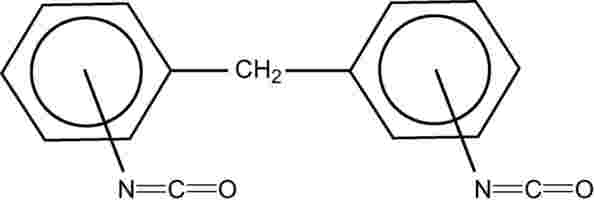
Structural formula: |
|
|
Einecs name: |
1,1’-methylenebis (isocyanatobenzene) |
|
IUPAC name: |
Methylenediphenyl diisocyanate Methylenebis (phenyl isocyanate) |
|
Rapporteur: |
Belgium |
|
Classification: |
Xn; R20 |
|
Xi; R36/37/38 |
|
|
R42/43 |
|
|
Proposed classification (to replace C&L from 28th ATP after 30th ATP is published) (10) |
|
|
Carc. Cat. 3; R40 |
|
|
Xn; R20-48/20 |
|
|
Xi; R36/37/38 |
|
|
R42/43 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the risk assessment forwarded to the Commission by the Member State Rapporteur (11).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used in the industrial production of rigid polyurethane foams. Many other uses are in the fields of wood binders, Coatings, Adhesives, Sealants and Elastomers (CASE), (semi) flexible and thermoplastic polyurethane foams and fibres. A limited but not negligible use is within consumer products, such as adhesives and one component foams (OCFs).
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS
|
1. |
is that there is a need for further information and/or testing. This conclusion is reached because:
|
|
2. |
that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
The conclusion of the assessment of the risks to
CONSUMERS
|
1. |
is that there is a need for further information and/or testing. This conclusion is reached because:
|
|
2. |
that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
The conclusion of the assessment of the risks to
HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ATMOSPHERE, AQUATIC ECOSYSTEM, TERRESTRIAL ECOSYSTEM, MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT and NON ECOSYSTEM-SPECIFIC EFFECTS RELEVANT TO THE FOOD CHAIN
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
STRATEGY FOR LIMITING RISKS
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed and shall apply.
Within this framework it is recommended:
|
— |
to establish at Community level occupational exposure limit values for MDI according to Council Directive 98/24/EC (12). |
For CONSUMERS
To consider at Community level marketing and use restrictions in Council Directive 76/769/EEC (13) for the use of MDI in consumer’s products.
PART 4
|
CAS No 110-65-6 |
Einecs No 203-788-6 |
|
Structural formula: |
|
|
Einecs name: |
But-2-yne-1,4-diol |
|
IUPAC name: |
But-2-yne-1,4-diol |
|
Rapporteur: |
Germany |
|
Classification: |
C; R34 |
|
T; R23/25 |
|
|
Xn; R21-48/22 |
|
|
R43 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the comprehensive Risk Assessment Reports as forwarded to the Commission by the Member State Rapporteur (14).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as an intermediate in the chemical industry for the production of butanediol and butenediol. Other professional uses include its use as an intermediate for the synthesis of polyols, insecticides, pharmaceuticals and auxiliaries for the paint and textile industry. The substance is directly used as a corrosion inhibitor in pickling solutions in technical cleaning products for metal surface treatment, as a brightener in galvanic baths and in organic paint removers. In consumer products it is used in cleaning agents and sanitary disinfectants.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for local respiratory tract irritation as a consequence of single inhalation exposure arising from production and further processing of the solid substance (flakes) in the large scale chemical industry. |
|
— |
concerns for local respiratory tract irritation as a consequence of repeated exposure arising from manufacturing and further processing of the solid substance (flakes) in the large scale chemical industry and in the preparation of formulations (in the absence of local exhaust ventilation). |
|
— |
concerns for sensitisation as a consequence of dermal exposure arising from production and further processing of the substance in the large scale chemical industry, in the preparation of formulations and its use in organic paint removers. |
The conclusion of the assessment of the risks to
CONSUMERS
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physicochemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
AQUATIC ECOSYSTEM, ATMOSPHERE and TERRESTRIAL ECOSYSTEM
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks related to the environmental spheres mentioned above are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks for
MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks related to the environmental spheres mentioned above are not expected. Risk reduction measures already being applied are considered sufficient. |
STRATEGY FOR LIMITING RISKS
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed and shall apply.
PART 5
|
CAS No 75-56-9 |
Einecs No 200-879-2 |
|
Structural formula: |
|
|
Einecs name: |
Methyloxirane |
|
IUPAC name: |
Propylene oxide |
|
Rapporteur: |
United Kingdom |
|
Classification: |
F+; R12 |
|
Carc. Cat. 2; R45 |
|
|
Muta. Cat. 2; R46 |
|
|
Xn; R20/21/22 |
|
|
Xi; R36/37/38 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the comprehensive Risk Assessment Reports as forwarded to the Commission by the Member State Rapporteur (15).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as a monomer in polymer production and as an intermediate in the synthesis of other substances. Other uses are as a stabiliser in dichloromethane and as an anti-corrosion additive. It was not possible to obtain information on the use of the total volume of substance produced in or imported into the European Community, therefore some uses may exist which are not covered by this risk assessment.
This substance has not been adequately tested for sensitisation and consequently the risk assessment does not evaluate the risks to any population of this end point. This test has not been required, as the substance has been identified as a non-threshold carcinogen.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS, CONSUMERS and HUMANS EXPOSED VIA THE ENVIRONMENT
is that the risk assessment shows that risks cannot be excluded for all exposure scenarios, as the substance is identified as a non-threshold carcinogen, however, the risk assessment indicates that risks are already low. This should be taken into account when considering the adequacy of existing controls and the feasibility and practicability of further specific risk reduction measures.
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ATMOSPHERE, AQUATIC ECOSYSTEM and TERRESTRIAL ECOSYSTEM
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks related to the environmental spheres mentioned above are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks for
MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks related to the environmental spheres mentioned above are not expected. Risk reduction measures already being applied are considered sufficient. |
STRATEGY FOR LIMITING RISKS
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed.
For CONSUMERS AND HUMANS EXPOSED VIA THE ENVIRONMENT
The existing legislative measures for the protection of consumers and humans exposed via the environment, in particular the provisions under the Council Directive 76/769/EEC (16) (marketing and use Directive) as regards CMR substances, Directive 2001/95/EC of the European Parliament and of the Council (17) (general product safety) as regards products, and Council Directive 96/61/EC (18) (integrated pollution prevention and control) are considered sufficient to address the risks identified.
PART 6
|
CAS No 62-53-3 |
Einecs No 200-539-3 |
|
Structural formula: |
C6H7N |
|
|
|
|
Einecs name: |
Aniline |
|
IUPAC name: |
Aminobenzene |
|
Rapporteur: |
Germany |
|
Classification (19): |
Carc. Cat. 3; R40 |
|
Muta. Cat. 3; R68 |
|
|
T; R23/24/25-48/23/24/25 |
|
|
Xi; R41 R43 |
|
|
N; R50 |
The risk assessment is based on current practices related to the life cycle of the substance produced in or imported into the European Community as described in the comprehensive Risk Assessment Report forwarded to the Commission by the Member State Rapporteur (20).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as an intermediate in the chemical industry, to produce methylenedianiline or rubber. Other uses are processing to dyes, pesticides, pharmaceuticals, fibres, etc.
Releases of aniline can occur during these production and processing scenarios. In addition, aniline is a residual component of dyes and adhesives.
The risk assessment has identified other sources of exposure to the substance to humans and the environment, in particular via microbial reduction of nitrobenzene, and from the coal and oil industry. The assessment of the risks arising from these exposures, which do not result from the life-cycle of the substance produced in or imported into the European Community are not part of this risk assessment. The comprehensive Risk Assessment Reports as forwarded to the Commission by the Member State Rapporteur does however provide information which could be used to assess those risks.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusions of the assessment of the risks to
WORKERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for acute toxicity as a consequence of:
|
|
— |
concerns for skin sensitisation as a consequence of dermal exposure arising from production and further processing in the large-scale chemical industry (in case of unsuitable gloves), and the use of dyes with residual aniline; |
|
— |
concerns for systemic toxic effects as a consequence of:
|
|
— |
concerns for mutagenicity and carcinogenicity in all workplace scenarios, as the substance is identified as a non-threshold carcinogen. However, for the following specific working scenarios risks are already low:
|
This should be taken into account when considering the adequacy of existing controls and the feasibility and practicability of further specific risk reduction measures;
|
— |
concerns for developmental toxicity as a consequence of dermal exposure in case of unsuitable gloves arising from production and further processing in the large-scale chemical industry. |
The conclusion of the assessment of the risks to
CONSUMERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for mutagenicity and carcinogenicity as a consequence of exposure arising from use of products containing the substance, as aniline is identified as a non-threshold carcinogen. |
The conclusions of the assessment of the risks to
HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for systemic toxic effects, developmental toxicity, mutagenicity and carcinogenicity as a consequence of exposure arising from point sources, |
|
— |
concerns for mutagenicity and carcinogenicity as a consequence of possible exposures at a regional level, as aniline is identified as a non-threshold carcinogen. However, exposures are already very low and this should be taken into account when considering the adequacy of existing controls and the feasibility and practicability of further specific risk reduction measures. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physicochemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because of:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
AQUATIC ECOSYSTEM and MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
|
1. |
is that there is a need for further information and/or testing. This conclusion is reached because of:
The information and/or test requirements are:
|
|
2. |
is that there is a need for specific measures to limit the risks; risk reduction measures which are already being applied shall be taken into account. This conclusion is reached because of:
|
The conclusion of the assessment of the risks to the
ATMOSPHERE
|
1. |
is that there is a need for further information and/or testing. This conclusion is reached because:
The information and/or test requirements are:
|
|
2. |
is that there is a need for specific measures to limit the risks; risk reduction measures which are already being applied shall be taken into account. This conclusion is reached because of:
|
The conclusion of the assessment of the risks to the
TERRESTRIAL ECOSYSTEM
is that there is a need for further information and/or testing. This conclusion is reached because:
|
— |
there is a need for better information to adequately characterise the risks to agricultural soils from aniline as a degradation product of phenylurea and carbamate derivatives used as plant protection products. |
The information and/or test requirements are:
|
— |
long term tests with plants, earthworms and micro-organisms. |
However, since the risk to soil from the breakdown of plant protection agents is not covered by Council Regulation (EEC) No 793/93 (21) it is proposed that this be considered within the frame of Council Directive 91/414/EEC (22).
STRATEGY FOR LIMITING RISKS
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed and shall apply.
Within this framework it is recommended:
|
— |
to establish at community level occupational exposure limit values for Aniline according to Council Directive 98/24/EC (23), taking the dermal uptake into account. |
PART 7
|
CAS No 103-11-7 |
Einecs No 203-080-7 |
|
Structural formula: |
|
|
EINECS name: |
2-ethylhexyl acrylate |
|
IUPAC name: |
2-ethylhexyl acrylate |
|
Rapporteur: |
Germany |
|
Classification: (24) |
Xi; R37/38 R43 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the risk assessment forwarded to the Commission by the Member State Rapporteur (25).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as a monomer in the chemical industry for the production of polymers and copolymers, which are mainly processed further to aqueous polymer dispersions. The polymers and polymer dispersions are used in adhesives and as binders for paints. Other applications include coatings raw materials and uses in the plastics and textiles industries. In addition, 2-ethylhexyl acrylate is used as a monomer in construction-industry, chemicals (e.g. floor coatings, road-marking substances).
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for local effects as a consequence of repeated inhalation exposure arising during the formulation of preparations containing 2-ethylhexyl acrylate. |
|
— |
concerns for skin sensitisation as a consequence of dermal exposure arising during the production of 2-ethylhexyl acrylate and polymerisation, the formulation of preparations and the use of formulations containing monomeric 2-ethylhexyl acrylate in the building trade. |
The conclusions of the assessment of the risks to
CONSUMERS and HUMANS EXPOSED VIA THE ENVIRONMENT
are that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusions of the assessment of the risks to the
ATMOSPHERE, AQUATIC ECOSYSTEM and TERRESTRIAL ECOSYSTEM
are that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to the
MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
STRATEGY FOR LIMITING THE RISK
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to workers to the extent needed and shall apply.
Within this framework it is recommended:
|
— |
to establish at Community level occupational exposure limit values for 2-ethylhexyl acrylate according to Council Directive 98/24/EEC (26). |
Existing controls are considered to be sufficient to limit the risks of skin sensitisation.
PART 8
|
CAS No 106-46-7 |
Einecs No 203-400-5 |
|
Structural formula: |
|
|
EINECS name: |
1,4-Dichlorobenzene |
|
IUPAC name: |
1,4-Dichlorobenzene |
|
Rapporteur: |
France |
|
Classification (27) |
Carc. Cat. 3; R40 |
|
Xi; R36; |
|
|
R 50/53 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the comprehensive Risk Assessment Reports as forwarded to the Commission by the Member State Rapporteur (28).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as an intermediate in the chemical industry, in the formulation of moth repellents, air fresheners and toilet blocks. Other uses are as a processing aid in the production of grinding wheels and as a carrier for textile dyes.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for general systemic toxicity, carcinogenicity and reproductive toxicity as a consequence of inhalation and dermal exposure arising from manufacture and use (intermediate, formulation of products containing the substance and production of grinding wheels), |
|
— |
concerns for ocular and nasal irritation as a consequence of exposure to vapours arising during use of formulation of products containing the substance and production of grinding wheels. |
The conclusion of the assessment of the risks for
CONSUMERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for carcinogenicity as a consequence of inhalation exposure arising from use of moth repellents, air fresheners and toilet blocks. |
The conclusion of the assessment of the risks for
HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ATMOSPHERE, AQUATIC ECOSYSTEM, TERRESTRIAL ECOSYSTEM MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT and NON ECOSYSTEM-SPECIFIC EFFECTS RELEVANT TO THE FOOD CHAIN
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks related to the environmental spheres mentioned above are not expected. Risk reduction measures already being applied are considered sufficient. |
STRATEGY FOR LIMITING RISKS
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed and shall apply.
Within this framework it is recommended:
|
— |
the Commission Scientific Committee on Occupational Exposure Limits (SCOEL) review the new information contained in the risk assessment report and recommend whether there is a need to revise the current community OEL. |
For CONSUMERS
It is recommended:
|
— |
to consider at Community level marketing and use restrictions in Council Directive 76/769/EEC (29) for the use of 1,4-dichlorobenzene in air fresheners, moth repellents and toilet blocks. |
PART 9
|
CAS No 81-14-1 |
Einecs No 201-328-9 |
|
Structural formula: |
|
|
EINECS name: |
4'-tert-butyl-2',6'-dimethyl-3',5'-dinitroacetophenone |
|
IUPAC name: |
3,5-dinitro-2,6-dimethyl-4-tert-butylacetophenone |
|
Rapporteur: |
The Netherlands |
|
Classification (30): |
At the June 2002 meeting for environment and at the January 2003 CMR meeting it was agreed as Carc. Cat. 3; R40 N; R50/53 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the risk assessment forwarded to the Commission by the Member State Rapporteur.
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as an ingredient in fragrance compositions for cosmetic products.
Other uses are detergents, fabric softeners, household cleaning products and other fragranced products.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS, CONSUMERS and HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is at present no need for further information and/or testing and no need for risk reduction measures beyond those, which are being applied already. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks arising from
COMBINED EXPOSURE
is that there is at present no need for further information and/or testing and no need for risk reduction measures beyond those, which are being applied already. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
given the physico-chemical data, musk ketone is considered not to form a risk with respect to flammability, and explosive and oxidizing properties. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ATMOSPHERE, the AQUATIC ECOSYSTEM and the TERRESTRIAL ECOSYSTEM
is that there is at present no need for further information and/or testing and no need for risk reduction measures beyond those, which are being applied already. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
PART 10
|
CAS No 117-81-7 |
Einecs No 204-211-0 |
|
Structural formula: |
|
|
EINECS name: |
Di-(2-ethylhexyl) phthalate (DEHP) |
|
IUPAC name: |
Bis(2-ethylhexyl)phthalate |
|
Rapporteur: |
Sweden |
|
Classification (31): |
Repr. Cat. 2; R60-61 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the risk assessment forwarded to the Commission by the Member State Rapporteur (32).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used (97 %) as a plasticizer (improving the polymer material’s flexibility and workability) in polymer products, mainly PVC.
Flexible PVC is used in many different articles e.g. toys, building material such as flooring, cables, profiles and roofs, as well as medical products like blood bags, dialysis equipment etc. DEHP is used also in other polymer products, e.g. other vinyl resins and cellulose ester plastics.
Other uses (3 %) are for non-polymer applications such as adhesives and sealant, lacquers and paints, printing inks for paper and plastics, printing inks for textiles, rubber and ceramics for electronic purposes. Another use is as a dielectric fluid in capacitors.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for testicular effects, fertility, toxicity to kidneys on repeated exposure, and developmental toxicity as a consequence of inhalation and dermal exposure during production, processing and industrial end-use of preparations or materials containing DEHP. |
The conclusion of the assessment of the risks to
CONSUMERS
is that there is a need for specific measures to limit the risks. This conclusion is reached because of:
|
— |
concerns for children with regard to testicular effects, fertility and toxicity to kidneys on repeated exposure, as a consequence of oral exposure arising from the use of toys and child-care articles; |
|
— |
concerns for children undergoing long-term blood transfusion and neonates undergoing transfusions with regard to testicular toxicity and fertility, as a consequence of exposure from materials in medical equipment containing DEHP; |
|
— |
concerns for adults undergoing long-term haemodialysis with regard to testicular effects, fertility, toxicity to kidneys on repeated exposure, and developmental toxicity, as a consequence of exposure from materials in medical equipment containing DEHP. |
The conclusion of the assessment of the risks to
HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is a need for specific measures to limit the risks; risk reduction measures which are already being applied shall be taken into account. This conclusion is reached because of:
|
— |
concerns for children with regard to testicular effects, fertility, and toxicity to kidneys on repeated exposure, as a consequence of exposure via food grown locally near sites processing polymers with DEHP, or sites producing sealants and/or adhesives, paints and lacquers or printing inks with DEHP. The scenarios that give concern are generic scenarios based on default emission data. There is no concern for the limited number of sites that have reported measured emission data. |
|
— |
concerns for children with regard to testicular toxicity, as a consequence of exposure via food grown locally near sites recycling paper or municipal sewage treatment plants. The scenarios that give concern are generic scenarios based on default emission data. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ATMOSPHERE
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to the
AQUATIC ECOSYSTEM
is that there is a need for limiting the risks; risk reduction measures which are already being applied shall be taken into account. This conclusion is reached because of:
|
— |
concern for birds consuming mussels exposed to DEHP near sites processing polymers with DEHP or sites producing sealants and/or adhesives with DEHP. The scenarios that give concern are generic scenarios based on default emission data. There is no concern for the limited number of sites that have reported measured emission data. |
There is a need for further information and/or testing. This conclusion is reached because of:
|
— |
concern for sediment-dwelling organisms as a consequence of exposure to DEHP near sites processing polymers with DEHP or sites producing lacquers, paints, printing inks, sealants and/or adhesives with DEHP. The scenarios that give concern are generic scenarios based on default emission data. There is no concern for the limited number of sites that have reported measured emission data. |
Further refinement of the assessment may remove some concern. However implementation of risk management measures to address the risks identified for other environmental spheres will eliminate the need for further information on sediment-dwelling organisms.
The conclusion of the assessment of the risks to the
TERRESTRIAL ECOSYSTEM
is that there is a need for limiting the risks; risk reduction measures which are already being applied shall be taken into account. This conclusion is reached because of:
|
— |
concern for mammals consuming earthworms exposed to DEHP near sites processing polymers with DEHP or sites producing lacquers, paints, printing inks, sealants and/or adhesives with DEHP. The scenarios that give concern are generic scenarios based on default emission data. There is no concern for the limited number of sites that have reported measured emission data. |
There is a need for further information and/or testing. This conclusion is reached because of:
|
— |
concern for soil organisms exposed to DEHP near sites processing polymers with DEHP or sites producing printing inks, sealants and/or adhesives with DEHP. The scenarios that give concern are generic scenarios based on default emission data. There is no concern for the limited number of sites that have reported measured emission data. |
Further refinement of the assessment may remove some concern. However implementation of risk management measures to address the risks identified for other environmental spheres will eliminate the need for further information on soil organisms.
The conclusion of the assessment of the risks to
MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
STRATEGY FOR LIMITING THE RISKS
A. HUMAN HEALTH
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed and shall apply. Within this framework it is recommended:
|
— |
to establish at community level Occupational Exposure Limit values for DEHP according to Council Directive 98/24/EC (33). |
For CONSUMERS
It is recommended:
|
— |
to restrict the use of DEHP in packaging materials for foods (plastic materials in contact with food (Directive 2002/72/EC (34)). |
|
— |
to consider restricting the use of DEHP in medical devices giving rise to possible exposure of neonates and identified groups of concern following the procedure laid down in Council Directive 93/42/EEC (35) concerning medical devices, assuming the availability of safe alternatives. |
As regards consumer use of DEHP, the existing legislative measures for consumer protection, in particular the provisions under Council Directive 76/769/EEC (36) (marketing and use Directive) as regards CMR substances and Directive 2005/84/EC of the European Parliament and of the Council (37) on phthalates in toys and child care articles are considered sufficient to address risks identified to consumers.
HUMANS indirectly EXPOSED VIA THE ENVIRONMENT
Within the framework of existing legislative measures under Council Directive 76/769/EEC (marketing and use Directive) it is recommended:
|
— |
to consider at Community level restrictions for the use of DEHP in industrial installations for processing polymers with DEHP (extrusion, calendaring, spread coating) and for producing sealants and/or adhesives, paints and lacquers or printing inks with DEHP, exempting installations with no emission of DEHP to the environment as well as installations where DEHP emissions are adequately controlled. Adequate control could e.g. be achieved through efficient treatment of exhaust air and aqueous effluents. The efficiency in emissions’ reduction should be documented to enable follow up by Member State authorities; |
|
— |
to consider, within a reasonable time period, the need for Community level restrictions due to emissions to water from products containing DEHP, taking into account any additional information. |
PART 11
|
CAS No 108-95-2 |
Einecs No 203-632-7 |
|
Structural formula: |
|
|
EINECS name: |
Phenol |
|
IUPAC name: |
Phenol |
|
Rapporteur: |
Germany |
|
Classification (38): |
T; R23/24/25 |
|
C; R34 |
|
|
Xn; R48/20/21/22 |
|
|
Muta Cat. 3; R68 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the risk assessment forwarded to the Commission by the Member State Rapporteur (39).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as an intermediate in the production of bisphenol A, phenol resins, alkylphenols, caprolactam, salicylic acid, nitrophenols, diphenyl ethers, halogen phenols and other chemicals.
Other uses are as a component in cosmetics and medical preparations as well as in non-agricultural biocides, adhesives and impregnating agents.
The risk assessment has identified other sources of exposure to the substance to human and the environment, in particular, releases of phenol as a product of human metabolism and livestock farming, from processing of coal and pulp manufacture and from landfills, which do not result from the life-cycle of the substance produced in or imported into the European Community. The assessment of the risks arising from these exposures is not part of this risk assessment. The comprehensive Risk Assessment Reports as forwarded to the Commission by the Member State Rapporteur does however provide information that could be used to assess these risks.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS
is that there is a need for specific measures to limit the risks. The conclusion is reached because of:
|
— |
concerns for acute toxic effects (systemic) as a consequence of inhalation exposure, arising from the formulation of phenolic resins; |
|
— |
concerns for acute toxic effects (systemic) as a consequence of dermal exposure, arising from the use of phenolic resins in spraying techniques; |
|
— |
concerns for corrosivity following skin contact and contact to the eyes arising from all dermal exposure scenarios (production and further processing, formulation and use of phenolic resins); |
|
— |
concerns for systemic effects as a consequence of repeated inhalation exposure arising from all scenarios (production and further processing, formulation and use of phenolic resins); |
|
— |
concerns for systemic effects as a consequence of repeated dermal exposure arising from the formulation of phenolic resins and use of phenolic resins in spraying techniques. |
The conclusion of the assessment of the risks to
CONSUMERS
is that there is a need for specific measures to limit the risks. The conclusion is reached because of:
|
— |
concerns for skin irritation as a consequence of exposure arising from the use of phenol containing disinfectants; |
|
— |
concerns for systemic effects as a consequence of repeated inhalation exposure arising from phenol in floor waxes; |
|
— |
concerns for systemic effects as a consequence of repeated dermal exposure arising from phenol in disinfectants. |
The conclusion of the assessment of the risks to
HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is a need for specific measures to limit the risks. The conclusion is reached because of:
|
— |
concerns for systemic effects as a consequence of repeated oral exposure arising from local indirect exposure via plant shoots. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ATMOSPHERE, AQUATIC ECOSYSTEM and TERRESTRIAL ECOSYSTEM
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
MICRO-ORGANISMS IN THE SEWAGE TREATMENT PLANT
is that there is a need for specific measures to limit the risks. The conclusion is reached because of:
|
— |
concerns for effects on micro-organisms in industrial waste water treatment plants at eight production and processing or mere processing sites |
STRATEGY FOR LIMITING RISKS
For WORKERS
The legislation for workers’ protection currently in force at Community level is generally considered to give an adequate framework to limit the risks of the substance to the extent needed and shall apply.
ENVIRONMENT AND HUMANS EXPOSED VIA THE ENVIRONMENT
The risk assessment has identified other sources of phenol emissions (from non isolated phenol, e.g. from cooking, gasification and liquefaction of coal, refineries and pulp manufacture, as a product of human or livestock metabolism or from landfills), than those from the produced or imported chemical. The need to consider if additional risk management is needed can best be considered under Directive 2000/60/EC of the European Parliament and of the Council (40) (Water Framework Directive) and forthcoming EU legislation with regard to soil protection using the information in the comprehensive risk assessment report.
The existing legislative measures for the protection of the environment are considered sufficient to address potential risks from landfills without landfill leachate collecting systems (Council Directive 1999/31/EC (41)).
PART 12
|
CAS No 81-15-2 |
Einecs No 201-329-4 |
|
Structural formula: |
|
|
EINECS name: |
5-tert-butyl-2,4,6-trinitro-m-xylene |
|
IUPAC name: |
1-tert-butyl-3,5-dimethyl-2,4,6-trinitrobenzene |
|
Rapporteur: |
The Netherlands |
|
Classification (42): |
Carc. Cat. 3; R40 E; R2 N; R50/53 |
The risk assessment is based on current practices related to the life-cycle of the substance produced in or imported into the European Community as described in the risk assessment forwarded to the Commission by the Member State Rapporteur (43).
The risk assessment has, based on the available information, determined that in the European Community the substance is mainly used as an ingredient in fragrance compositions for cosmetic products.
Other uses are detergents, fabric softeners, household cleaning products and other fragranced products.
RISK ASSESSMENT
A. HUMAN HEALTH
The conclusion of the assessment of the risks to
WORKERS, CONSUMERS and HUMANS EXPOSED VIA THE ENVIRONMENT
is that there is at present no need for further information and/or testing and no need for risk reduction measures beyond those, which are being applied already. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks arising from
COMBINED EXPOSURE
is that there is at present no need for further information and/or testing and no need for risk reduction measures beyond those, which are being applied already. This conclusion is reached because:
|
— |
the risk assessment shows that risks are not expected. Risk reduction measures already being applied are considered sufficient. |
The conclusion of the assessment of the risks to
HUMAN HEALTH (physico-chemical properties)
is that there is at present no need for further information and/or testing or for risk reduction measures beyond those which are being applied. This conclusion is reached because:
|
— |
given the physico-chemical data, 5-tert-butyl-2,4,6-trinitro-m-xylene (musk xylene) is considered not to form a risk with respect to oxidizing properties. |
|
— |
It is noted that musk xylene is flammable and explosive by shock and heat, and should be labelled with respect to these aspects. Therefore, measures to avoid flammability and explosion are indicated. If the appropriate conditions of handling and storage are adhered to, there are no concerns for risks to human health arising from the physicochemical properties of musk xylene. |
B. ENVIRONMENT
The conclusion of the assessment of the risks to the
ENVIRONMENT
is that there is need for further information and/or testing. This conclusion is reached because the substance is considered a PBT candidate chemical. A further PBT-testing strategy is proposed.
(1) Commission Directive 2001/59/EC of 6 August adapting to technical progress for the 28th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 225, 21.8.2001).
(2) Commission Directive adapting to technical progress for the 30th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (not yet published).
(3) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(4) OJ L 224, 18.8.1990, p. 1.
(6) OJ L 131, 5.5.1998, p. 11.
(7) Commission Directive 2004/73/EC of 29 April 2004 adapting to technical progress for the 29th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 152, 30.4.2004, corrected by OJ L 216, 16.6.2004, p. 3).
(8) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European chemicals Bureau: http://ecb.jrc.it/existing-substances/
(9) OJ L 262, 27.9.1976, p. 201.
(10) Commission Directive, adapting to technical progress for the 30th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (not yet published).
(11) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(12) OJ L 131, 5.5.1998, p. 11.
(13) OJ L 262, 27.9.1976, p. 201.
(14) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(15) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(16) OL L 262, 27.9.1976, p. 201.
(17) OJ L 11, 15.1.2002, p. 4.
(18) OJ L 257, 10.10.1996, p. 26.
(19) Commission Directive 2004/73/EC of 29 April 2004 adapting to technical progress for the 29th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 152, 30.4.2004, as corrected by OJ L 216, 16.6.2004, p. 3).
(20) The comprehensive Risk Assessment Report can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(23) OJ L 131, 5.5.1998, p. 11.
(24) Commission Directive 2004/73/EC of 29 April 2004 adapting to technical progress for the 29th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 152, 30.4.2004, corrected by OJ L 216, 16.6.2004, p. 3).
(25) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(26) OJ L 131, 5.5.1998, p. 11.
(27) The classification of the substance is established by Commission Directive 2004/73/EC of 29 April 2004 adapting to technical progress for the 29th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 152, 30.4.2004, corrected by OJ L 216, 16.6.2004, p. 3).
(28) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(29) OJ L 262, 27.9.1976, p. 201.
(30) The classification of the substance is established by Commission Directive [to be published in the 31st ATP)] adapting to technical progress for the 31st time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances, as last amended by Directive 2004/73/EC.
(31) The classification of the substance is established by Commission Directive 2001/59/EC of 6 August 2001 adapting to technical progress for the 28th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 225, 21.8.2001, p. 1.).
(32) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(33) OJ L 131, 5.5.1998, p. 11.
(34) OJ L 220, 15.8.2002, p. 18.
(35) OJ L 169, 12.7.1993, p. 1.
(36) OJ L 262, 27.9.1976, p. 201.
(37) OJ L 344, 27.12.2005, p. 40.
(38) Commission Directive 2004/73/EC of 29 April 2004, adapting to technical progress for the 29th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 152, 30.4.2004, corrected by OJ L 216, 16.6.2004, p. 3).
(39) The comprehensive Risk Assessment Report, as well as a summary thereof, can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/
(40) OJ L 327, 22.12.2000, p. 1.
(41) OJ L 182, 16.7.1999, p. 1.
(42) The classification of the substance is established by Commission Directive 2004/73/EC of 29 April 2004 adapting to technical progress for the 29th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances (OJ L 152, 30.4.2004, corrected by OJ L 216, 16.6.2004, p. 3).
(43) The comprehensive Risk Assessment Report can be found on the internet site of the European Chemicals Bureau: http://ecb.jrc.it/existing-substances/