EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document 02008R0798-20130326
Commission Regulation (EC) No 798/2008 of 8 August 2008 laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements (Text with EEA relevance)
Consolidated text: Commission Regulation (EC) No 798/2008 of 8 August 2008 laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements (Text with EEA relevance)
Commission Regulation (EC) No 798/2008 of 8 August 2008 laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements (Text with EEA relevance)
2008R0798 — EN — 26.03.2013 — 013.001
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
COMMISSION REGULATION (EC) No 798/2008 of 8 August 2008 laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements (OJ L 226, 23.8.2008, p.1) |
Amended by:
Corrected by:
COMMISSION REGULATION (EC) No 798/2008
of 8 August 2008
laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements
(Text with EEA relevance)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Directive 90/539/EEC of 15 October 1990 on animal health conditions governing intra-Community trade in, and imports from third countries of, poultry and hatching eggs ( 1 ), and in particular Article 21(1), Article 22(3) Article 23, Article 24(2) and Articles 26 and 27a thereof,
Having regard to Council Directive 91/496/EEC of 15 July 1991 laying down the principles governing the organisation of veterinary checks on animals entering the Community from third countries and amending Directives 89/662/EEC, 90/425/EEC and 90/675/EEC ( 2 ), and in particular Articles 10 and 18 thereof,
Having regard to Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC ( 3 ), and in particular the fourth subparagraph of Article 29(1), thereof,
Having regard to Council Directive 97/78/EC of 18 December 1997 laying down the principles governing the organisation of veterinary checks on products entering the Community from third countries ( 4 ), and in particular Article 22(1) thereof,
Having regard to Council Directive 2002/99/EC of 16 December 2002 laying down the animal health rules governing the production, processing, distribution and introduction of products of animal origin for human consumption ( 5 ), and in particular Article 8, Article 9(2)(b) and Article 9(4) thereof,
Having regard to Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of salmonella and other specified food-borne zoonotic agents ( 6 ), and in particular Article 10(2) thereof,
Having regard to Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin ( 7 ), and in particular Article 9 thereof,
Having regard to Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption ( 8 ), and in particular Article 11(1) thereof,
Whereas:|
(1) |
Directive 90/539/EEC lays down animal health conditions governing imports into the Community from third countries of poultry and hatching eggs. It provides that poultry and hatching eggs are to satisfy the conditions laid down therein and originate in a third country or part thereof included on a list drawn up in accordance with that Directive. |
|
(2) |
Directive 2002/99/EC lays down rules governing the introduction from third countries of products of animal origin and products obtained there from intended for human consumption. It provides that such products are only to be imported into the Community if they comply with the requirements applicable to all stages of the production, processing and distribution of those products in the Community or if they offer equivalent animal health guarantees. |
|
(3) |
Commission Decision 2006/696/EC of 28 August 2006 laying down a list of third countries from which poultry, hatching eggs, day-old chicks, meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may be imported into and transit through the Community and the applicable veterinary certification conditions ( 9 ) sets out a list of third countries from which the commodities concerned may be imported into, and transit through, the Community and lays down the veterinary certification conditions. |
|
(4) |
Commission Decision 93/342/EEC of 12 May 1993 laying down the criteria for classifying third countries with regard to avian influenza and Newcastle disease in relation to imports of live poultry and hatching eggs ( 10 ) and Commission Decision 94/438/EC of 7 June 1994 laying down the criteria for classifying third countries and parts thereof with regard to avian influenza and Newcastle disease in relation to imports of fresh poultrymeat ( 11 ) lay down criteria for classifying third countries with regard to avian influenza and Newcastle disease in relation to imports of live poultry, hatching eggs and poultrymeat. |
|
(5) |
Community legislation for the control of avian influenza has recently been updated by Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza ( 12 ), to take account of the most recent scientific knowledge and developments on the epidemiology of avian influenza in the Community and worldwide. The scope of the control measures to be applied in the event of an outbreak has been extended from highly pathogenic avian influenza (HPAI) to deal also with outbreaks of low pathogenic avian influenza (LPAI), and to introduce compulsory active surveillance for avian influenza and a wider use of vaccination against this disease. |
|
(6) |
Imports from third countries should therefore meet conditions equivalent to those applied within the Community and which are in line with the revised requirements for international trade in poultry and poultry products laid down by the standards of the Terrestrial Animal Health Code of the World Organisation for Animal health (OIE) ( 13 ) and the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals ( 14 ) of the OIE. |
|
(7) |
Argentina and Israel have submitted their avian influenza surveillance programmes to the Commission for evaluation. The Commission has examined these programmes and they conform to the relevant Community provisions and therefore a positive evaluation of these programmes should be indicated in the column 7 of part 1 of Annex I to this Regulation. |
|
(8) |
Article 21(2) of Directive 90/539/EEC sets out certain matters that are to be taken into account when deciding whether or not a third country or part thereof maybe included in the list of third countries from which poultry and hatching eggs may be imported into the Community, such as the state of health of poultry, the regularity and rapidity of the supply of information by a third country relating to the existence of certain contagious animal diseases, including avian influenza and Newcastle disease and the rules for animal disease prevention and control in the third country concerned. |
|
(9) |
Article 8 of Directive 2002/99/EC provides that when drawing up lists of third countries or regions of third countries thereof from which imports of specified products of animal origin are permitted into the Community, particular account is to be taken of certain matters, such as the health status of livestock, the regularity, speed and accuracy with which the third country supplies information on the existence of certain infectious or contagious animal diseases in its territory, in particular avian influenza and Newcastle disease and the general health situation in the third country concerned which might pose a risk to public or animal health in the Community. |
|
(10) |
In the interests of animal health, this Regulation should provide that commodities should only be imported into the Community from third countries, territories, zones or compartments which have in place avian influenza surveillance programmes and avian influenza vaccination plans, where such vaccination is carried out. |
|
(11) |
Pursuant to Regulation (EC) No 2160/2003, admission to or retention on the lists of third countries provided for in Community legislation from which Member States are authorised to import certain poultry commodities covered by that Regulation is subject to the submission to the Commission by the third country concerned of a programme equivalent to national control programmes for Salmonella to be established by the Member States, and its approval by the Commission. A positive evaluation of these programmes should be indicated in part 1 of Annex I to this Regulation. |
|
(12) |
The Community and certain third countries wish to permit trade in poultry and poultry products coming from approved compartments and therefore the principle of compartmentalisation for imports of poultry and poultry products should be further laid down in Community legislation. The compartmentalisation principle has been laid down recently by the OIE in order to facilitate world wide trade in poultry and poultry products and therefore it should be incorporated into Community legislation. |
|
(13) |
Currently Community legislation does not provide for certificates for the import into the Community of minced meat and mechanically separated meat of poultry, ratites and wild game-birds, for certain health reasons, in particular the traceability of meat used for its production. Accordingly, model veterinary certificates covering those commodities should be provided for in this Regulation following further scientific investigations. |
|
(14) |
In order to provide more flexibility in certain situations for the competent authorities for veterinary certificates purposes, and based on several requests from third countries exporting day-old chicks of poultry and ratites to the Community, this Regulation should provide that such commodities should be examined at the time of dispatch of the consignment instead of at the time of issue of the veterinary certificate. |
|
(15) |
In order to avoid any interruption in trade, imports into the Community of commodities that have been produced before the introduction of animal health restrictions, as set out in Part 1 of Annex I to this Regulation, should continue to be permitted for 90 days following the introduction of import restrictions for the commodity concerned. |
|
(16) |
Specific conditions for transit via the Community of consignments to and from Russia should be provided for owing to the geographical situation of Kaliningrad which affects only Latvia, Lithuania and Poland. |
|
(17) |
Council Regulation (EC) No 1234/2007 of 22 October 2007 establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products (Single CMO Regulation) ( 15 ) set out general Community health rules applicable to the import into, and transit through, the Community of the commodities covered by the Regulation. |
|
(18) |
In addition, Council Directive 96/93/EC of 17 December 1996 on the certification of animals and animal products ( 16 ) lays down standards of certification which are necessary to ensure valid certification and to prevent fraud. It is, therefore, appropriate to ensure in this Regulation that the rules and principles applied by third country certifying officers provide guarantees that are equivalent to those laid down in that Directive and that the model veterinary certificates laid down in this Regulation reflect only such facts as may be attested at the time the certificate is issued. |
|
(19) |
In the interests of clarity and coherence of Community legislation, Decisions 93/342/EEC, 94/438/EC and 2006/696/EC should be repealed and replaced by this Regulation. |
|
(20) |
It is appropriate to provide for a transitional period to permit Member States and industry to take the necessary measures to comply with the applicable veterinary certification requirements laid down in this Regulation. |
|
(21) |
The measures provided for in this Regulation are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS REGULATION:
CHAPTER I
SUBJECT MATTER, SCOPE AND DEFINITIONS
Article 1
Subject matter and scope
1. This Regulation lays down veterinary certification requirements for imports into and transit, including storage during transit, through the Community of the following commodities (the commodities):
(a) poultry, hatching eggs, day-old chicks and specified pathogen-free eggs;
(b) meat, minced meat and mechanically separated meat of poultry, including ratites and wild game-birds, eggs and egg products.
It lays down a list of third countries, territories, zones or compartments from which the commodities may be imported into the Community.
2. This Regulation shall not apply to poultry for exhibitions, shows or contests.
3. This Regulation shall apply without prejudice to specific certification requirements provided for in Community agreements with third countries.
Article 2
Definitions
For the purposes of this Regulation, the following definitions shall apply:
(1) ‘poultry’ means fowl, turkeys, guinea fowl, ducks, geese, quails, pigeons, pheasants, partridges and ratites (ratitae), reared or kept in captivity for breeding, the production of meat or eggs for consumption, or for restocking supplies of game;
(2) ‘hatching eggs’ means eggs for incubation, laid by poultry;
(3) ‘day-old chicks’ means all poultry less than 72 hours old, not yet fed and muscovy ducks (Cairina moschata) or their crosses, less than 72 hours old whether or not fed;
(4) ‘breeding poultry’ means poultry 72 hours old or more, intended for the production of hatching eggs;
(5) ‘productive poultry’ means poultry 72 hours old or more, reared for:
(a) the production of meat and/or eggs for consumption; or
(b) the restocking of supplies of game;
(6) ‘specified pathogen-free eggs’ means hatching eggs which are derived from ‘chicken flocks free from specified pathogens’, as described in the European Pharmacopoeia ( 17 ), and which are intended solely for diagnostic, research or pharmaceutical use;
(7) ‘meat’ means edible parts of the following animals:
(a) poultry, which, when relating to meat, means farmed birds, including birds that are farmed as domestic animals without being considered as such, with the exception of ratites;
(b) wild game-birds that are hunted for human consumption;
(c) ratites;
(8) ‘mechanically separated meat’ means the product obtained by removing meat from flesh-bearing bones after boning or from poultry carcases, using mechanical means resulting in the loss or modification of the muscle fibre structure;
(9) ‘minced meat’ means de-boned meat that has been minced into fragments and contains less than 1 % salt;
(10) ‘zone’ means a clearly defined part of a third country containing an animal subpopulation with a distinct health status with respect to a specific disease for which the required surveillance, control and biosecurity measures have been applied for the purpose of imports under this Regulation;
(11) ‘compartment’ means one or more poultry establishments in a third country under a common biosecurity management system containing a poultry subpopulation with a distinct health status with respect to a specific disease or diseases for which the required surveillance, control and biosecurity measures have been applied for the purpose of imports under this Regulation;
(12) ‘establishment’ means a facility or part of a facility which occupies a single site and is devoted to one or more of the following activities:
(a) pedigree breeding establishment: an establishment which produces hatching eggs for the production of breeding poultry;
(b) breeding establishment: an establishment which produces hatching eggs for the production of productive poultry;
(c) rearing establishment either:
(i) a breeding poultry-rearing establishment which rears breeding poultry prior to the reproductive stage; or
(ii) a productive poultry-rearing establishment which rears egg-laying productive poultry prior to the laying stage;
(d) keeping of other productive poultry;
(13) ‘hatchery’ means an establishment which incubates and hatches eggs and supplies day-old chicks;
(14) ‘flock’ means all poultry of the same health status kept on the same facilities or in the same enclosure and constituting a single epidemiological unit; as regards housed poultry, this definition includes all birds sharing the same airspace;
(15) ‘avian influenza’ means an infection of poultry caused by any influenza A virus:
(a) of the subtypes H5 or H7;
(b) with an intravenous pathogenicity index (IVPI) in six-week old chickens greater than 1,2; or
(c) causing at least 75 % mortality in four- to 8-week-old chickens infected intravenously;
(16) ‘highly pathogenic avian influenza’ (HPAI) means an infection of poultry caused by:
(a) avian influenza viruses of the subtypes H5 or H7 with genome sequences codifying for multiple basic amino acids at the cleavage site of the haemagglutinin molecule similar to that observed for other HPAI viruses, indicating that the haemagglutinin molecule can be cleaved by a host ubiquitous protease;
(b) avian influenza as defined in point 15(b) and (c);
(17) ‘low pathogenic avian influenza’ (LPAI) means an infection of poultry caused by avian influenza viruses of subtypes H5 or H7 other than HPAI;
(18) ‘Newcastle disease’ means an infection of poultry:
(a) caused by any avian strain of the paramyxovirus 1 with an intracerebral pathogenicity index (ICPI) in day-old chicks greater than 0,7; or
(b) multiple basic amino acids have been demonstrated in the virus (either directly or by deduction) at the C-terminus of the F2 protein and phenylalanine at residue 117, which is the N-terminus of the F1 protein; the term ‘multiple basic amino acids’ refers to at least three arginine or lysine residues between residues 113 and 116; failure to demonstrate the characteristic pattern of amino acid residues as described in this point requires characterisation of the isolated virus by an ICPI test; in this definition, amino acid residues are numbered from the N-terminus of the amino acid sequence deduced from the nucleotide sequence of the F0 gene, 113-116 corresponds to residues -4 to -1 from the cleavage site;
(19) ‘official veterinarian’ means the veterinarian designated by the competent authority;
(20) ‘differentiating infected from vaccinated animal (DIVA) strategy’ means a vaccination strategy which enables a differentiation to be made between vaccinated/infected and vaccinated/non-infected animals through the application of a diagnostic test designed to detect antibodies against the field virus and the use of non-vaccinated sentinel birds.
CHAPTER II
GENERAL CONDITIONS FOR IMPORTS AND TRANSIT
Article 3
Lists of third countries, territories, zones or compartments of origin from which commodities may be imported into and transit through the Community
The commodities shall only be imported into and transit through the Community from the third countries, territories, zones or compartments listed in columns 1 and 3 of the table in Part 1 of Annex I.
Article 4
Veterinary certification
1. Commodities imported into the Community shall be accompanied by a veterinary certificate, as referred to in column 4 of the table in Part 1 of Annex I, for the commodity concerned, completed in accordance with the notes and the model veterinary certificates set out in Part 2 of that Annex (the certificate).
2. A declaration by the master of the ship, as set out in Annex II, shall be attached to veterinary certificates for imports of poultry and day-old chicks, where the transport of those commodities includes transport by ship, even for part of the journey.
3. Poultry, hatching eggs and day-old chicks transiting through the Community shall be accompanied by:
(a) a veterinary certificate as referred to in paragraph 1 which shall bear the words ‘for transit through the EC’, and
(b) a certificate required by the third country of destination.
4. Specified pathogen-free eggs, meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds, eggs and egg products transiting through the Community shall be accompanied by a certificate drawn up in accordance with the model certificate set out in Annex XI and complying with the conditions set out therein.
5. For the purposes of this Regulation, transit may include storage during transit in accordance with Articles 12 and 13 of Directive 97/78/EC.
6. Electronic certification and other agreed systems harmonised at Community level may be used.
Article 5
Conditions for imports and transit
1. Commodities imported into and transiting through the Community shall comply with the conditions laid down in Articles 6 and 7 and in Chapter III.
2. Paragraph 1 shall not apply to single consignments of fewer than 20 units of poultry other than ratites, hatching eggs or day-old chicks thereof. However, such single consignments may only be imported from third countries, territories, zones or compartments thereof that are approved for such imports and they comply with the following conditions:
(a) third country, territory, zone or compartment is listed in columns 1 and 3 of the table in Part 1 of Annex I and column 4 of that table provides for a model veterinary certificate for the commodity concerned;
(b) they are not covered by an import ban for animal health reasons;
(c) the importation conditions include the requirement for post-import isolation or quarantine.
3. Commodities referred to in paragraph 1 shall comply with the following:
(a) the additional guarantees, as specified in column 5 of the table in Part 1 of Annex I;
(b) the specific conditions set out in column 6, and where appropriate, the closing dates set out in column 6A and the opening dates set out in column 6B, of the table in Part 1 of Annex I;
(c) the animal health additional guarantees, where required by the Member State of destination and referred to in the certificate;
(d) the restrictions in relation to the approval of a Salmonella control programme, shall only apply when indicated in the appropriate column of the table in Part 1 of Annex I.
Article 6
Examination, sampling and testing procedures
Where examination, sampling and testing for avian influenza, Mycoplasma, Newcastle disease, Salmonella, and other pathogens of animal or public health significance is required for imports of commodities into the Community in accordance with the certificates, such commodities shall only be imported into the Community where those examinations, sampling and testing have been carried out by the competent authority of the third country concerned or where appropriate by the competent authority of the Member State of destination in accordance with Annex III.
Article 7
Disease reporting requirements
Commodities shall only be imported into the Community from third countries, territories, zones or compartments, where the third country concerned:
(a) informs the Commission of the disease situation within 24 hours of confirmation of any initial outbreaks of LPAI, HPAI or Newcastle disease;
(b) submits virus isolates from initial outbreaks of HPAI and Newcastle disease, without undue delay to the Community reference laboratory for avian influenza and Newcastle disease ( 18 ); such virus isolates shall not be required for imports of eggs, egg products and specified pathogen-free eggs from third countries, territories, zones or compartments from which the import of such commodities into the Community is authorised;
(c) submits to the Commission regular updates on the disease situation.
CHAPTER III
ANIMAL HEALTH STATUS OF THIRD COUNTRIES, TERRITORIES, ZONES OR COMPARTMENTS OF ORIGIN WITH REGARD TO AVIAN INFLUENZA AND NEWCASTLE DISEASE
Article 8
Avian influenza free third countries, territories, zones or compartments
1. For the purposes of this Regulation, a third country, territory, zone or compartment from which commodities are imported into the Community shall be considered as free from avian influenza where:
(a) avian influenza has not been present in the third country, territory, zone or compartment for a period of at least 12 months preceding the certification by the official veterinarian;
(b) an avian influenza surveillance programme, in accordance with Article 10 has been carried out during a period of at least six months preceding the certification referred to in point (a) of this paragraph where required in the certificate.
2. Where an outbreak of avian influenza occurs in a third country, territory, zone or compartment previously free of that disease, as referred to in paragraph 1, that third country, territory, zone or compartment shall again be considered as free from avian influenza provided the following conditions have been met:
(a) in the case of HPAI, a stamping out policy has been implemented to control the disease;
(b) in the case of LPAI, either a stamping out policy has been implemented or the poultry have been slaughtered to control the disease;
(c) adequate cleansing and disinfection has been carried out on all previously infected establishments;
(d) avian influenza surveillance has been carried out in accordance with Part II of Annex IV during a three-month period following completion of the cleansing and disinfection referred to in point (c) of this paragraph with negative results.
Article 9
HPAI free third countries, territories, zones and compartments
1. For the purposes of this Regulation, a third country, territory, zone or compartment from which commodities are imported into the Community shall be considered as free from HPAI where that disease has not been present in the third country, territory, zone or compartment for a period of at least 12 months preceding the certification by the official veterinarian.
2. Where an outbreak of HPAI occurs in a third country, territory, zone or compartment previously free of that disease as referred to in paragraph 1, that third country, territory, zone or compartment shall again be considered as free of HPAI, provided the following conditions are met:
(a) a stamping out policy has been implemented to control the disease, including adequate cleansing and disinfection carried out on all previously infected establishments;
(b) avian influenza surveillance has been carried out in accordance with Part II of Annex IV during a three-month period following completion of the stamping out policy and cleansing and disinfection referred to in point (a).
Article 10
Avian influenza surveillance programmes
Where an avian influenza surveillance programme is required in the certificate, commodities shall only be imported into Community from third countries, territories, zones or compartments where:
(a) the third country, territory, zone or compartment has had in place for a period of at least six months an avian influenza surveillance programme, which is indicated in column 7 of the table in Part 1 of Annex I, and that programme meets the requirements:
(i) set out in Part I of Annex IV; or
(ii) of the Terrestrial Animal Health Code of the OIE ( 19 );
(b) the third country informs the Commission of any changes to its avian influenza surveillance programme.
Article 11
Vaccination against avian influenza
Where vaccination against avian influenza is carried out in third countries, territories, zones or compartments, poultry or other commodities derived from vaccinated poultry shall only be imported into the Community, where:
(a) the third country carries out vaccination against avian influenza in accordance with a vaccination plan indicated in column 8 of the table in Part 1 of Annex I, and that plan meets the requirements set out in Annex V;
(b) the third country informs the Commission of any changes to its avian influenza vaccination plan.
Article 12
Newcastle disease free third countries, territories, zones and compartments
1. For the purposes of this Regulation, a third country, territory, zone or compartment from which commodities are imported into the Community shall be considered as free from Newcastle disease where the following conditions are met:
(a) no outbreaks of Newcastle disease have occurred in poultry in the third country, territory, zone or compartment for a period of at least 12 months preceding the certification by the official veterinarian;
(b) no vaccination against Newcastle disease using vaccines that do not comply with the criteria for recognised Newcastle disease vaccines set out in Annex VI has been carried out for at least the period referred to in point (a) of this paragraph.
2. Where an outbreak of Newcastle disease occurs in a third country, territory, zone or compartment previously free from that disease, as referred to in paragraph 1, that third country, territory, zone or compartment shall again be considered as free from that disease provided the following conditions are met:
(a) a stamping out policy has been implemented to control the disease;
(b) adequate cleansing and disinfection has been carried out on all previously infected establishments;
(c) during a period of at least three months following the completion of the stamping out policy and cleansing and disinfection referred to in points (a) and (b):
(i) the competent authority of a the third country can demonstrate the absence of that disease in the third country, territory, zone or compartment by intensified investigations including laboratory testing in relation to the outbreak;
(ii) no vaccination against Newcastle disease using vaccines that do not comply with the criteria for recognised Newcastle disease vaccines set out in Annex VI has been carried out.
Article 13
Derogations concerning the use of vaccines against Newcastle disease
1. For commodities referred to in Article 1(1)(a), and by way of derogation from Article 12(1)(b) and Article 12(2)(c)(ii), a third country, territory, zone or compartment shall be considered as free from Newcastle disease where the following conditions are met:
(a) the third country, territory, zone or compartment allows the use of vaccines which comply with the general criteria set out in Part I of Annex VI, but not with the specific criteria set out in Part II of that Annex;
(b) the additional health requirements set out in Part I of Annex VII are complied with.
2. For commodities referred to in Article 1(1)(b), and by way of derogation from Article 12(1)(b) and Article 12(2)(c)(ii), a third country, territory, zone or compartment from which imports of poultrymeat into the Community are authorised, shall be considered as free from Newcastle disease where the additional health requirements laid down in Part II of Annex VII are complied with.
CHAPTER IV
SPECIFIC CONDITIONS FOR IMPORTS
Article 14
Specific conditions for imports of poultry, hatching eggs and day-old chicks
1. In addition to the conditions laid down in Chapters II and III, the following specific conditions shall apply to imports of:
(a) breeding and productive poultry other than ratites, hatching eggs and day-old chicks other than of ratites, the requirements set out in Annex VIII;
(b) ratites for breeding and production, hatching eggs and day-old chicks thereof, the requirements set out in Annex IX.
2. The conditions provided for in paragraph 1 shall not apply to single consignments of less than 20 units of poultry other than, ratites, hatching eggs or day-old chicks thereof.
Article 15
Specific conditions for imports of specified pathogen-free eggs
In addition to the requirements provided for in Articles 3 to 6, specified pathogen-free eggs imported into the Community shall comply with the following requirements:
(a) they shall be marked with a stamp bearing the ISO code of the third country of origin and the approval number of the establishment of origin;
(b) each package of specified pathogen-free eggs must only contain eggs from the same third country of origin, establishment and consignor, and must bear at least the following particulars:
(i) the information shown on the eggs as provided for in point (a);
(ii) a clearly visible and legible indication that the consignment contains specified pathogen-free eggs;
(iii) the consignor’s name or business name and address.
(c) specified pathogen-free eggs imported into the Community must be transported directly to their final destination after import controls have been completed satisfactorily.
Article 16
Specific conditions for transport of poultry and day-old chicks
Poultry and day-old chicks imported into the Community shall not be:
(a) loaded onto a means of transport carrying other poultry and day-old chicks of a lower health status;
(b) in the course of transport to the Community, shall not be moved through nor unloaded in a third country, territory, zone or compartment from which imports of such poultry and day-old chicks into the Community are not authorised.
Article 17
Specific conditions for imports of meat of ratites
Only meat derived from ratites which have undergone the protective measures in relation to Crimean-Congo haemorrhagic fever, laid down in Part II of Annex X, may be imported into the Community.
CHAPTER V
SPECIFIC CONDITIONS FOR TRANSIT
Article 18
Derogations for transit through Latvia, Lithuania and Poland
1. By way of derogation from Article 4(4), transit by road or by rail shall be authorised between the border inspection posts in Latvia, Lithuania and Poland listed in the Annex to Commission Decision 2009/821/EC ( 20 ), of consignments of meat, minced meat and mechanically separated meat of poultry including ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs coming from and bound for Russia, directly or via another third country, where the following conditions are met:
(a) the consignment is sealed with a serially numbered seal by the official veterinarian at the border inspection post of entry in Latvia, Lithuania or Poland;
(b) the documents accompanying the consignment, as provided for in Article 7 of Directive 97/78/EC, are stamped with the words ‘ONLY FOR TRANSIT TO RUSSIA VIA THE EU’ on each page by the official veterinarian at the border inspection post of entry in Latvia, Lithuania or Poland;
(c) the procedural requirements provided for in Article 11 of Directive 97/78/EC are complied with;
(d) the consignment is certified as acceptable for transit on the common veterinary entry document issued by the official veterinarian at the border inspection post of entry in Latvia, Lithuania or Poland.
2. By way of derogation from Article 4(4), transit by road or rail shall be authorised between the border inspection posts in Lithuania listed in the Annex to Decision 2009/821/EC, of consignments of eggs and egg products coming from Belarus and bound for the Russian territory of Kaliningrad, where the following conditions are met:
(a) the consignment is sealed with a serially numbered seal by the official veterinarian at the border inspection post of entry in Lithuania;
(b) the documents accompanying the consignment, as provided for in Article 7 of Directive 97/78/EC, are stamped with the words ‘ONLY FOR TRANSIT TO RUSSIA VIA LITHUANIA’ on each page by the official veterinarian at the border inspection post of entry in Lithuania;
(c) the procedural requirements provided for in Article 11 of Directive 97/78/EC are complied with;
(d) the consignment is certified as acceptable for transit on the common veterinary entry document issued by the official veterinarian at the border inspection post of entry in Lithuania.
3. The consignments, as referred to in paragraphs 1 and 2, may not be unloaded or put into storage, as referred to in Article 12(4) or in Article 13 of Directive 97/78/EC, within the Union.
4. Regular audits shall be conducted by the competent authority to ensure that the number of consignments, as referred to in paragraphs 1 and 2, and the corresponding quantities of products leaving the Union correspond with the number and quantities entering the Union.
CHAPTER VI
TRANSITIONAL AND FINAL PROVISIONS
Article 19
Repeals
Decisions 93/342/EEC, 94/438/EC and 2006/696/EC are repealed.
References to the repealed Decisions shall be construed as references to this Regulation and shall be read in accordance with the correlation table in Annex XII.
Article 20
Transitional provisions
Commodities in respect of which the relevant veterinary certificates have been issued in accordance with Decisions 93/342/EEC, 94/438/EC and 2006/696/EC may be imported into or transit through the Community until 15 February 2009.
Article 21
Entry into force
This Regulation shall enter into force on the 20th day following its publication in the Official Journal of the European Union.
It shall apply from 1 January 2009.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
ANNEX I
POULTRY, HATCHING EGGS, DAY-OLD CHICKS, SPECIFIED PATHOGEN-FREE EGGS, MEAT, MINCED MEAT, MECHANICALLY SEPARATED MEAT, EGGS AND EGG PRODUCTS
PART 1
List of third countries, territories, zones or compartments
|
ISO code and name of third country or territory |
Code of third country, territory, zone or compartment |
Description of third country, territory, zone or compartment |
Veterinary certificate |
Specific conditions |
Specific conditions |
Avian influenza surveillance status |
Avian influenza vaccination status |
Salmonella control status |
||
|
Model(s) |
Additional guarantees |
Closing date (1) |
Opening date (2) |
|||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
6A |
6B |
7 |
8 |
9 |
|
AL – Albania |
AL-0 |
Whole country |
EP, E |
S4 |
||||||
|
AR – Argentina |
AR-0 |
Whole country |
SPF |
|||||||
|
POU, RAT, EP, E |
A |
S4 |
||||||||
|
WGM |
VIII |
|||||||||
|
AU – Australia |
AU-0 |
Whole country |
SPF |
|||||||
|
EP, E |
S4 |
|||||||||
|
BPP, DOC, HEP, SRP |
S0, ST0 |
|||||||||
|
BPR |
I |
|||||||||
|
DOR |
II |
|||||||||
|
HER |
III |
|||||||||
|
POU |
VI |
|||||||||
|
RAT |
VII |
|||||||||
|
BR – Brazil |
BR-0 |
Whole country |
SPF |
|||||||
|
BR-1 |
States of: Rio Grande do Sul, Santa Catarina, Paraná, São Paulo and Mato Grosso do Sul |
RAT, BPR, DOR, HER, SRA |
N |
A |
||||||
|
BR-2 |
States of: Mato Grosso, Paraná, Rio Grande do Sul, Santa Catarina and São Paulo |
BPP, DOC, HEP, SRP |
N |
S5, ST0 |
||||||
|
BR-3 |
Distrito Federal and States of: Goiás, Minas Gerais, Mato Grosso, Mato Grosso do Sul, Paraná, Rio Grande do Sul, Santa Catarina and São Paulo |
WGM |
VIII |
|||||||
|
EP, E, POU |
N |
S4 |
||||||||
|
BW – Botswana |
BW-0 |
Whole country |
SPF |
|||||||
|
EP, E |
S4 |
|||||||||
|
BPR |
I |
|||||||||
|
DOR |
II |
|||||||||
|
HER |
III |
|||||||||
|
RAT |
VII |
|||||||||
|
BY- Belarus |
BY-0 |
Whole country |
EP, E (both for transit only through Lithuania) |
IX |
||||||
|
CA – Canada |
CA-0 |
Whole country |
SPF |
|||||||
|
EP, E |
S4 |
|||||||||
|
BPR, BPP, DOR, HER, SRA, SRP |
N |
A |
S1, ST1 |
|||||||
|
DOC, HEP |
L, N |
|||||||||
|
WGM |
VIII |
|||||||||
|
POU, RAT |
N |
|||||||||
|
CH – Switzerland |
CH-0 |
Whole country |
A |
|||||||
|
CL – Chile |
CL-0 |
Whole country |
SPF |
|||||||
|
EP, E |
S4 |
|||||||||
|
BPR, BPP, DOC, DOR, HEP, HER, SRA, SRP |
N |
A |
S0, ST0 |
|||||||
|
WGM |
VIII |
|||||||||
|
POU, RAT |
N |
|||||||||
|
CN – China |
CN-0 |
Whole country |
EP |
|||||||
|
CN-1 |
Province of Shandong |
POU, E |
VI |
P2 |
6.2.2004 |
— |
S4 |
|||
|
GL – Greenland |
GL-0 |
Whole country |
SPF |
|||||||
|
EP, WGM |
||||||||||
|
HK – Hong Kong |
HK-0 |
The whole territory of the Hong Kong Special Administrative Region |
EP |
|||||||
|
HR – Croatia |
HR-0 |
Whole country |
SPF |
|||||||
|
BPR, BPP, DOR, DOC, HEP, HER, SRA, SRP |
N |
A |
ST0 |
|||||||
|
EP, E, POU, RAT, WGM |
N |
|||||||||
|
IL – Israel |
IL-0 |
Whole country |
SPF |
|||||||
|
EP, E |
S4 |
|||||||||
|
IL-1 |
Area of Israel excluding IL-2, IL-3 and IL-4 |
BPR, BPP, DOC, DOR, HEP, HER, SRP |
N |
A |
S5, ST1 |
|||||
|
WGM |
VIII |
|||||||||
|
POU, RAT |
N |
|||||||||
|
IL-2 |
Area of Israel inside the following boundaries: — to the west: road number 4, — to the south: road number 5812 connecting to road number 5815, — to the east: the security fence until road number 6513, — to the north: road number 6513 until the junction with road 65. From this point in a straight line to the entrance of Givat Nili and from there in a straight line to the junction of roads 652 and 4. |
BPR, BPP, DOC, DOR, HEP, HER, SRP |
N, P2 |
1.5.2010 |
A |
S5, ST1 |
||||
|
WGM |
VIII |
P2 |
1.5.2010 |
|||||||
|
POU, RAT |
N, P2 |
1.5.2010 |
||||||||
|
IL-3 |
Area of Israel inside the following boundaries: — to the north: road 386 until municipal boundaries of Jerusalem, the Refaim river, the former Israel Jordan border (‘green line’), — to the east: road 356, — to the south: roads 8670, 3517 and 354, — to the west: a straight line going north until road 367, following 367 west and then north until road 375 and west of the village of Matta a north-north east line to road 386. |
BPR, BPP, DOC, DOR, HEP, HER, SRP |
N, P2 |
14.6.2011 |
A |
S5, ST1 |
||||
|
WGM |
VIII |
P2 |
14.6.2011 |
|||||||
|
POU, RAT |
N, P2 |
14.6.2011 |
||||||||
|
IL-4 |
Area of Israel inside the following boundaries: — Junction of the Israel-Palestinian Authority (Gaza strip) border with the Israel-Egypt border. — South along the Israel-Egypt border until latitude 31° 06′ N. — East on latitude 31° 06′ N until longitude 34° 26′ E. — A straight line north until the Nassi junction (junction of routes 264 and 25). — Route 264 north until the Bet Kama junction (junction of routes 264 and 40). — East on latitude 31° 27′ N until longitude 34° 52′ E. — North on longitude 34° 52′ E until route 353. — Straight line until the meeting of route 40 and latitude 31° 40′ N. — West on latitude 31° 40′ N until the sea. — South along the Mediterranean coast until the Israel-Palestinian Authority (Gaza strip) border. — South along the Israel-Palestinian Authority (Gaza strip) border. |
BPR, BPP, DOC, DOR, HEP, HER, SRP |
N, P2 |
8.3.2012 |
22.6.2012 |
A |
S5, ST1 |
|||
|
WGM |
VIII |
P2 |
8.3.2012 |
22.6.2012 |
||||||
|
POU, RAT |
N, P2 |
8.3.2012 |
22.6.2012 |
|||||||
|
IN – India |
IN-0 |
Whole country |
EP |
|||||||
|
IS – Iceland |
IS-0 |
Whole country |
SPF |
|||||||
|
EP, E |
S4 |
|||||||||
|
KR – Republic of Korea |
KR-0 |
Whole country |
EP, E |
S4 |
||||||
|
ME – Montenegro |
ME-O |
Whole country |
EP |
|||||||
|
MG – Madagascar |
MG-0 |
Whole country |
SPF |
|||||||
|
EP, E, WGM |
S4 |
|||||||||
|
MY – Malaysia |
MY-0 |
— |
— |
|||||||
|
MY-1 |
Western Peninsular |
EP |
||||||||
|
E |
P2 |
6.2.2004 |
S4 |
|||||||
|
MK – former Yugoslav Republic of Macedonia (4) |
MK-0 (4) |
Whole country |
EP |
|||||||
|
MX – Mexico |
MX-0 |
Whole country |
SPF |
|||||||
|
EP |
||||||||||
|
NA – Namibia |
NA-0 |
Whole country |
SPF |
|||||||
|
BPR |
I |
|||||||||
|
DOR |
II |
|||||||||
|
HER |
III |
|||||||||
|
RAT, EP, E |
VII |
S4 |
||||||||
|
NC – New Caledonia |
NC-0 |
Whole country |
EP |
|||||||
|
NZ – New Zealand |
NZ-0 |
Whole country |
SPF |
|||||||
|
BPR, BPP, DOC, DOR, HEP, HER, SRA, SRP |
S0, ST0 |
|||||||||
|
WGM |
VIII |
|||||||||
|
EP, E, POU, RAT |
S4 |
|||||||||
|
PM – Saint Pierre and Miquelon |
PM-0 |
Whole territory |
SPF |
|||||||
|
RS – Serbia (5) |
RS-0 (5) |
Whole country |
EP |
|||||||
|
RU – Russia |
RU-0 |
Whole country |
EP, E, POU |
S4 |
||||||
|
SG – Singapore |
SG-0 |
Whole country |
EP |
|||||||
|
TH – Thailand |
TH-0 |
Whole country |
SPF, EP |
|||||||
|
WGM |
VIII |
1.7.2012 |
||||||||
|
POU, RAT |
1.7.2012 |
|||||||||
|
E |
1.7.2012 |
S4 |
||||||||
|
TN – Tunisia |
TN-0 |
Whole country |
SPF |
|||||||
|
DOR, BPR, BPP, HER |
S0, ST0 |
|||||||||
|
WGM |
VIII |
|||||||||
|
EP, E, POU, RAT |
S4 |
|||||||||
|
TR – Turkey |
TR-0 |
Whole country |
SPF |
|||||||
|
EP, E |
S4 |
|||||||||
|
UA – Ukraine |
UA-0 |
Whole country |
E, EP, POU, RAT, WGM |
S4 |
||||||
|
US – United States |
US-0 |
Whole country |
SPF |
|||||||
|
BPR, BPP, DOC, DOR, HEP, HER, SRA, SRP |
N |
A |
S3, ST1 |
|||||||
|
WGM |
VIII |
|||||||||
|
EP, E, POU, RAT |
N |
S4 |
||||||||
|
UY – Uruguay |
UY-0 |
Whole country |
SPF |
|||||||
|
EP, E, RAT |
S4 |
|||||||||
|
ZA – South Africa |
ZA-0 |
Whole country |
SPF |
|||||||
|
EP, E |
S4 |
|||||||||
|
BPR |
I |
P2 |
9.4.2011 |
A |
||||||
|
DOR |
II |
|||||||||
|
HER |
III |
|||||||||
|
RAT |
VII |
P2 |
9.4.2011 |
|||||||
|
ZW – Zimbabwe |
ZW-0 |
Whole country |
RAT |
VII |
||||||
|
EP, E |
S4 |
|||||||||
|
(1) Commodities, including those transported on the high seas, produced before this date may be imported into the Union during a period of 90 days from this date. (2) Only commodities produced after this date may be imported into the Union. (3) In accordance with the agreement between the European Union and the Swiss Confederation on trade in agricultural products (OJ L 114, 30.4.2002, p. 132). (4) The former Yugoslav Republic of Macedonia; provisional code that does not prejudge in any way the definitive nomenclature for this country, which will be agreed following the conclusion of negotiations currently taking place on this subject in the United Nations. (5) Not including Kosovo, as defined by United Nations Security Council Resolution 1244 of 10 June 1999. (6) Only for transit in accordance with Article 4(4) and Article 5. |
||||||||||
PART 2
Model veterinary certificates
Model(s):
|
‘BPP’ |
: |
Model veterinary certificate for breeding or productive poultry other than ratites |
|
‘BPR’ |
: |
Model veterinary certificate for breeding or productive ratites |
|
‘DOC’ |
: |
Model veterinary certificate for day-old chicks FORM than of ratites |
|
‘DOR’ |
: |
Model veterinary certificate for day-old chicks of ratites |
|
‘HEP’ |
: |
Model veterinary certificate for hatching eggs of poultry other than ratites |
|
‘HER’ |
: |
Model veterinary certificate for hatching eggs of ratites |
|
‘SPF’ |
: |
Model veterinary certificate for specified pathogen-free eggs |
|
‘SRP’ |
: |
Model veterinary certificate for slaughter poultry and poultry for restocking game supplies other than ratites |
|
‘SRA’ |
: |
Model veterinary certificate for slaughter ratites |
|
‘POU’ |
: |
Model veterinary certificate for meat of poultry |
|
‘POU-MI/MSM’ |
: |
Model veterinary certificate for minced meat and mechanically separated meat of poultry |
|
‘RAT’ |
: |
Model veterinary certificate for meat of farmed ratites for human consumption |
|
‘RAT-MI/MSM’ |
: |
Model veterinary certificate for minced meat and mechanically separated meat of farmed ratites for human consumption |
|
‘WGM’ |
: |
Model veterinary certificate for wild game-bird meat |
|
‘WGM-MI/MSM’ |
: |
Model veterinary certificate for wild game-bird minced meat and mechanically separated meat |
|
‘E’ |
: |
Model veterinary certificate for eggs |
|
‘EP’ |
: |
Model veterinary certificate for egg products |
Additional guarantees (AG):
|
‘I’ |
: |
Guarantees for breeding and productive ratites coming from a third country, territory or zone not free from Newcastle disease, certified in accordance with model BPR |
|
‘II’ |
: |
Guarantees for day-old chicks of ratites coming from a third country, territory or zone not free from Newcastle disease, certified in accordance with model DOR |
|
‘III’ |
: |
Guarantees for hatching eggs of ratites coming from a third country, territory or zone not free from Newcastle disease certified in accordance with model HER |
▼M1 —————
|
‘V’ |
: |
Guarantees for slaughter ratites coming from a third country, territory or zone not free from Newcastle disease, certified in accordance with model SRA |
|
‘VI’ |
: |
additional guarantees covering poultrymeat certified in accordance with model POU |
|
‘VII’ |
: |
additional guarantees covering meat of farmed ratites for human consumption certified in accordance with model RAT |
|
‘VIII’ |
: |
additional guarantees for wild game-bird meat certified in accordance with model WGM |
|
‘IX’ |
: |
only transit through Lithuania of consignments of eggs and egg products originating in Belarus and bound for the Russian territory of Kaliningrad shall be permitted provided that Article 18(2), (3) and (4) is complied with. |
Salmonella control programme:
|
‘S0’ |
Prohibition to export into the Community breeding or productive poultry (BPP) of Gallus gallus, day-old chicks (DOC) of Gallus gallus, slaughter poultry and poultry for restocking (SRP) of Gallus gallus and hatching eggs (HEP) of Gallus gallus because a relevant Salmonella control programme in accordance with Regulation (EC) No 2160/2003 has not been submitted to the Commission or approved by it. |
|
‘S1’ |
Prohibition to export into the Community breeding or productive poultry (BPP) of Gallus gallus, day-old chicks (DOC) of Gallus gallus and slaughter poultry and poultry for restocking (SRP) of Gallus gallus for other purposes than breeding, because a relevant Salmonella control programme in accordance with Regulation (EC) No 2160/2003 has not been submitted to the Commission or approved by it. |
|
‘S2’ |
Prohibition to export into the Community breeding or productive poultry (BPP) of Gallus gallus, day-old chicks (DOC) of Gallus gallus and slaughter poultry and poultry for restocking (SRP) of Gallus gallus for other purposes than breeding or laying, because a relevant Salmonella control programme in accordance with Regulation (EC) No 2160/2003 has not been submitted to the Commission or approved by it. |
|
‘S3’ |
Prohibition to export into the Community breeding or productive poultry (BPP) of Gallus gallus and slaughter poultry and poultry for restocking (SRP) of Gallus gallus for other purposes than breeding, because a relevant Salmonella control programme in accordance with Regulation (EC) No 2160/2003 has not been submitted to the Commission or approved by it. |
|
‘S4’ |
Prohibition to export into the Community eggs (E) of Gallus gallus others than eggs classed B in accordance with Regulation (EC) No 557/2007 because a relevant Salmonella control programme in accordance with Regulation (EC) No 2160/2003 has not been submitted to the Commission or approved by it. |
|
‘S5’ |
Prohibition to export into the Union breeding and productive poultry of Gallus gallus (BPP), slaughter poultry and poultry for restocking (SRP) of Gallus gallus because a Salmonella control programme in accordance with Regulation (EC) No 2160/2003 has not been submitted to the Commission or approved by it. |
|
‘ST0’ |
Prohibition to export into the Union breeding or productive poultry (BPP) of turkeys, day-old chicks (DOC) of turkeys, slaughter poultry and poultry for restocking (SRP) of turkeys and hatching eggs (HEP) of turkeys because a relevant Salmonella control programme in accordance with Regulation (EC) No 2160/2003 has not been submitted to the Commission or approved by it. |
|
‘ST1’ |
Prohibition to export into the Union breeding or productive poultry (BPP) of turkeys and slaughter poultry and poultry for restocking (SRP) of turkeys because a relevant Salmonella control programme in accordance with Regulation (EC) No 2160/2003 has not been submitted to the Commission or approved by it. |
Specific conditions:
|
‘P2’ |
: |
Prohibition to import into or transit through the Community due to restrictions related to a HPAI outbreak |
|
‘P3’ |
: |
Prohibition to import into or transit through the Community due to restrictions related to a ND outbreak |
|
‘N’ |
: |
Guarantees have been provided that the legislation on the control of Newcastle disease in the third country or territory is equivalent to that applied in the Union. In the case of an outbreak of Newcastle disease, imports may continue to be authorised from the third country or territory with no change in the third country code or territory code. However, imports into the Union from any areas which are placed under official restrictions by the competent authority of the third country or territory concerned due to an outbreak of that disease shall be automatically prohibited |
|
‘L’ |
: |
Guarantees have been provided that the legislation on the control of avian influenza in the third country or territory is equivalent to that applied in the Union. In the case of an outbreak of low pathogenic avian influenza, imports may continue to be authorised from the third country or territory with no change in the third country code or territory code. However, imports into the Union from any areas which are placed under official restrictions by the competent authority of the third country or territory concerned due to an outbreak of that disease shall be automatically prohibited |
Avian influenza surveillance programme and avian influenza vaccination plan:
|
‘A’ |
: |
Third country, territory, zone or compartment carries out an avian influenza surveillance programme in accordance with Regulation (EC) No 798/2008 |
|
‘B’ |
: |
Third country, territory, zone or compartment carries out vaccination against avian influenza in accordance with Regulation (EC) No 798/2008 |
Notes
General notes:
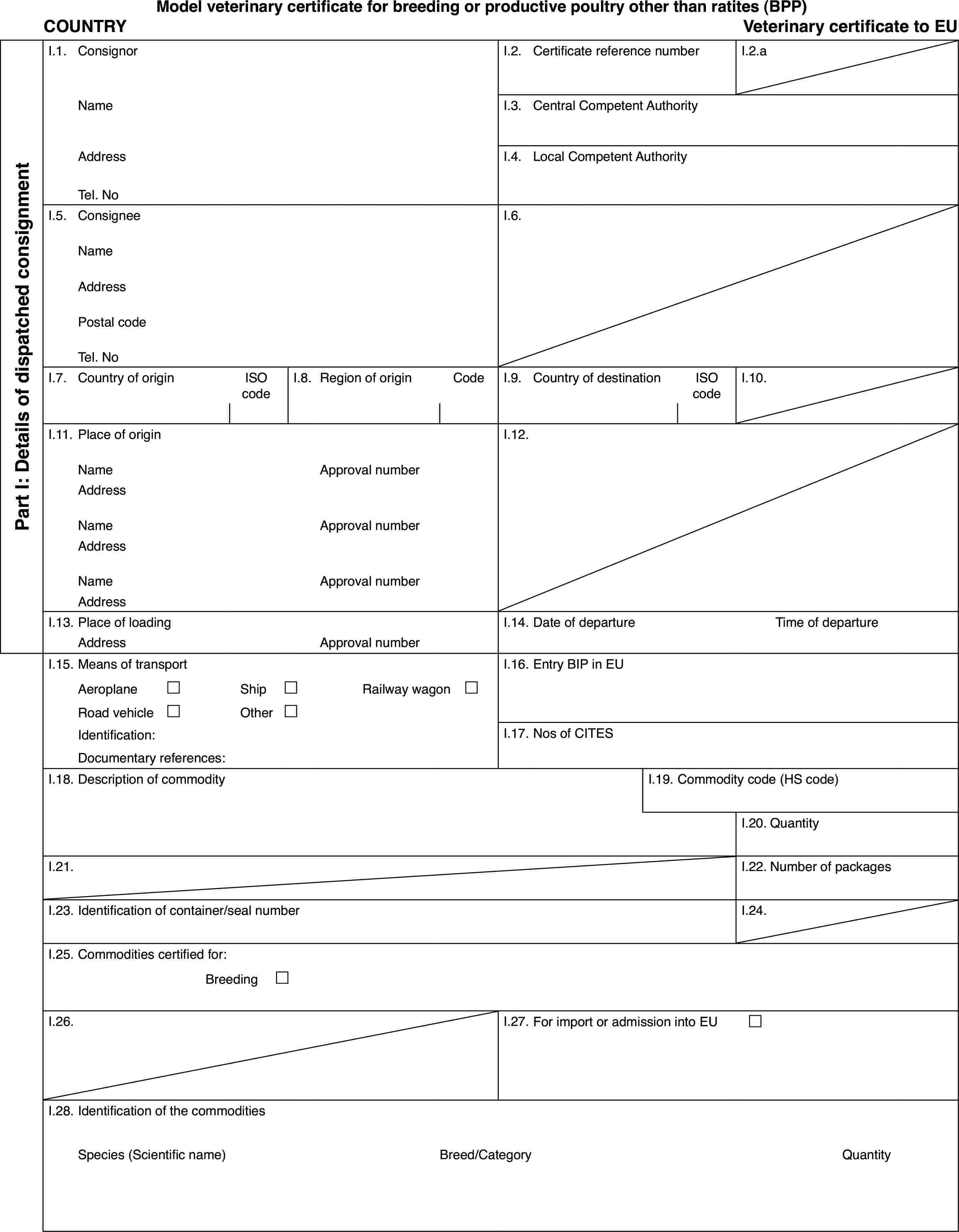
(a) Veterinary certificates based on the models in Part 2 of this Annex and following the layout of the model that corresponds to the commodity concerned shall be issued by the exporting country, territory, zone or compartment. They shall contain, in the order appearing in the model, the attestations that are required for any third country and, where applicable, those additional health requirements required for the exporting country, territory, zone or compartment.
Where additional guarantees are required by the EU Member State of destination for the commodity concerned, these shall also be entered on the original of the veterinary certificate.
(b) A separate, single certificate must be presented for each consignment of the commodity concerned, exported to the same destination from a territory appearing in columns 2 and 3 of Part 1 of this Annex and transported in the same railway wagon, lorry, aircraft or ship.
(c) The original of certificates shall consist of a single page printed on both sides or, where more text is required, such that all the pages form a whole and cannot be separated.
(d) The certificate shall be drawn up in at least one official language of the EU Member State where the border inspection takes place and in one official language of the EU Member State of destination. However, those Member States may allow another Community language instead of their own, accompanied, if necessary, by an official translation.
(e) Where additional pages are attached to the certificate for the purposes of identifying the items making up the consignment, such additional pages shall also be considered to form part of the original of the certificate, provided the signature and stamp of the certifying official veterinarian appear on each page.
(f) Where the certificate, including any additional pages as provided for in (e), comprises more than one page, each page shall be numbered ‘–x(page number) of y(total number of pages)–’ on the bottom and shall bear the code number of the certificate allocated by the competent authority on the top.
(g) The original of the certificate must be completed and signed by an official veterinarian not more than 24 hours prior to loading of the consignment for imports to the Community, unless otherwise stated. To that end, the competent authorities of the exporting country shall ensure that principles of certification equivalent to those laid down in Directive 96/93/EC are followed.
The colour of the signature shall be different from that of the printing. The same rule shall apply to stamps other than embossed stamps or watermarks.
(h) The original of the certificate must accompany the consignment as far as the EU border inspection post.
Additional notes for poultry and day-old chicks:
(i) The certificate shall be valid for 10 days from the date of issue, unless otherwise stated.
In the case of transport by ship, the term of validity shall be extended by the time taken by the voyage. To that end, the original of a declaration by the ship's master, drawn up in accordance with Annex II, shall be attached to the veterinary certificate.
(j) Poultry and day-old chicks shall not be transported with other poultry and day-old chicks that are either not intended for the European Community or of a lower health status.
(k) Poultry and day-old chicks shall not in the course of transport to the Community be moved through nor unloaded in a third country, territory, zone or compartment from which imports of such poultry and day-old chicks into the Community are not authorised.
(4) or[compartment(s) …;]for at least threemonths or since hatching where it is less than three months old; where itwas imported into the country, territory, zone or compartment of origin, thistook place in accordance with veterinary conditions at least as strict asthe relevant requirements of Directive 2009/158/EC and any subsidiary Decisions;II.1.3come from:(2) (3) (12) either[theterritory of code …;](3) (4) or[compartment(s) …;]a)which, at the date of issue of this certificate, was (were) free fromNewcastle disease as defined in Regulation (EC) No 798/2008;b)where a surveillance programme for avian influenza according to Regulation(EC) No 798/2008 is carried out;II.1.4come from:(2)(3) either[theterritory of code. …;](3) (4) or[compartment(s) …;](3) either[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic and low pathogenic avian influenza as defined in Regulation(EC) No 798/2008;](3) or[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic avian influenza as defined in Regulation (EC) No 798/2008,and(3) either[(a)the poultry comefrom an establishment in which within the 21 days prior to import to the Unionavian influenza surveillance has been carried out with negative results;](3) or[(a)during the past21 days prior to import to the Union the poultry have been kept separatelyfrom other birds and a virus detection test with negative testing resultsfor avian influenza has been carried out on a random sample of cloacal andtracheal/or oropharyngeal swabs taken from at least 60 poultry in the consignmentor from all poultry if less than 60 are present in the consignment;](b)the poultry come from an establishment:—around which within a 1 km radius low pathogenic avian influenza hasnot been present within the last 30 days on any establishment;—where there has been no epidemiological link to an establishment whereavian influenza has been detected within the last 30 days;]II.1.5come from a flock where vaccination against avian influenza has notbeen carried out;II.1.6come from establishment(s)defined in Box I.11 of Part I officially approved in accordance with requirementswhich are at least equivalent to those laid down in Annex II to Directive2009/158/EC, where it has been kept since hatching or for at least six weeksimmediately prior to export, and(a)the approval of which has not been suspended or withdrawn;(b)which, at the time of consignment, was (were) not subject to any animalhealth restriction;(c)within a 10 km radiusof which, including, where appropriate, the territory of a neighbouring country,there has been no outbreak of highly pathogenic avian influenza or Newcastledisease for at least the previous 30 days;II.1.7comes from a flock which:(a)has been examined no more than 24 hours before loading and showed noclinical signs of or grounds for suspecting any disease;](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01001001.tif.jpg)
 or[Salmonella arizonae (serogroup O:18(K)), S. Pullorum and S. Gallinarum, Mycoplasma meleagridis and M. gallisepticum (turkeys);](3) or[Salmonella Pullorum and S. Gallinarum (guinea fowl, quails,pheasants, partridges and ducks);]in accordance with Chapter III ofAnnex II to Directive 2009/158/EC and was not found to be infected, nor showedany grounds for suspecting any infection, by these agents;(3) either[(c)has not been vaccinated against Newcastle disease;](3) or[(c)has been vaccinated against Newcastle disease using:(name and type (live or inactivated)of Newcastle disease virus strain used in vaccine(s))at the age of … weeks;](5) and/or[(d)has been vaccinated using officially approved vaccineson… against …(repeat as necessary);]II.1.8have been examinedat the date of issue of this certificate and showed no clinical signs of orgrounds for suspecting any disease;II.1.9during the periodmentioned in II.1.6, have had no contact with poultry not complying with therequirements laid down in this certificate or with wild birds.II.2Public health additional guarantees(6)[II.2.1The Salmonella control programme referred to in Article 10 of Regulation(EC) No 2160/2003 and the specific requirements for the use of antimicrobialsand vaccines in Regulation (EC) No 1177/2006, have been applied to the flockof origin and the flock has been tested for Salmonella serotypes of public health significance.Date of last samplingof the flock from which the testing result is known: … (dd/mm/yyyy);Result of all testingin the flock:(3) (7) either[positive;](3) (7) oder[negative;]Forreasons other than the Salmonella control programme, within the last three weeks prior to import:(3) either[antimicrobialswere not administered to the breeding and productive poultry other than ratites;](3) (8) or[the following antimicrobials were administeredto the breeding and productive poultry other than ratites: …;]](6)[II.2.2If breeding poultry, neither Salmonella Enteritidis nor Salmonella Typhimurium were detected within the control programme referredto in point II.2.1.]II.3Animal health additionalguaranteesI, the undersigned official veterinarian,further certify that:(9) [II.3.1where the consignment is intended for a MemberState the status of which has been established pursuant to Article 15(2) ofDirective 2009/158/EC, the poultry described in this certificate:(a)have not been vaccinated against Newcastle disease;(b)were kept in isolation for 14 days before consignment in an establishmentunder the supervision of an official veterinarian. In this connection, nopoultry at the establishment of origin or quarantine station, as applicable,were vaccinated against Newcastle disease during the 21 days preceding consignmentand no bird which was not intended for consignment entered during that time;(c)underwent a serological examination for the presence of Newcastle diseaseantibodies in the 14 days preceding consignment and tested negative;]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01001101.tif.jpg)
[II.3.3if the Member State of destination is Finland or Sweden:(3) either[the breeding poultry has tested negative in accordancewith the rules laid down in Decision 2003/644/EC;](3) or[the laying hens (productive poultry reared witha view to producing eggs for consumption) have tested negative in accordancewith the rules laid down in Decision 2004/235/EC.]]II.4Additional health requirements(10) [I, the undersigned officialveterinarian, further certify that:although the useof vaccines against Newcastle disease which do not fulfil the specific requirementsof Annex VI (II) to Regulation (EC) No 798/2008 is not prohibited in:(2)(3) either[the territory ofcode…;](3) (4) or[compartment(s)…;]the poultry describedin this certificate:(a)have not been vaccinatedfor at least the previous 12 months with such vaccines;(b)comes from a flock or flocks which underwent a virus isolation testfor Newcastle disease, carried out in an official laboratory not earlier than14 days preceding consignment on a random sample of cloacal swabs from atleast 60 birds in each flock and in which no avian paramyxoviruses with anIntracerebral Pathogenicity Index (ICPI) of more than 0,4 were found;(c)in the 60 days before consignment was not in contact with poultry whichdoes not fulfil the conditions in (a) and (b);(d)were kept in isolation under official surveillance on the establishmentof origin during the 14 days mentioned in (b).](11)II.5Animal transport AttestationI, the undersignedofficial veterinarian, further certify that poultry is transported in cratesor cages which:(a)contain only poultryof the same species, category and type coming from the same establishment;(b)bear the approval number of the establishment of origin;(c)are closed in accordance with the instructions of the competent authorityto avoid any possibility of substitution of the contents;(d)in addition to the vehicles in which they are transported, are designedto:(i)prevent any excrement escapingand reduce to a minimum any loss of feathers during transport;(ii)allow visual inspection ofthe poultry;(iii)allow cleansing and disinfection;(e)have been cleansed and disinfected, as have the vehicles in which theyare transported, before loading in accordance with the instructions of thecompetent authority.NotesPart I:—Box I.8: Provide the code for the zone or the compartment of origin,if necessary, as defined under code of column 2 of Part 1 of Annex I to Regulation(EC) No 798/2008.—Box I.11: Name,address and approval number of breeding and rearing establishment.—Box I.15: Indicate the registration number(s) of railway wagons andlorries, the names of ships and, if known, the flight numbers of aircraft.In the case of transport in containers or boxes, the total number of theseand their registration and where there is a serial number of the seal it hasto be indicated in box I.23.—Box I.19: Use theappropriate Harmonised System (HS) code of the World Customs Organisation:01.05 or 01.06.39.—Box I.28 (Category):Select one of the following: Pure line/grandparents/parents/laying pullets/others.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01001201.tif.jpg)


 (4) or[compartment(s) …;]for at least threemonths or since hatching where it is less than three months old; where itwas imported into the country, territory, zone or compartment of origin, thistook place in accordance with veterinary conditions at least as strict asthe relevant requirements of Directive 2009/158/EC and any subsidiary Decisions;II.1.3come from:(2) (3) (9) either[the territory ofcode …;](3) (4) or[compartment(s) …;](3) either[(a)which was (were)free from Newcastle disease as defined in Regulation (EC) No 798/2008;](3)(5) or[(a)which was not free from Newcastle disease as defined in Regulation(EC) No 798/2008;](b)where a surveillanceprogramme for avian influenza according to Regulation (EC) No 798/2008 iscarried out;II.1.4come from:(2)(3) either[the territory ofcode …](3) (4) or[compartment(s) …;](3) either[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic and low pathogenic avian influenza as defined in Regulation(EC) No 798/2008;](3) or[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic avian influenza as defined in Regulation (EC) No 798/2008,and(3) either[(a)the ratites comefrom an establishment in which within the 21 days prior to import to the Unionavian influenza surveillance has been carried out with negative results;](3) or[(a)during the past21 days prior to import to the Union ratites have been kept separately fromother birds and a virus detection test with negative testing results for avianinfluenza has been carried out on a random sample of cloacal and tracheal/ororopharyngeal swabs taken from at least 60 ratites in the consignment or fromall ratites if less than 60 are present in the consignment;](b)the ratites come from an establishment:—around which within a 1 km radius low pathogenic avian influenza hasnot been present on any establishment;—where there has been no epidemiological link to an establishment whereavian influenza has been detected within the last 30 days;]II.1.5come from a flock where vaccination against avian influenza has notbeen carried out;II.1.6come from establishment(s)defined in Box I.11 of Part I officially approved in accordance with requirementswhich are at least equivalent to those laid down in Annex II to Directive2009/158/EC, where they have been kept since hatching or for at least sixweeks immediately prior to export, and(i)the approval of which has not been suspended or withdrawn;(ii)which is (are) not subject to any animal health restriction;(iii)within a 10 km radius of which, including, where appropriate, the territoryof a neighbouring country, there has been no outbreak of highly pathogenicavian influenza or Newcastle disease for at least the previous 30 days;](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01001501.tif.jpg)
 oder[(b)has been vaccinatedagainst Newcastle disease using:(nameand type (live or inactivated) of Newcastle disease virus strain used invaccine(s))at the age of …weeks;](6)and/or[(c)has been vaccinatedusing officially approved vaccines on…against … (repeat as necessary);](6) [II.1.8where they come from countries in Asia or Africa:(3)either[were kept in isolation in tick-proofed surroundingsunder an officially approved programme for rodent control for at least 21days prior import to the Union;](3)or[underwent treatment to ensure that all ticks onthem were destroyed before they were moved to the tick-proofed surroundings;specification of the treatment: …;](3)or[after spending 14 days in tick-proofed surroundings,underwent the competitive ELISA test for antibodies to Crimean-Congo haemorrhagicfever and all ratites leaving isolation tested negative;]]II.1.9have been examined at the date of issue of this certificate and showedno clinical signs of or grounds for suspecting any disease;II.1.10during the period mentioned in II.1.6 have had no contact with ratitesnot complying with the requirements laid down in this certificate or withother birds.II.2Additional guaranteesI, the undersignedofficial veterinarian, further certify that:(7) [II.2.1where the consignment is intended for a MemberState the status of which has been established pursuant to Article 15(2) ofDirective 2009/158/EC, the ratites described in this certificate:(a)have not been vaccinated against Newcastle disease;(b)were kept in isolation for 14 days before consignment at an establishmentunder the supervision of an official veterinarian. In this connection no ratites andother poultry at the establishment were vaccinated against Newcastle diseaseduring the 21 days preceding consignment and no bird which was not intendedfor consignment entered during that time;(c)underwent a serological examination for the presence of Newcastle diseaseantibodies in the 14 days preceding consignment and tested negative;](6) [II.2.1the following additional guarantees laid down bythe Member State of destination in accordance with Articles 16 and/or 17of Directive 2009/158/EC are provided:;](7) [II.2.2if the Member State of destination is Finland orSweden:(3)either[the breeding ratites have tested negative in accordancewith the rules laid down in Decision 2003/644/EC;](3)or[the laying hens (productive ratites reared witha view to producing eggs for consumption) have tested negative in accordancewith the rules laid down in Decision 2004/235/EC.]]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01001601.tif.jpg)
 II.4Animal transportAttestationI, the undersigned official veterinarian, further certify that theratites are transported in crates or cages which:(a)contain only ratitesof the same species, category and type coming from the same establishment;(b)bear the approvalnumber of the establishment of origin;(c)are closed in accordancewith the instructions of the competent authority to avoid any possibilityof substitution of the contents;(d)in addition to the vehicles in whichthey are transported, are designed to:(i)prevent any excrement escaping and reduce to a minimum any loss offeathers during transport;(ii)allow visual inspectionof the ratites;(iii)allow cleansing and disinfection;(e)have been cleansedand disinfected, as have the vehicles in which they are transported, beforeloading in accordance with the instructions of the competent authority.NotesPart I:—Box I.8: Provide the code for the zone or the compartment of origin,if necessary, as defined under code of column 2 of Part 1 of Annex I to Regulation(EC) No 798/2008.—Box I.11: Name,address and approval number of breeding and rearing establishment.—Box I.15: Indicate the registration number(s) of railway wagons andlorries, the names of ships and, if known, the flight numbers of aircraft.In the case of transport in containers or boxes, the total number of theseand their registration and where there is a serial number of the seal it hasto be indicated in box I.23.—Box I.28 (Category):Select one of the following: Pure line/grandparents/parents/others; (IdentificationSystem and Identification Number): Neck-tags and microchips must include theISO code of the country of origin; microchips must comply with ISO standards.Part II:(1)“Ratites” means birds of the order Struthioniformes (Casuariidae, Rheidae,Struthionidae) reared or kept in captivity for breeding and production.(2)Code of the territory as it appears in column 2 of Part 1 of AnnexI to Regulation (EC) No 798/2008.(3)Keep as appropriate.(4)Insert the name of compartment(s).(5)This is applicable only to the countries withthe entry “I” in column 5 of Part 1 of AnnexI to Regulation (EC) No 798/2008. However, thisdoes not apply to breeding and productive ratites coming from compartments.(6)Keep if appropriate.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01001701.tif.jpg)

Model veterinary certificate for day-old chicks FORM than of ratites
(DOC)
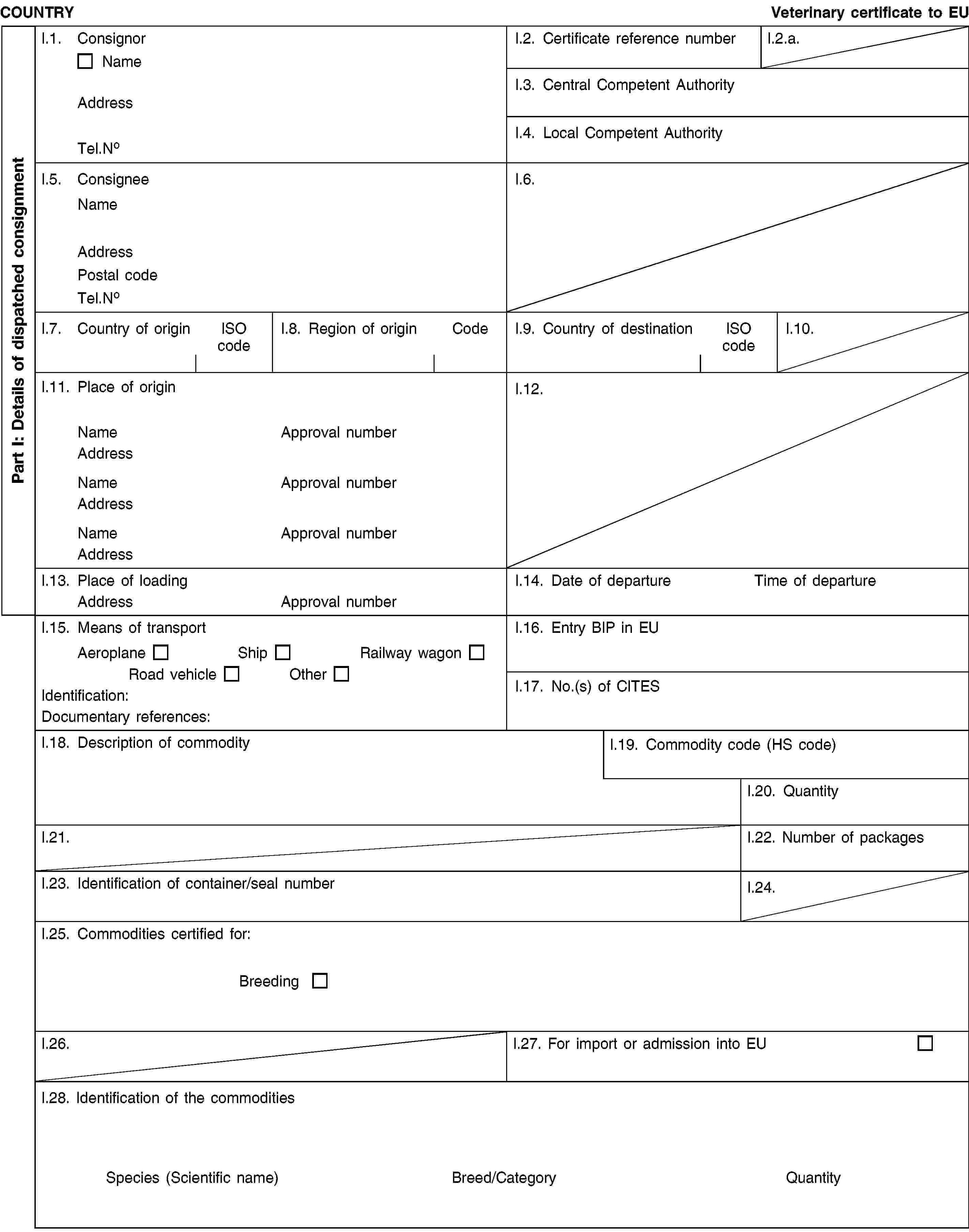
(4) or[compartment(s) …;]where the flocks from which the hatching eggs come were imported into the country, territory, zone or compartment of origin, this took place in accordance with veterinary conditions at least as strict as the relevant requirements of Directive 2009/158/EC and any subsidiary Decisions;II.1.3come from:(2)(3)(12) either[the territory of code …;](3)(4) or[compartment(s) …;](a) which, at the date of issue of this certificate, was (were) free from Newcastle disease as defined in Regulation (EC) No 798/2008;(b) where a surveillance programme for avian influenza according to Regulation (EC) No 798/2008 is carried out;II.1.4come from:(2)(3)(13) either[the territory of code …;](3)(4) or[compartment(s) …;](3) either[II.1.4.1which, at the date of issue of this certificate was (were) free from highly pathogenic and low pathogenic avian influenza as defined in Regulation (EC) No 798/2008;](3) or[II.1.4.1which, at the date of issue of this certificate was (were) free from highly pathogenic avian influenza as defined in Regulation (EC) No 798/2008, and(3) either[(a) were derived from parent flocks which have been kept in an establishment in which avian influenza surveillance has been carried out with negative results within 21 days prior to the time of collection of eggs from which the day-old chicks were hatched;](3) or[(a) were derived from parent flocks which have been kept in an establishment in which during the past 21 days prior to the collection of the eggs from which the day-old chicks were hatched a virus detection test with negative testing results for avian influenza has been carried out on a random sample of cloacal and tracheal/or oropharyngeal swabs taken from at least 60 poultry in the establishment or from all poultry if less than 60 are present in the establishment;](b) the day-old chicks come from an establishment:around which within a 1 km radius low pathogenic avian influenza has not been present within the last 30 days on any establishment, where there has been no epidemiological link to an establishment where avian influenza has been detected within the last 30 days;],](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010077EN.01001101.tif.jpg)
 or[have been vaccinated against avian influenza in accordance with a vaccination plan under Regulation (EC) No 798/2008 using:(name and type of used vaccine(s))at the age of … weeks;]II.1.6have been hatched in the establishment(s) defined in Box I.11 of Part I officially approved in accordance with requirements which are at least equivalent to those laid down in Annex II to Directive 2009/158/EC, and(a) the approval of which has not been suspended or withdrawn;(b) which, at the time of consignment, was (were) not subject to any animal health restriction;(c) within a 10 km radius of which, including, where appropriate, the territory of a neighbouring country, there has been no outbreak of highly pathogenic avian influenza or Newcastle disease for at least the previous 30 days;II.1.7have been hatched from eggs coming from flocks which:(a) have been kept for at least six weeks immediately prior to import to the Union in officially approved establishments, the approval of which, at the time of consignment of the hatching eggs to the hatchery, had not been suspended or withdrawn;(b) at the time of consignment, were not subject to any animal health restriction;(c) have undergone a disease surveillance programme for:(3) either[Salmonella Pullorum, S. Gallinarum and Mycoplasma gallisepticum (fowls);](3) or[Salmonella arizonae (serogroup O:18(K)), S. Pullorum and S. Gallinarum, Mycoplasma meleagridis and M. gallisepticum (turkeys);](3) or[Salmonella Pullorum and S. Gallinarum (guinea fowls, quails, pheasants, partridges and ducks);]in accordance with Chapter III of Annex II to Directive 2009/158/EC and have not been found to be infected, or showed any grounds for suspecting infection, by these agents;(3) either[(d) have not been vaccinated against Newcastle disease;](3) or[(d) have been vaccinated against Newcastle disease using:(name and type (live or inactivated) of Newcastle disease virus strain used in vaccine(s)) at the age of … weeks;](5) and/or[(e) have been vaccinated using officially approved vaccineson … against … (repeat as necessary);]II.1.8have been hatched from eggs which:(a) prior to consignment to the hatchery, had been marked in accordance with the instructions of the competent authority;(b) had been disinfected in accordance with the instructions of the competent authority;(5) [II.1.9have been vaccinated using officially approved vaccines on … against … (repeat as necessary);]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010077EN.01001201.tif.jpg)
(7) or[negative;]The specific requirements for the use of antimicrobials and vaccines in Regulation (EC) No 1177/2006, have been applied to the day-old chicks.For reasons other than the Salmonella control programme:(3) either[antimicrobials were not administered to the day-old chicks (including in-ovo injection);](3)(8) or[the following antimicrobials were administered to the day-old chicks (including in-ovo injection) …;]](6) [II.2.2If the day-old chicks are intended for breeding, neither Salmonella Enteritidis nor Salmonella Typhimurium were detected within the control programme referred to in point II.2.1.]II.3.Animal health additional guaranteesI, the undersigned official veterinarian, further certify that:(9) [II.3.1where the consignment is intended for a Member State the status of which has been established pursuant to Article 15(2) of Directive 2009/158/EC, the day-old chicks described in this certificate come from hatching eggs coming from flocks which:(3) either[have not been vaccinated against Newcastle disease;](3) or[have been vaccinated against Newcastle disease using an inactivated vaccine;](3) or[have been vaccinated against Newcastle disease using a live vaccine at the latest 60 days before the date the eggs were collected;]](5) [II.3.2the following additional guarantees, laid down by the Member State of destination under Articles 16 and/or 17 of Directive 2009/158/EC, are provided:…;](9) [II.3.3if the Member State of destination is Finland or Sweden, the day-old chicks for introduction into flocks of breeding poultry or flocks of productive poultry come from flocks which have tested negative in accordance with the rules laid down in Decision 2003/644/EC.]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010077EN.01001301.tif.jpg)
(4) or[compartment(s) …;]the breeding poultry from which the day-old chicks are derived:(a) have not been vaccinated for at least the previous 12 months with such vaccines;(b) come from a flock or flocks which underwent a virus isolation test for Newcastle disease, carried out in an official laboratory not earlier than 14 days preceding consignment on a random sample of cloacal swabs from at least 60 birds in each flock and in which no avian paramyxoviruses with an Intracerebral Pathogenicity Index (ICPI) of more than 0,4 were found;(c) have not been in contact during the last 60 days before consignment with poultry which does not fulfil the conditions in (a) and (b);(d) have been kept in isolation under official surveillance on the establishment of origin in the 14-day period mentioned in (b);](10) [II.4.2the hatching eggs from which the day-old chicks have been hatched have not been in contact in the hatchery or during transport with eggs or poultry which do not fulfil the abovementioned requirements.](11) II.5.Animal transport attestationI, the undersigned official veterinarian, further certify that:II.5.1the day-old chicks described in this certificate are transported in perfectly clean, disposable boxes used for the first time and:(a)contain only day-old chicks of the same species, category and type coming from the same establishment;(b)bear the following information:the name of the country, territory, zone or compartment of consignment, the species of poultry concerned, the number of chicks, the category and type of production for which they are intended, the name, address and approval number of the production establishment, the approval number of the establishment of origin, the Member State of destination;(c)are closed in accordance with the instructions of the competent authority to avoid any possibility of substitution of the contents.The containers and vehicles in which the boxes mentioned above have been transported have been cleansed and disinfected before loading in accordance with the instructions of the competent authority.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010077EN.01001401.tif.jpg)



 (4) or[compartment(s) …;]where the flocksfrom which the hatching eggs come were imported into the country, territory,zone or compartment of origin, this took place in accordance with veterinaryconditions at least as strict as the relevant requirements of Directive 2009/158/ECand any subsidiary Decisions;II.1.3come from:(2)(3) (9) either[the territory of code …;](3)(4) or[compartment(s)…;](3) either[(a)which at the date of issue of this certificate was (were) free fromNewcastle disease as defined in Regulation (EC) No 798/2008;](3)(5) or[(a)which at the date of issue of this certificate was not free from Newcastledisease as defined in Regulation (EC) No 798/2008;](b)where a surveillance programme for avian influenza according to Regulation(EC) No 798/2008 is carried out;II.1.4come from:(2)(3) either[the territory ofcode …;](3) (4) or[compartment(s) …;](3) either[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic and low pathogenic avian influenza as defined in Regulation(EC) No 798/2008;](3) or[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic avian influenza as defined in Regulation (EC) No 798/2008,and(3) either[(a)were derived fromparent flocks which have been kept in an establishment in which avian influenzasurveillance has been carried out with negative results within 21 days priorto the time of collection of eggs from which the day-old chicks were hatched;](3) or[a)were derived fromparent flocks which have been kept in an establishment in which during thepast 21 days prior to the collection of the eggs from which the day-old chickswere hatched a virus detection test with negative testing results for avianinfluenza has been carried out on a random sample of cloacal and tracheal/ororopharyngeal swabs taken from at least 60 birds in the establishment or fromall birds if less than 60 are present in the establishment;](b)the day-old chicks come from an establishment:—around which within a 1 km radius low pathogenic avian influenza hasnot been present within the last 30 days on any establishment;—where there has been no epidemiological link to an establishment whereavian influenza has been detected within the last 30 days;]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01002601.tif.jpg)
 or[have been vaccinatedagainst avian influenza in accordance with a vaccination plan under Regulation(EC) No 798/2008 using:(name and type of used vaccine(s))at the age of … weeks;]II.1.6have been hatchedin the establishment(s) defined in Box I.11 of Part I, officially approvedin accordance with requirements which are at least equivalent to those laiddown in Annex II to Directive 2009/158/EC:(a)the approval of which has not been suspended or withdrawn;(b)which is (are), at the time of consignment, not subject to any animalhealth restriction;(c)withina 10 km radius of which, including, where appropriate, the territory of aneighbouring country, there has been no outbreak of highly pathogenic avian influenza or Newcastle disease forat least the previous 30 days;II.1.7have been hatched from eggs from flocks which:(a)have been kept for at least the previous six weeks in officially approvedestablishments, the approval of which, at the time of consignment of the hatchingeggs to the hatchery, had not been suspended or withdrawn;(3) either[(b)have been kept inestablishments located in a country, territory, zone or compartment whichis free from Newcastle disease;](3) (5) or[(b)have been kept in establishments located in a country, territory orzone which is not free from Newcastle disease;](c)at the time of consignment, was (were) not subject to any animal healthrestriction;(3) either[(d)have not been vaccinated against Newcastle disease;](3) or[(d)have been vaccinatedagainst Newcastle disease using(name and type (liveor inactivated) of Newcastle disease virus strain used in vaccine(s))at the age of …weeks;](7) and/or[(e)have been vaccinatedusing officially approved vaccineson …against … (repeat as necessary);]II.1.8have been hatched from eggs which:(a)prior to the consignment to the hatchery had been marked in accordancewith the instructions of the competent authority;(b)were disinfected in accordance with the instructions of the competentauthority;II.1.9hatched on …(dd/mm/yyyy);(7) [II.1.10have been vaccinatedusing officially approved vaccines on … against …(repeat as necessary);]II.1.11at the time of consignmentwere examined and showed no clinical signs of or grounds for suspecting anydisease;II.1.12have had no contact with ratites or other poultry not meeting the requirementslaid down in this certificate.II.2Additional guaranteesI, the undersignedofficial veterinarian, further certify that:(6) [II.2.1where the consignment is intended for a MemberState the status of which has been established in accordance with Article15(2) of Directive 2009/158/EC, the day-old chicks described in this certificatecome from:(a)hatchingeggs from flocks which:(3) either[have not been vaccinated against Newcastle disease;](3) or[have been vaccinated against Newcastle disease using an inactivated vaccine;]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01002701.tif.jpg)
a hatchery where working practices ensure that such eggs are incubatedat completely separate times and locations from eggs not satisfying the requirementsin (a);](7) [II.2.2the following additional guarantees, laid downby the Member State of destination pursuant to Articles 16 and/or 17 of Directive2009/158/EC are provided:(6) [II.2.3if the Member Stateof destination is Finland or Sweden, the day-old chicks for introduction intoflocks of breeding ratites or flocks of productive ratites come from flockswhich have tested negative in accordance with the rules laid down in Decision2003/644/EC.]II.3Additional health requirements for countries notfree from Newcastle diseaseI, the undersigned official veterinarian, further certify that:(5) [II.3.1the breeding ratitesfrom which the day-old chicks are derived:(a)were placed in isolationunder official surveillance not less than 30 days before laying the hatchingeggs from which the day-old chicks for import to the Union are derived;(b)underwent a virusisolation test for Newcastle disease, carried out in an official laboratoryseven to ten days after their entry into isolation on either cloacal swabsor faeces samples from each bird and in which no avian paramyxovirus type1 isolates with an Intracerebral Pathogenicity Index (ICPI) of more than 0,4were found. Favourable results were available for all tests carried out beforethe day-old chicks left the hatchery for import to the Union;(c)during the last30 days prior to and during laying of the hatching eggs from which the day-oldchicks for import to the Union are derived, have not been in contact withpoultry (including ratites) which do not fulfil the guarantees mentioned under(a), (b) and (d);(d)come from flocks in which surveillance for Newcastle disease has beencarried out under a statistically based sampling plan which produced negativeresults for at least six months immediately prior to import to the Union;](5) [II.3.2the hatching eggsfrom which the day-old chicks have been hatched and the day-old chicks havenot been in contact in the hatchery or during transport with eggs or poultryincluding ratites which do not fulfil the abovementioned requirements.](8) II.4Animal transportAttestationI, the undersigned official veterinarian, further certify that theday-old chicks are transported in perfectly clean, disposable boxes used forthe first time which:(a)contain only day-old chicks of the same species, category and typecoming from the same establishment;(b)bear the following information inlegible writing and in at least one Union language:—the name of the country, territory, zone or compartment of consignment,—the species of ratites concerned,—the number of chicks,—the category and type of production for which they are intended,—the name, address and approval number of the breeding establishment,—the name, address and approval number of the establishment of origin,—the date of dispatch,—the Member State of destination;(c)are closed in accordancewith the instructions of the competent authority to avoid any possibilityof substitution of the contents.The containers and vehicles in which the boxes mentioned above havebeen transported have been cleansed and disinfected before loading in accordancewith the instructions of the competent authority.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01002801.tif.jpg)

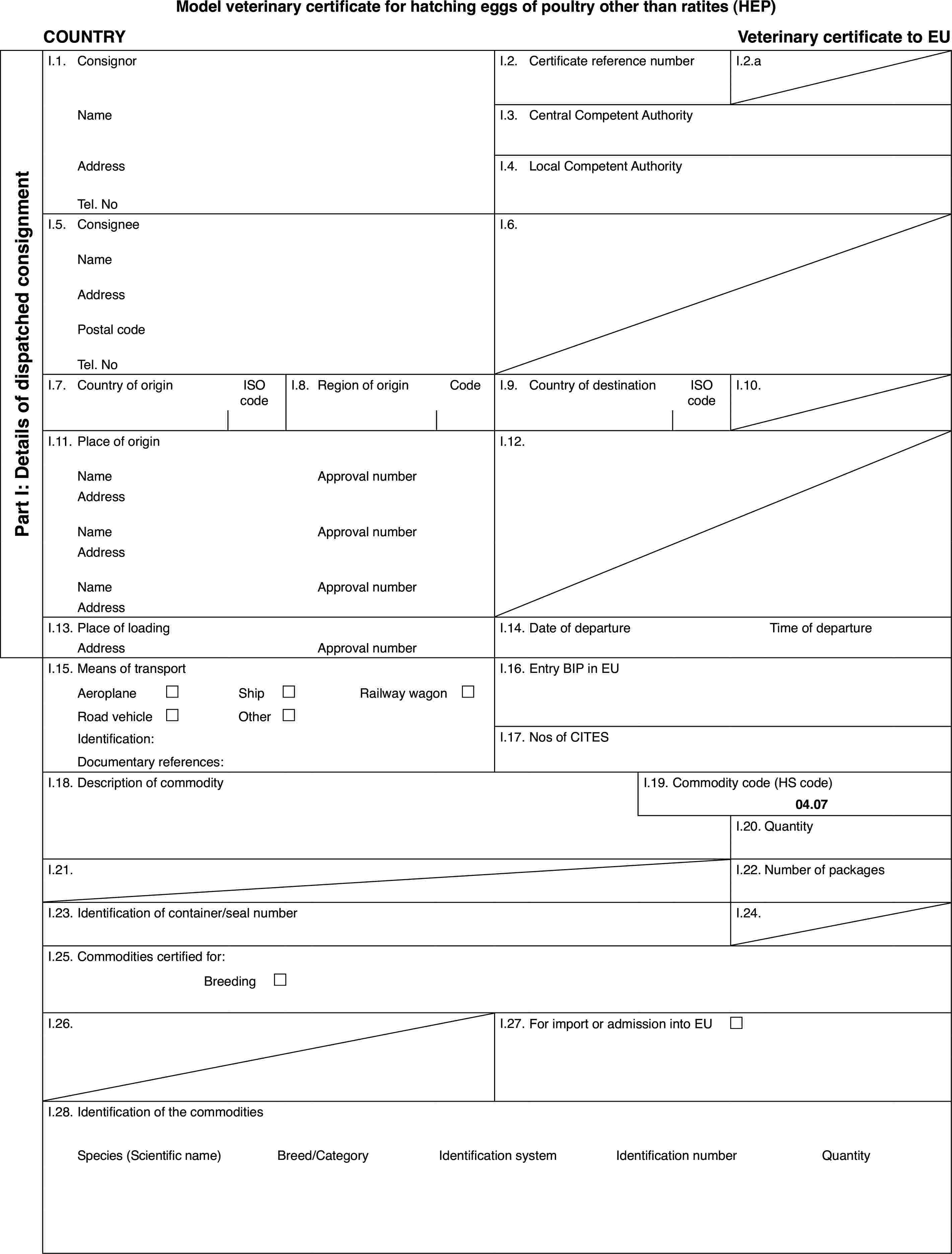
 (4) or[compartment(s) …;]for at least threemonths. Where the flocks from which the hatching eggs come were imported intothe country, territory, zone or compartment of origin, this took place inaccordance with veterinary conditions at least as strict as the relevant requirementsof Directive 2009/158/EC and any subsidiary Decisions;II.1.3come from:(2) (3) (10) either[the territory ofcode …;](3) (4) or[compartment(s) …;](a)which, at the date of issue of this certificate, was (were) free fromNewcastle disease as defined in Regulation(EC) No 798/2008;(b)where a surveillance programme for avian influenza according to Regulation(EC) No 798/2008 is carried out;II.1.4come from:(2)(3) (11) either[the territory of code …;](3)(4) or[compartment(s)…;](3) either[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic and low pathogenic avian influenza as defined in Regulation(EC) No 798/2008;](3) or[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic avian influenza as defined in Regulation (EC) No 798/2008,and(3) either[(a)were derived fromparent flocks which have been kept in an establishment in which avian influenzasurveillance has been carried out with negative results within 21 days priorto the time of collection of eggs;](3) or[(a)were derived fromparent flocks which have been kept in an establishment in which during thepast 21 days prior to the collection of the eggs a virus detection test withnegative testing results for avian influenza has been carried out on a randomsample of cloacal and tracheal/or oropharyngeal swabs taken from at least60 poultry in the establishment or from all poultry if less than 60 are presentin the establishment;](b)the hatching eggscome from an establishment:—around which within a 1 km radius low pathogenic avian influenza hasnot been present within the last 30 days on any establishment;—where there has been no epidemiological link to an establishment whereavian influenza has been detected within the last 30 days;]II.1.5were derived from parent flocks which:(3) either[have not been vaccinated against avian influenza;](3) or[have been vaccinated against avian influenza inaccordance with a vaccination plan under Regulation (EC) No 798/2008 using:(name and type ofused vaccine(s))at the age of … weeks;]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01003101.tif.jpg)
 or[Salmonella arizonae (serogroup O:18(K)), S. Pullorum and S. Gallinarum, Mycoplasma meleagridis and M. gallisepticum (turkeys);](3) or[Salmonella Pullorum and S. Gallinarum(guinea fowls, quails, pheasants, partridges and ducks);]in accordance with Chapter III ofAnnex II to Directive 2009/158/EC and were not found to be infected, or showedany grounds for suspecting infection, by these agents;(3) either[(e)have not been vaccinated against Newcastle disease;](3) oder[(e)have been vaccinated against Newcastle diseaseusing:(name and type (live or inactivated) of Newcastle disease virus strain used in vaccine(s))at the age of … weeks;](8) and/or[(f)have been vaccinated using officially approvedvaccineson … against …(repeat as necessary);](9) II.1.7have been marked as indicated in point I.28 ofthe certificate using …(colour ink);II.1.8have been disinfectedin accordance with my instructions, using …(name of the product and active substance) for …(time in minutes);II.1.9have been collectedfrom … (dd/mm/yyyy) to …(dd/mm/yyyy);II.1.10have been examinedat the date of issue of this certificate and showed no clinical signs of orgrounds for suspecting any disease.II.2Public health additionalguaranties(5) [II.2.1The Salmonella control programmereferred to in Article 10 of Regulation (EC) No 2160/2003 and the specificrequirements for the use of antimicrobials and vaccines in Regulation (EC)No 1177/2006, have been applied to the parent flock of origin and this parentflock has been tested for Salmonella serotypes of public health significance.Date of last samplingof the parent flock from which the testing result is known: … (dd/mm/yyyy);Result of all testingin the parent flock:(3) (6) either[positive;](3) (6) or[negative;](5) [II.2.2Neither Salmonella Enteritidis nor Salmonella Typhimurium were detected within the control programme referredto in point II.2.1.]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.CL2008R0798EN0020020.0001.tif.jpg)
 or[have been vaccinated against Newcastle diseaseusing an inactivated vaccine;](3) or[have been vaccinated against Newcastle diseaseusing a live vaccine at the latest 60 days before the initial date mentionedunder point II.1.9;]](8) [II.3.2the following additionalguarantees, laid down by the Member State of destination in accordance withArticles 16 and/or 17 of Directive 2009/158/EC, are provided:;](7) [II.3.3if the Member State of destination is Finland orSweden, the hatching eggs come from flocks which have tested negative in accordancewith the rules laid down in Decision 2003/644/EC.]II.4Additional health requirementsI, the undersignedofficial veterinarian, further certify that:(8) [II.4.1although the use of vaccines against Newcastledisease which do not fulfil the specific requirements of Annex VI (II) toRegulation (EC) No 798/2008 is not prohibited in:(2)(3) either[the territory ofcode …;](3) (4) or[compartment(s) …;]the poultry fromwhich the hatching eggs are derived:(a)has not been vaccinated for at least the previous 12 months with suchvaccines;(b)comes from a flockor flocks that underwent a virus isolation test for Newcastle disease, carriedout in an official laboratory not earlier than 14 days preceding consignmenton a random sample of cloacal swabs from at least 60 birds in each flock concernedand in which no avian paramyxoviruses with an Intracerebral PathogenicityIndex (ICPI) of more than 0,4 have been found;(c)has not been in contact during the last 60 days before consignmentwith poultry that does not fulfil the conditions in (a) and (b);(d)has been kept in isolation under official surveillance on the establishmentof origin in the 14-day period mentioned in (b).]II.5Animal transport attestationI, the undersignedofficial veterinarian, further certify that:II.5.1the hatching eggs are transported in perfectly clean disposable boxesused for the first time and which:(a)contain only hatching eggs of the same species, category and type comingfrom the same establishment;(b)bear the following indications:—the word “hatching”,—the name of the country, territory, zone or compartment of consignment,—the species of poultry concerned,—the number of eggs,—the category and type of production for which they are intended,—the name, address and approval number of the production establishment,—the approval number of the establishment of origin,—the Member State of destination;](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01003301.tif.jpg)


(4) or[compartment(s)…;]for at least three months. Where the flocks havebeen imported into the country, territory, zone or compartment of origin,this took place in accordance with veterinary conditions at least as strictas the relevant requirements of Directive 2009/158/EC and any subsidiary Decisions;II.1.3come from:(2) (3) (9) either[the territory ofcode …;](3) (4) or[compartment(s) …;](3) either[(a)which at the dateof issue of this certificate was (were) free from Newcastle disease as definedin Regulation (EC) No 798/2008;](3) (5) or[(a)which at the dateof issue of this certificate was not free from Newcastle disease as definedin Regulation (EC) No 798/2008;](b)where a surveillanceprogramme for avian influenza according to Regulation (EC) No 798/2008 iscarried out;II.1.4come from:(2)(3) either[the territory ofcode …;](3) (4) or[compartment(s) …;](3) either[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic and low pathogenic avian influenza as defined in Regulation(EC) No 798/2008;](3) or[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic avian influenza as defined in Regulation (EC) No 798/2008,and(3) either[(a)were derived fromparent flocks which have been kept in an establishment in which avian influenzasurveillance has been carried out with negative results within 21 days priorto the time of collection of eggs;](3) or[(a)were derived fromparent flocks which have been kept in an establishment in which during thepast 21 days prior to the collection of the eggs a virus detection test withnegative testing results for avian influenza has been carried out on a randomsample of cloacal and tracheal/or oropharyngeal swabs taken from at least60 birds in the establishment or from all birds if less than 60 are presentin the establishment;](b)the hatching eggscome from an establishment:—around which within a 1 km radius low pathogenic avian influenza hasnot been present within the last 30 days on any establishment;—where there has been no epidemiological link to an establishment whereavian influenza has been detected within the last 30 days;]II.1.5were derived from parent flocks which:(3) either[have not been vaccinated against avian influenza;](3) or[have been vaccinated against avian influenza inaccordance with a vaccination plan under Regulation (EC) No 798/2008 using:(name and type ofused vaccine(s))at the age of … weeks;]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01003601.tif.jpg)
 or[(d)have been vaccinatedagainst Newcastle disease using:(name and type (liveor inactivated) of Newcastle disease virus strain used in vaccine(s))at the age of …weeks;](8)[(e)have been vaccinated using officially approved vaccineson …against … (repeat as necessary);](6) II.1.7have been marked as indicated in point I.28 ofthe certificate using…(colour ink)II.1.8have been disinfected in accordance with my instructions, using …(name of the product and active substance) for …(time in minutes);II.1.9have been collectedfrom … (dd/mm/yyyy) to …(dd/mm/yyyy);II.1.10have been examinedat the date of issue of this certificate and showed no clinical signs of orgrounds for suspecting any disease.II.2Additional guaranteesI, the undersignedofficial veterinarian, further certify that:(7) [II.2.1where the consignment is intended for a MemberState the status of which has been established in accordance with Article15(2) of Directive 2009/158/EC, the hatching eggs described in this certificateare derived from ratites which:(3) either[have not been vaccinated against Newcastle disease;](3) or[have been vaccinated against Newcastle diseaseusing an inactivated vaccine;](3) or[were vaccinated against Newcastle disease usinga live vaccine at the latest 60 days before the initial date mentioned underpoint II.1.9;]](8) [II.2.2the following additionalguarantees, laid down by the Member State of destination in accordance withArticles 16 and/or 17 of Directive 2009/158/EC are provided:;](7) [II.2.3if the Member State of destination is Finland orSweden, the hatching eggs come from flocks which have tested negative in accordancewith the rules laid down in Decision 2003/644/EC.]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01003701.tif.jpg)
![II.3Additional healthrequirements for countries not free from Newcastle disease(5) [I, the undersigned officialveterinarian, further certify that the breeding ratites from which the hatchingeggs are derived:(a)were placed in isolation under officialsurveillance not less than 30 days prior to laying the hatching eggs for importto the Union;(b)underwent a virus isolatio n test for Newcastle disease, carried outin an official laboratory seven to ten days after their entry into isolationon either cloacal swabs or faeces samples from each bird and in which no avianparamyxovirus type 1 isolates with an Intracerebral Pathogenicity Index (ICPI)of more than 0,4 were found. Favourable results were available from all birdsbefore the eggs left isolation for import to the Union;(c)during the last30 days prior to and during the laying of the hatching eggs for import tothe Union, were not in contact with poultry (including ratites) that doesnot fulfil the conditions in (a), (b) and (d);(d)come from flockson which surveillance for Newcastle disease has been carried out under a statisticallybased sampling plan which produced negative results for at least six monthsimmediately prior to import to the Union.]II.4Animal transportattestationI, the undersigned official veterinarian, further certify that thehatching eggs are transported in perfectly clean, disposable boxes used forthe first time and which:(a)contain only hatching eggs of thesame species, category and type coming from the same establishment;(b)bear the followinginformation in legible writing in at least one Union language:—the word “hatching”,—the name of the country, territory, zone or compartment of consignment,—the species of ratites concerned,—the number of eggs,—the category and type of production for which they are intended,—the name, address and approval number of the breeding establishment,—the name and address of the establishment of origin,—the date of dispatch,—the Member State of destination;(c)are closed in accordancewith the instructions of the competent authority to avoid any possibilityof substitution of the contents.The containers and vehicles in which the boxes mentioned above havebeen transported have been cleansed and disinfected before loading in accordancewith the instructions of the competent authority.NotesPart I:—Box I.8: Provide the code for the zone or the compartment of origin,if necessary, as defined under code in column 2 of Part 1 of Annex I to Regulation(EC) No 798/2008.—Box I.11: Name,address and approval number of the breeding establishment.—Box I.15: Indicate the registration number(s) of railway wagons andlorries, the names of ships and, if known, the flight numbers of aircraft.In the case of transport in containers or boxes, the total number of theseand their registration and where there is a serial number of the seal it hasto be indicated in box I.23.—Box I.28 (Category):Select one of the following: Pure line/grandparents/parents/others; (Identificationsystem & Identification number): introduce the egg mark.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01003801.tif.jpg)

Model veterinary certificate for specified pathogen-free eggs (SPF)
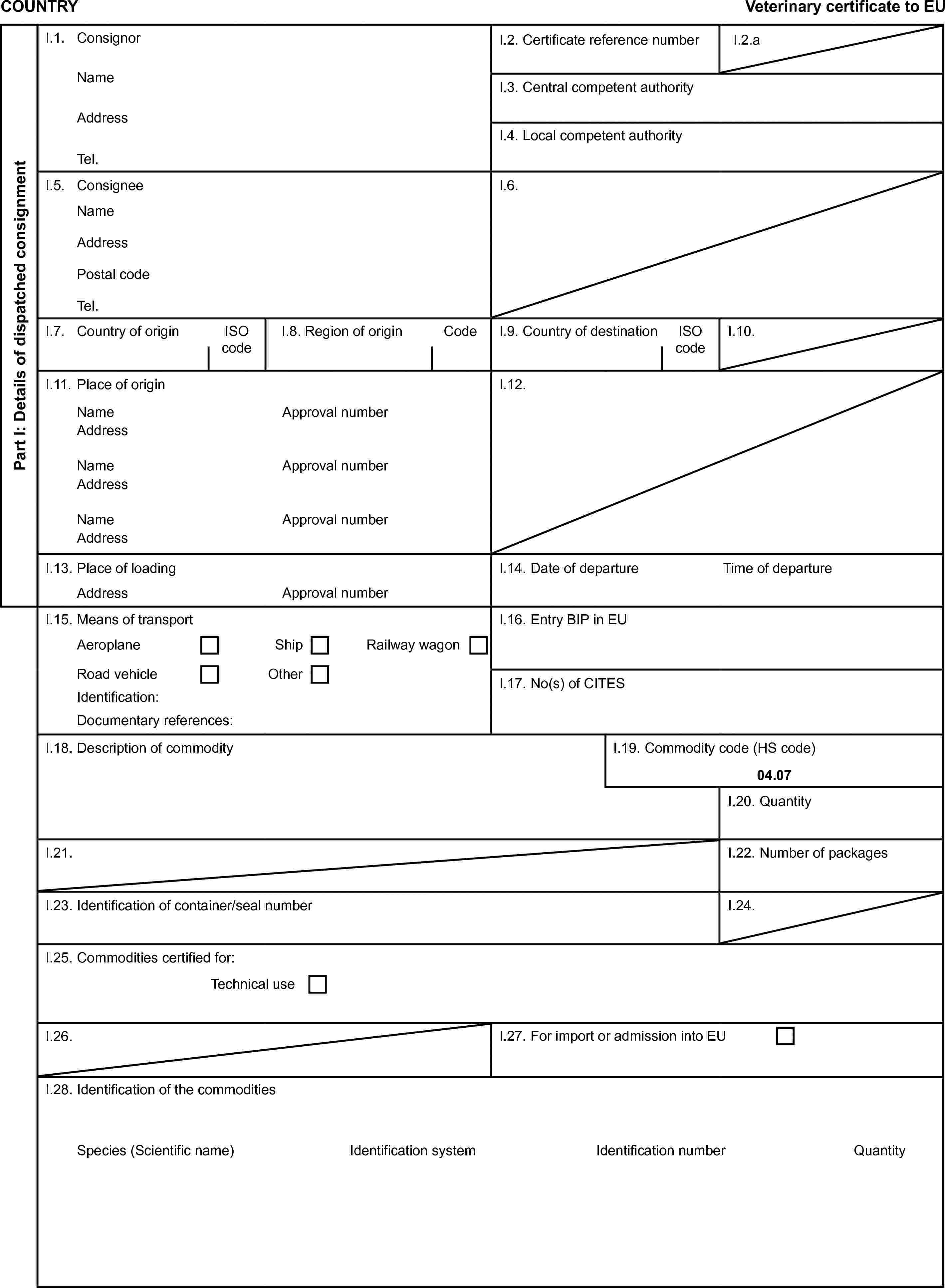
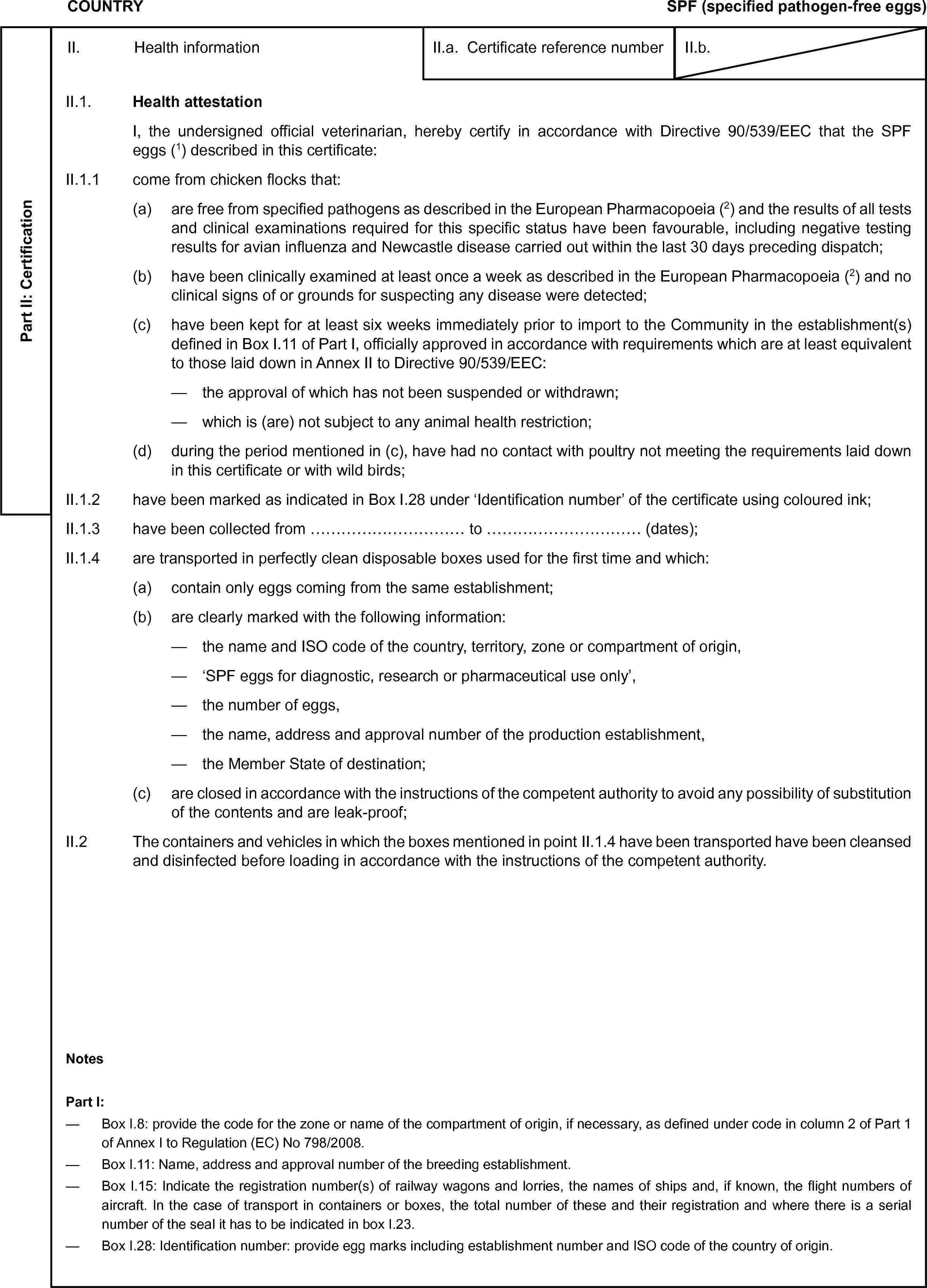


 (4) or[compartment(s) …;]for at least sixweeks or since hatching if less than six weeks old prior to import to theUnion. Where it was imported into the country, territory, zone or compartmentof origin, this took place in accordance with veterinary conditions at leastas strict as the relevant requirements of Directive 2009/158/EC and any subsidiaryDecisions;II.1.3come from:(2) (3) (12) either[the territory of code …;](3)(4) or[compartment(s)…;](a)which, at the dateof issue of this certificate, was (were) free from Newcastle disease as definedin Regulation (EC) No 798/2008;(b)where a surveillanceprogramme for avian influenza according to Regulation (EC) No 798/2008 iscarried out;II.1.4come from:(2)(3) either[the territory ofcode …;](3) (4) or[compartment(s) …;](3) either[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic and low pathogenic avian influenza as defined in Regulation(EC) No 798/2008;](3) or[II.1.4.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic avian influenza as defined in Regulation (EC) No 798/2008,and(3) either[(a)the poultry comefrom an establishment in which within the 21 days prior to import to the Unionavian influenza surveillance has been carried out with negative results;](3) oder[a)during the past21 days prior to import to the Union the poultry have been kept separatelyfrom other poultry and a virus detection test with negative testing resultsfor avian influenza has been carried out on a random sample of cloacal andtracheal/or oropharyngeal swabs taken from at least 60 poultry in the consignmentor from all poultry if less than 60 are present in the consignment;](b)the poultry come from an establishment:—around which within a 1 km radius low pathogenic avian influenza hasnot been present within the last 30 days on any establishment;—where there has been no epidemiological link to an establishment whereavian influenza has been detected within the last 30 days;]II.1.5come from a flock where vaccination against avian influenza has notbeen carried out;II.1.6have been kept sincehatching or for at least the previous 30 days on the establishment(s) of origin;(a)which is (are) not subject to any animal health restriction;(b)within a 10 km radius of which, including, where appropriate, the territoryof a neighbouring country, there has been no outbreak of highly pathogenicavian influenza or Newcastle disease for at least the previous 30 days;](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01004201.tif.jpg)
 or[(b)have been vaccinatedagainst Newcastle disease using:(name and type (liveor inactivated) of Newcastle disease virus strain used in vaccine(s))at the age of …weeks;](5) [(c)have been vaccinated using officially approvedvaccines on… against… (repeat as necessary);]II.1.8during the period mentioned in II.1.6 has had no contact with poultrynot meeting the requirements laid down in this certificate or with wild birds.II.2Public health additional guarantees(6)[The Salmonella control programmereferred to in Article 10 of Regulation (EC) No 2160/2003 and the specificrequirements for the use of antimicrobials and vaccines in Regulation (EC)No 1177/2006, have been applied to the flock of origin and the flock has beentested for Salmonella serotypesof public health significance.Date of last samplingof the flock from which the testing result is known: … (dd/mm/yyyy);Result of all testingin the flock:(3) (7) either[positive;](3) (7) or[negative;]Forreasons other than the Salmonella control programme, within the last three weeks prior to import:(3) either[antimicrobialswere not administered to the slaughter poultry;](3) (8) or[the following antimicrobials were administeredto the slaughter poultry: …;]]II.3Additional guaranteesI, the undersignedofficial veterinarian, further certify that:(9)[II.3.1where the consignment is intended for a Member State the status ofwhich has been established in accordance with Article 15(2) of Directive 2009/158/EC,the poultry described in this certificate come from flocks which:(3) either[have not been vaccinated against Newcastle diseaseand underwent serological examination for the presence of Newcastle diseaseantibodies in the 14 days preceding consignment and tested negative;](3) or[have been vaccinated against Newcastle diseasebut not with a live vaccine in the 30 days preceding consignment and underwenta virus isolation test for Newcastle disease in the 14 days preceding consignmenton a random sample of cloacal swabs or faeces samples from at least 60 birdsand tested negative;]](5) [II.3.2the following additionalguarantees, laid down by the Member State of destination in accordance withArticles 16 and/or 17 of Directive 2009/158/EC, are provided:…;](9) [II.3.3if the Member State of destination is Finland orSweden the poultry:(3) either[underwent a microbiologicaltest by sampling on the holding of origin and tested negative in accordancewith Decision 95/410/EC;](3) or[comes from a holding subject to a programme recognisedby the European Commission as equivalent to the national programme of Finlandor Sweden, as appropriate;]]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01004301.tif.jpg)
 (4) or[compartment(s) …;]the poultry described in this certificate:(a)have not been vaccinatedfor at least the previous 12 months with such vaccines;(b)come from a flockwhich underwent a virus isolation test for Newcastle disease, carried outin an official laboratory not earlier than 14 days preceding consignment ona random sample of cloacal swabs from at least 60 birds in each flock concernedand in which no avian paramyxoviruses with an Intracerebral PathogenicityIndex (ICPI) of more than 0,4 were found;(c)during the last60 days before consignment, have not been in contact with poultry that doesnot fulfil the conditions in (a) and (b);(d)have been kept inisolation under official surveillance on the establishment of origin in the14-day period mentioned in (b).](11) II.5Animal transportattestationI, the undersigned official veterinarian, furthercertify that thepoultry are transported in crates or cages which:(a)contain only poultryof the same species, category and type coming from the same establishment;(b)are closed in accordancewith the instructions of the competent authority to avoid any possibilityof substitution of the contents;(c)in addition to the vehicles in whichthey are transported, are designed to:(i)prevent any excrement escaping and reduce to a minimum any loss offeathers during transport;(ii)allow visual inspectionof the poultry;(iii)allow cleansing and disinfecting;(d)in addition to thevehicles in which they are transported, have been cleansed and disinfectedbefore loading in accordance with the instructions of the competent authority.NotesPart I:—Box I.8: Provide the code for the zone or the compartment of origin,if necessary, as defined under code of column 2 of Part 1 of Annex I to Regulation(EC) No 798/2008.—Box I.15: Indicatethe registration number(s) of railway wagons and lorries, the names of shipsand, if known, the flight numbers of aircraft. In the case of transport incontainers or boxes, the total number of these and their registration andwhere there is a serial number of the seal it has to be indicated in box I.23.—Box I.19: Use the appropriate Harmonised System (HS) code of the WorldCustoms Organisation: 01.05 or 01.06.39.Part II:(1)Poultry as defined in Regulation (EC) No 798/2008with the exception of ratites.(2)Code of the territory as it appears in column 2 of Part 1 of AnnexI to Regulation (EC) No 798/2008.(3)Keep as appropriate.(4)Insert the name of compartment(s).(5)Complete if appropriate.(6)Thisguarantee applies only for poultry belonging to the species of Gallus gallus.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.CL2008R0798EN0020010.0004.tif.jpg)


(4) or[compartment(s)…;]where they have remained for at least six weeksor since hatching if less than six weeks old prior to import to the Union.If they were imported into the country, territory, zone or compartment oforigin, this took place in accordance with veterinary conditions at leastas strict as the relevant requirements of Directive 2009/158/EC and any subsidiaryDecisions;II.1.2come from:(2) (3) (9) either[the territory of code …;](3)(4) or[compartment(s)…;](3) either[(a)which was (were) free from Newcastle disease as defined in Regulation(EC) No 798/2008;](3) (5) or[(a)which was not freefrom Newcastle disease as defined in Regulation (EC) No 798/2008;](b)where a surveillance programme for avian influenza according to Regulation(EC) No 798/2008 is carried out;II.1.3come from:(2)(3) either[the territory ofcode …;](3) (4) or[compartment(s) …;](3) either[II.1.3.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic and low pathogenic avian influenza as defined in Regulation(EC) No 798/2008;](3) or[II.1.3.1which, at the date of issue of this certificate was (were) free fromhighly pathogenic avian influenza as defined in Regulation (EC) No 798/2008,and(3) either[(a)the ratites comefrom an establishment in which within the 21 days prior to import to the Unionavian influenza surveillance has been carried out with negative results;](3) or[(a)during the past21 days prior to import to the Union the ratites have been kept separatelyfrom other birds and a virus detection test with negative testing resultsfor avian influenza has been carried out on a random sample of cloacal andtracheal/or oropharyngeal swabs taken from at least 60 birds in the consignmentor from all birds if less than 60 are present in the consignment;](b)the ratites come from an establishment:—around which within a 1 km radius low pathogenic avian influenza hasnot been present on any establishment;—where there has been no epidemiological link to an establishment whereavian influenza has been detected within the last 30 days;]II.1.4come from a flock where vaccination against avian influenza has notbeen carried out;II.1.5have been kept sincehatching or for at least the previous 30 days on the establishment(s) of origin;(a)which is (are) not subject to any animal health restriction;(b)within a 10 km radius of which, including, where appropriate, the territoryof a neighbouring country, there has been no outbreak of highly pathogenicavian influenza or Newcastle disease for at least the previous 30 days;](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01004701.tif.jpg)
 or[(b)have been vaccinated against Newcastle disease using:(name and type (live or inactivated) of Newcastle disease virus strainused in vaccine(s))at the age of … weeks;](7) [(c)have been vaccinatedusing officially approved vaccines on… against … (repeat as necessary);]II.1.7have been examined at the date of issue of this certificate and showedno clinical signs of or grounds for suspecting any disease;II.1.8during the period mentioned in II.1.5, have had no contact with poultrynot meeting the requirements laid down in this certificate or with wild birds.II.2Additional guaranteesI, the undersignedofficial veterinarian, further certify that:(6) [II.2.1where the consignment is intended for a MemberState the status of which has been established in accordance with Article15(2) of Directive 2009/158/EC, the ratites:(3) either[have not been vaccinated against Newcastle diseaseand underwent a serological examination for the presence of Newcastle diseaseantibodies in the 14 days preceding consignment and tested negative;](3) or[have been vaccinated against Newcastle diseasebut not with a live vaccine in the 30 days preceding consignment and underwenta virus isolation test for Newcastle disease in the 14 days preceding consignmenton a random sample of cloacal swabs or faeces samples from at least 60 birdsand tested negative;]](7) [II.2.2The following additionalguarantees, laid down by the Member State of destination pursuant to Articles 16and/or 17 of Directive 2009/158/EC, are provided:;](6) [II.2.3If the Member State of destination is Finland orSweden, the ratites:(3) either[have undergonea microbiological test by sampling on the establishment of origin and testednegative in accordance with Decision 95/410/EC;](3) or[come from an establishment subject to a programmerecognised by the European Commission as equivalent to the national programmeof Finland or Sweden, as appropriate.]]II.3.Additional health requirements for countries not free from Newcastledisease(5)[I,the undersigned official veterinarian, further certify that the ratites described in this certificate:(a)were placed under officialsurveillance for at least 21 days prior to import to the Union in a quarantinestation as defined in Article 2 of Directive 2009/158/EC and approved by thecompetent authority:(approval number and address of the quarantine station …);(b)underwent a virus isolationtest for Newcastle disease, carried out in an official laboratory seven toten days after their entry into a quarantine station on either cloacal swabsor faeces samples from each bird and in which no avian paramyxovirus type1 isolates with an Intracerebral Pathogenicity Index (ICPI) of more than 0,4were found. Favourable results were available from all birds in the consignmentbefore they left the quarantine station for import to the Union;(c)come from flocks in whichsurveillance for Newcastle disease has been carried out under a statisticallybased sampling plan which produced negative results for at least six monthsimmediately prior to import to the Union.]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01004801.tif.jpg)

Model veterinary certificate for meat of poultry (POU)
![Part II: CertificationCOUNTRYPOU (meat of poultry)II. Health informationII.a. Certificate reference numberII.b.II.1. Public Health AttestationI, the undersigned official veterinarian, declare that I am aware of the relevant provisions of Regulations (EC) Nos 178/2002, 852/2004, 853/2004 and 854/2004 and hereby certify that the meat of poultry (1) described in this certificate has been obtained in accordance with those requirements, and in particular that:(a) it comes from (an) establishments(s) implementing a programme based on the HACCP principles in accordance with Regulation (EC) No 852/2004;(b) it has been produced in compliance with the conditions set out in Sections II and V of Annex III to Regulation (EC) No 853/2004;(c) it has been found fit for human consumption following ante and post-mortem inspections carried out in accordance with Section IV, Chapter V of Annex I to Regulation (EC) No 854/2004;(d) it has been marked with an identification mark in accordance with Section I of Annex II to Regulation (EC) No 853/2004;(e) it satisfies the relevant criteria set out in Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs;(f) the guarantees covering live animals and products thereof provided by the residue plans submitted in accordance with Directive 96/23/EC, and in particular Article 29 thereof, are fulfilled;(2) [(g) it fulfils the requirements of Regulation (EC) No 1688/2005 implementing Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards special guarantees concerning Salmonella for consignments to Finland and Sweden of certain meat and eggs.]II.2. Animal health attestationI, the undersigned official veterinarian, hereby certify that the meat of poultry described in this certificate:II.2.1. comes from:(3) (4) (6) either [the territory of code …;](4) (5) or [compartment(s) …;]which at the date of issue of the certificate was (were) free from:highly pathogenic avian influenza as defined in Regulation (EC) No 798/2008, and Newcastle disease as defined in Regulation (EC) No 798/2008;II.2.2. has been obtained from poultry which:(4) either [has not been vaccinated against avian influenza;](4) or [has been vaccinated against avian influenza in accordance with vaccination plan under Regulation (EC) No 798/2008 using:(name and type of used vaccine(s))at the age of … weeks;]II.2.3. has been obtained from poultry which has been kept in:(3) (4) (9) either [the territory(ies) of code …;](4) (5) (9) or [compartment(s) …;]since hatching or has been imported as day-old chicks or slaughter poultry from (a) third country(ies) listed for that commodity in Part 1 of Annex I to Regulation (EC) No 798/2008 under conditions at least equivalent to those in that Regulation;II.2.4. has been obtained from poultry coming from establishments:(a) which are not subject to any animal health restriction;(b) within a 10 km radius of which, including, where appropriate, the territory of a neighbouring country, there has been no outbreak of highly pathogenic avian influenza or Newcastle disease for at least the previous 30 days;II.2.5. has been obtained from poultry that:](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010279EN.01000701.tif.jpg)
![COUNTRYPOU (meat of poultry)II. Health informationII.a. Certificate reference numberII.b.(7) (a) has been slaughtered on … (dd/mm/yyyy) or between … (dd/mm/yyyy) and …(dd/mm/yyyy);(b) has not been slaughtered under any animal-health scheme for the control or eradication of poultry diseases;(c) during transport to the slaughterhouse, did not come into contact with poultry infected with highly pathogenic avian influenza or Newcastle disease;II.2.6. (a) comes from approved slaughterhouses which, at the time of slaughter, were not under restrictions owing to a suspected or confirmed outbreak of highly pathogenic avian influenza or Newcastle disease and within a 10 km radius of which there has been no outbreak of highly pathogenic avian influenza or Newcastle disease for at least the previous 30 days:(b) has not been in contact at any time during slaughter, cutting, storage or transport with poultry or meat of lower health status;(8) [II.2.7. comes from slaughter poultry that:(a) has not been vaccinated with live attenuated vaccines prepared from a Newcastle disease virus master seed showing a higher pathogenicity than lentogenic strains of the virus;(b) underwent a virus isolation test for Newcastle disease, carried out in an official laboratory at the time of slaughter on a random sample of cloacal swabs from at least 60 birds in each flock concerned and in which no avian paramyxoviruses with an Intracerebral Pathogenicity Index (ICPI) of more than 0,4 were found;(c) has not been in contact in 30 days preceding slaughter with poultry that does not fulfil the conditions in (a) and (b).]II.3. Animal welfare attestationI, the undersigned official veterinarian, hereby certify that I have read and understood Directive 93/119/EC and that the meat described in this certificate comes from poultry that has been treated in accordance with the relevant provisions of Directive 93/119/EC in the slaughterhouse before and at the time of slaughter or killing.NotesPart I:Box I.8: Provide the code for the zone or the compartment of origin, if necessary, as defined under the code in column 2 of Part 1 of Annex I to Regulation (EC) No 798/2008.Box I.11: Name, address and approval number of the establishment of dispatch.Box I.15: Indicate the registration number(s) of railway wagons and lorries, the names of ships and, if known, the flight numbers of aircraft. In the case of transport in containers or boxes, the total number of these and their registration and where there is a serial number of the seal it has to be indicated in box I.23.Box I.19: Use the appropriate Harmonised System (HS) code of the World Customs Organisation: 02.07 or 02.08.90.Part II:(1) “Poultry meat” means the edible parts of farmed birds, including birds that are not considered as domestic but which are farmed as domestic animals, with the exception of ratites, which have not undergone any treatment other than cold treatment to ensure preservation; vacuum-wrapped meat or meat wrapped in a controlled atmosphere must also be accompanied by a certificate in accordance with this model.(2) Delete if the consignment is not intended for import to Sweden or Finland.(3) Code of the territory as it appears in column 2 of Part 1 of Annex I to Regulation (EC) No 798/2008.(4) Keep as appropriate.(5) Insert the name of compartment(s).(6) For countries or territories with the entry “N” in column 6 of Part 1 of Annex I to Regulation (EC) No 798/2008, for meat of poultry (POU) only, this means that in the case of an outbreak of Newcastle disease as defined in Regulation (EC) No 798/2008 then the country code or territory code shall continue to be used but this will exclude any area under official restrictions, by the third country concerned in relation to Newcastle disease, at the date of issue of this certificate.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.CL2008R0798EN0130010.0001.tif.jpg)
Model veterinary certificate for minced meat and mechanically separated meat of poultry (POU-MI/MSM)
(Not yet established)

 (4) or[compartment(s) …;]which at the date of issue of the certificate was (were) free from:highly pathogenic avian influenza as defined in Regulation (EC) No 798/2008, and(6) [Newcastle disease as defined in Regulation (EC) No 798/2008;]II.2.2has been obtained from ratites which:(2) either[have not been vaccinated against avian influenza;](2) or[have been vaccinated against avian influenza in accordance with vaccination plan under Regulation (EC) No 798/2008 using:(name and type of used vaccine(s))at the age of … weeks;](7) have been slaughtered on … (dd/mm/yyyy) or between … (dd/mm/yyyy) and …(dd/mm/yyyy);II.2.3has been:(2) (6) either[II.2.3.1obtained from farmed ratites which have been kept uninterruptedly in:(2) (3) either[the territory of code …;](2) (4) or[compartment(s) …;]for at least three months before slaughter or since hatching;](2) (8) or[II.2.3.1boned and skinned and has been obtained from farmed ratites which have been kept uninterruptedly in:(2) (3) either[the territory of code …;](2) (4) or[compartment(s) …;]for at least three months before slaughter or since hatching;]](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01005601.tif.jpg)
(2) or[II.2.4.1boned and skinned and comes from ratites which were reared/ kept forat least three months prior to slaughter on establishments:(a)which receive regularveterinary inspection visits to detect diseases transmissible to humans oranimals;(b)which are not subject to animal-health restrictions in connection withany disease to which ratites and/or other poultry are susceptible;(c)on which there has been no outbreak of Newcastle disease or highlypathogenic avian influenza in the previous six months and around which nooutbreaks of highly pathogenic avian influenza or Newcastle disease have occurredfor at least three months within a distance of 10 km from the perimeter ofthat part of the establishment which contains the ratites, including whereappropriate the territory of a neighbouring country;](2) or[II.2.4.1boned and skinnedand comes from ratites from countries in Asia or Africa which:(a)were placed in isolation in tick-proofed surroundingsunder an officially approved programme for rodent control for at least 14days prior to slaughter;(b)before being movingto the tick-proofed surroundings, were:(2) either[examined to verifythat they were tick-free,](2) or[underwent treatmentto ensure that all ticks on them were destroyedby (specify the treatment):,and this treatment has not givenrise to any detectable residues in the ratite meat;](c)were checked forthe presence of ticks on arrival in the slaughterhouse (each batch), withnegative results;]II.2.5has not been obtainedfrom ratites that were slaughtered under any animal health scheme for thecontrol or eradication of poultry and/or ratite diseases;II.2.6comes from ratites:(2)(6) (9) either[II.2.6.1that have been vaccinatedagainst Newcastle disease using a live vaccine in the 30 days preceding slaughter;](2)(6) or[II.2.6.1that have not been vaccinated against Newcastle disease using a livevaccine in the 30 days preceding slaughter;](2)(8) either[II.2.6.1that have not been vaccinated against Newcastle disease;](2)(8) or[II.2.6.1that have been vaccinated against Newcastle disease using a live vaccinewhich does not meet the requirements of Annex VI of Regulation (EC) No 798/2008but were not vaccinated in 30 days preceding slaughter;](2)(8) or[II.2.6.1that have been vaccinated against Newcastle disease using an inactivatedvaccine which meets the requirements of Annex VI of Regulation (EC) No 798/2008;](8)(10) [II.2.7comes from ratitesfrom establishments on which surveillance for Newcastle disease has been carriedout under a statistically based sampling plan which produced negative resultsfor at least the previous six months immediately prior to import to the Union;]II.2.8comes from ratites that during transport to the slaughterhouse, didnot come into contact with poultry and/or ratites infected with highly pathogenicavian influenza or Newcastle disease;](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2010076EN.01005701.tif.jpg)

Model veterinary certificate for minced meat and mechanically separated meat of farmed ratites for human consumption (RAT-MI/MSM)
(Not yet established)
Model veterinary certificate for wild game-bird meat (WGM)

 (4) or [compartment(s) …;]in which no animal health restrictions have been in force for at least the previous 30 days in response to outbreaks of highly pathogenic avian influenza and Newcastle disease;(b) was obtained from animals which, after killing, were transported within 12 hours to a collection centre and/or an approved wild game processing house for chilling;II.2.2 comes from(2) either [a collection centre;](2) or [an approved wild game processing house;](2) or [a collection centre and an approved wild game processing house;]which at the time of dressing, was (were) not subject to restrictions owing to a suspected or actual outbreak of highly pathogenic avian influenza or Newcastle disease;II.2.3 was obtained and inspected in accordance with Regulations (EC) Nos 853/2004 and 854/2004;(2) either [II.2.4 In the case of fresh meat or plucked and eviscerated wild game-birds, the meat was obtained and inspected in accordance with Regulations (EC) Nos 853/2004 and 854/2004;](2) or [In the case of non-plucked and non-eviscerated wild game-birds:(a) the meat was chilled to +4 °C or below for a maximum of 15 days prior to the intended time of import but has not been frozen or deep-frozen;(b) an official veterinary health inspection has been carried out on a representative sample of the carcases and the meat was obtained and inspected in accordance with Regulations (EC) Nos 853/2004 and 854/2004;(c) the meat has been identified by affixing an official mark of origin, the details of which are recorded in the box 1.28;](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2008226EN.01006901.tif.jpg)
 (6) or [have been unplucked and uneviscerated but they will be transported by aeroplane.]NotesPart I:— Box I.8: provide the code for the zone or name of the compartment of origin, if necessary, as defined under code of column 2 of Part 1 of Annex I to Regulation (EC) No 798/2008.— Box I.11: Name, address and approval number of establishment of dispatch.— Box I.15: Indicate the registration number(s) of railway wagons and lorries, the names of ships and, if known, the flight numbers of aircraft. In the case of transport in containers or boxes, the total number of these and their registration and where there is a serial number of the seal it has to be indicated in box I.23.— Box: I.28 (Nature of commodity): select one of the following: Plucked and eviscerated game-birds/non-plucked and non-eviscerated game-birds.Part II:(1) ‘Wild game-bird meat’ means edible parts of wild game-birds hunted for human consumption and excluding offal, except for non-plucked and non-eviscerated wild game-birds which have not undergone any treatment other than cold treatment to ensure preservation; vacuum-wrapped meat or meat wrapped in a controlled atmosphere must also be accompanied by a certificate in accordance with this model.(2) Keep as appropriate.(3) Code of the territory as it appears in column 2 of Part 1 of Annex I to Regulation (EC) No 798/2008.(4) Insert the name of compartment(s).(5) Indicate the date or dates of slaughter. Imports of this meat shall not be allowed when obtained from birds slaughtered in the territory mentioned under (3) or the compartment mentioned under (4) during a period where restrictive measures have been adopted by the European Community against imports of this meat from this territory.(6) Applicable only to the countries with the entry ‘VIII’ in column 5 (‘AG’) of Part 1 of Annex I to Regulation (EC) No 798/2008.Official veterinarianName (in capital letters):Date:Stamp:Qualification and title:Signature:](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2008226EN.01007001.tif.jpg)
Model veterinary certificate for wild game-bird minced meat and mechanically separated meat (WGM-MI/MSM)
(Not yet established)
Model veterinary certificate for eggs (E)
![COUNTRYE (Eggs)Part II: CertificationII. Health informationII.a. Certificate reference numberII.b.II.1 Animal health attestationI, the undersigned official veterinarian, hereby certify that eggs described in this certificate come from an establishment which at the date of issue of the certificate is free from highly pathogenic avian influenza as defined in Regulation (EC) No 798/2008.II.2 Public Health attestationI, the undersigned official veterinarian, declare that I am aware of the relevant provisions of Regulations (EC) Nos 178/2002, 852/2004, 853/2004 and 2160/2003 and hereby certify that the eggs described in this certificate have been obtained in accordance with those requirements, and in particular that:II.2.1 they come from (an) establishments(s) implementing a programme based on the HACCP principles in accordance with Regulation (EC) No 852/2004;II.2.2 they have been kept, stored, transported and delivered in accordance with the relevant conditions laid down in Section X, Chapter I of Annex III to Regulation (EC) No 853/2004;(1) [II.2.3 they fulfil the requirements of Commission Regulation (EC) No 1688/2005 implementing Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards special guarantees concerning Salmonella for consignments to Finland and Sweden of certain meat and eggs;]II.2.4 the guarantees covering live animals and products thereof provided by the residue plans submitted in accordance with Directive 96/23/EC, and in particular Article 29 thereof, are fulfilled;II.2.5 they fulfil the requirements in Article 10(6) of Regulation (EC) No 2160/2003. In particular:(i) eggs shall not be imported from flocks of laying hens in which Salmonella spp. has been detected as a result of the epidemiological investigation of a food-borne outbreak or if no equivalent guarantees have been provided unless the eggs are marked as class B eggs;(ii) eggs shall not be imported from flocks of laying hens with unknown health status, that are suspected of being infected or from flocks infected by Salmonella Enteritidis and/or Salmonella Typhimurium for which a target for reduction has been set in Community legislation and on which monitoring equivalent to the monitoring laid down in the provisions in the Annex to Regulation (EC) No 1168/2006 is not applied, or if no equivalent guarantees have been provided unless the eggs are marked as class B eggs.NotesPart I:— Box I.8: provide the code for the zone or name of the compartment of origin, if necessary, as defined under code of column 2 of Part 1 of Annex I to Regulation (EC) No 798/2008.— Box I.11: Name, address and approval number of establishment of dispatch.— Box I.15: Indicate the registration number(s) of railway wagons and lorries, the names of ships and, if known, the flight numbers of aircraft. In the case of transport in containers or boxes, the total number of these and their registration and where there is a serial number of the seal it has to be indicated in box I.23.— Box 1.18: indicate the class of eggs according to Article 3 of Regulation (EC) No 1028/2006.Part II:(1) Delete if the consignment is not intended for import to Sweden or Finland.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2008226EN.01007301.tif.jpg)

Model veterinary certificate for egg products (EP)
![Part II: CertificationCOUNTRYEP (egg products)II. Health informationII.a. Certificate reference numberII.b.II.1. Animal health attestationI, the undersigned official veterinarian, hereby certify that the egg products described in this certificate were produced from eggs coming from an establishment which at the date of issue of the certificate is free from highly pathogenic avian influenza as defined in Regulation (EC) No 798/2008 andeither(1) II.1.1 [within a 10 km radius of which, including, where appropriate, the territory of a neighbouring country, there has been no outbreak of highly pathogenic avian influenza or Newcastle disease for at least the previous 30 days.]or(1) II.1.2 [the egg products were processed:(1) either [liquid egg white was treated:(1) either [with 55,6 °C for 870 seconds.](1) or [with 56,7 °C for 232 seconds.]](1) or [10 % salted yolk was treated with 62,2 °C for 138 seconds.](1) or [dried egg white was treated:(1) either [with 67 °C for 20 hours.](1) or [with 54,4 °C for 513 hours.]](1) or [whole eggs were at least treated:(1) either [with 60 °C for 188 seconds.](1) or [completely cooked.]](1) or [whole egg blends were at least treated:(1) either [with 60 °C for 188 seconds.](1) or [with 61,1 °C for 94 seconds.]]]II.2. Public health attestationI, the undersigned, official veterinarian/official inspector declare that I am aware of the relevant provisions of Regulations (EC) No 178/2002, (EC) No 852/2004 and (EC) No 853/2004 and hereby certify that the egg products described in this certificate have been obtained in accordance with those requirements, and in particular that:II.2.1 they come from (an) establishments(s) implementing a programme based on the HACCP principles in accordance with Regulation (EC) No 852/2004;II.2.2 they have been produced from raw material which meets the requirements of Section X, Chapter II (II) of Annex III to Regulation (EC) No 853/2004;II.2.3 they have been manufactured in compliance with the hygiene requirements laid down in Section X, Chapter II (III) of Annex III to Regulation (EC) No 853/2004;II.2.4 they satisfy the analytical specifications in Section X, Chapter II (IV) of Annex III to Regulation (EC) No 853/2004 and the relevant criteria in Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs;II.2.5 they have been marked with an identification mark in accordance with Section I of Annex II and Section X, Chapter II (V) of Annex III to Regulation (EC) No 853/2004;II.2.6 the guarantees covering live animals and products thereof provided by the residue plans submitted in accordance with Directive 96/23/EC, and in particular Article 29 thereof, are fulfilled.](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2011100EN.01003501.tif.jpg)
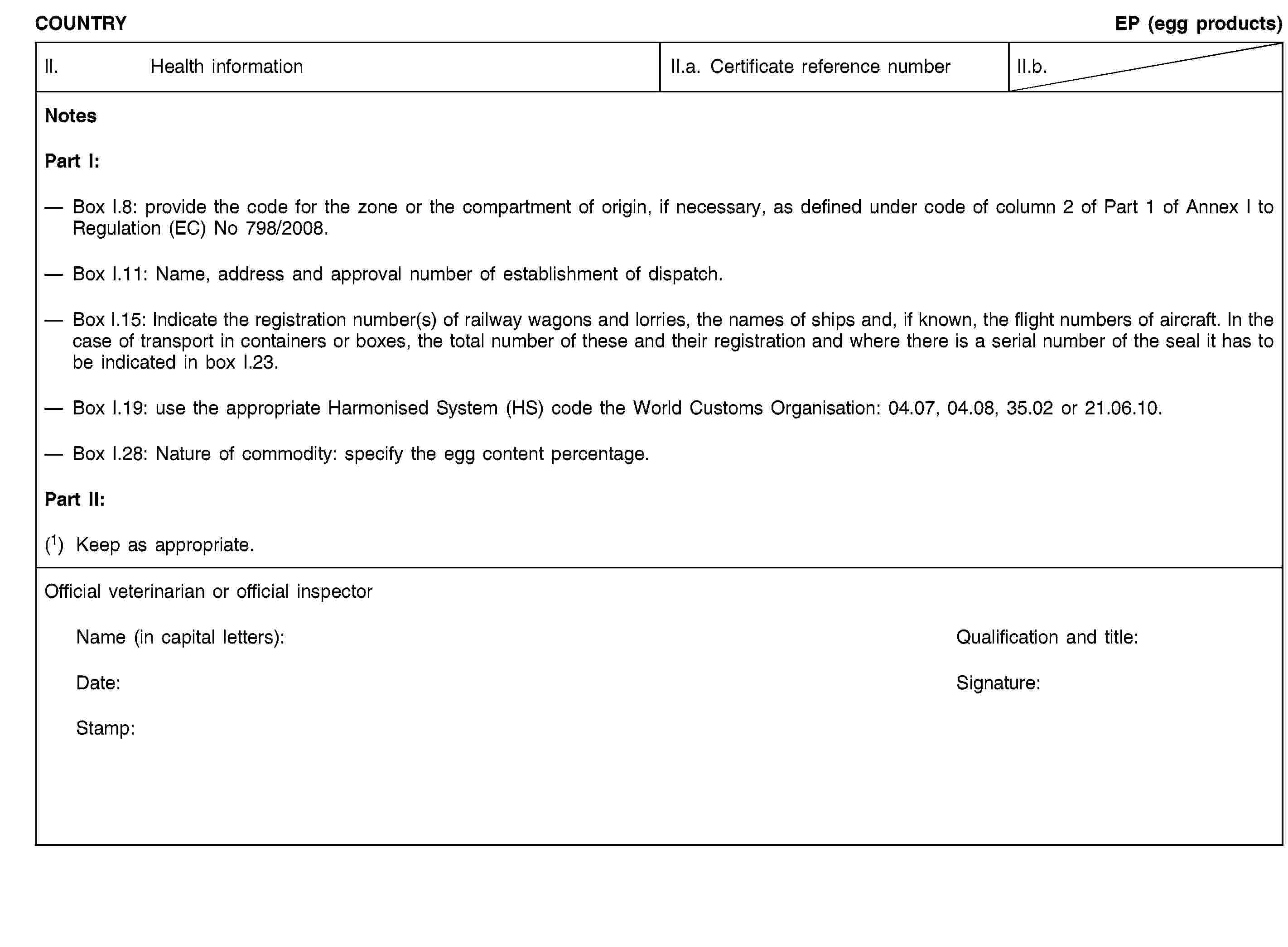
ANNEX II
(as referred to in Article 4)
(To be completed and attached to the veterinary certificate where transport of poultry and day-old chicks to the European Community border includes transport by ship, even for part of the journey.)

ANNEX III
COMMUNITY ACTS, INTERNATIONAL STANDARDS AND PROCEDURES FOR EXAMINATION, SAMPLING AND TESTING AS REFERRED TO IN ARTICLE 6
I. Before import into the Community
Methods for standardisation of materials and procedures for examination, sampling and testing for:
1. Avian influenza
— Diagnostic manual for avian influenza as laid down in Commission Decision 2006/437/EC ( 21 ); or
— Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for animal Health (OIE) ( 22 ).
2. Newcastle disease
— Annex III to Council Directive 92/66/EEC ( 23 ); or
— Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for animal Health (OIE);
— Where Article 12 of Directive 90/539/EEC applies, the sampling and testing methods must comply with the methods described in Annexes to Commission Decision 92/340/EEC ( 24 ).
3. Salmonella pullorum and Salmonella gallinarum
— Chapter III of Annex II to Directive 90/539/EEC; or
— Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for animal Health (OIE).
4. Salmonella arizonae
— Chapter III of Annex II to Directive 90/539/EEC; or
— Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for animal Health (OIE).
5. Mycoplasma gallisepticum
— Chapter III of Annex II to Directive 90/539/EEC; or
— Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for animal Health (OIE).
6. Mycoplasma meleagridis
Chapter III of Annex II to Directive 90/539/EEC.
7. Salmonella of public health significance
The detection method recommended by the Community reference laboratory (CRL) for Salmonella in Bilthoven, the Netherlands, or an equivalent method shall be used. That method is described in the current version of draft Annex D of ISO 6579 (2002): ‘Detection of Salmonella spp. in animal faeces and in samples of the primary production stage’. In that detection method, a semi-solid medium (modified semi-solid Rappaport-Vassiladis medium, MSRV) is used as the single selective enrichment medium.
Serotyping shall be carried out following the Kauffmann-White scheme or an equivalent method.
II. After import into the Community
Sampling and testing procedures for avian influenza and Newcastle disease:
During the period referred to in point II.1 of Annex VIII, the official veterinarian shall take samples from the imported poultry for virological examination, to be tested as follows:
— Between the seventh and the fifteenth day following the date of commencement of the isolation period, cloacal swabs must be taken from all birds where the consignment contains less than 60 birds, and from at least 60 birds where consignments contain more than 60 birds;
— Testing of samples must be carried out in official laboratories designated by the competent authority, using diagnostic procedures for:
—(i) avian influenza as laid down in the diagnostic manual in Commission Decision 2006/437/EC;
(ii) Newcastle disease as laid down in Annex III to Council Directive 92/66/EEC.
III. General requirements
— Samples may be pooled, subject to a maximum of five samples from individual birds in each pool.
— Virus isolates must be sent without delay to the national reference laboratory.
ANNEX IV
(as referred to in Article 8(2)(d), Article 9(2)(b) and Article 10
REQUIREMENTS FOR AVIAN INFLUENZA SURVEILLANCE PROGRAMMES AND INFORMATION TO BE SUBMITTED ( 25 )
I. Requirements for avian influenza surveillance in poultry carried out in third countries, territories, zones or compartments as referred to in Article 10.
A. surveillance for avian influenza in poultry:
1. Description of objectives
2. Third country, territory, zone or compartment (keep as appropriate):
3. Type of surveillance:
— Serological surveillance
— Virological surveillance
— Targeted avian influenza subtypes
4. Sampling criteria:
— Targeted species (for example, turkeys, chicken, partridges)
— Targeted categories (for example, breeders, layers)
— Targeted husbandry systems (for example, commercial establishments, backyard flocks)
5. Statistical basis for number of establishments sampled:
— Number of establishments in area
— Number of establishments per category
— Number of establishments to be sampled per poultry category
6. Frequency of sampling
7. Number of samples taken per establishment/shed
8. Time period for sampling
9. Type of samples taken (tissue, faeces, cloacal/oropharyngeal/tracheal swabs)
10. Laboratory tests used (for example, AGID, PCR, HI, Virus isolation.)
11. Indication of laboratories carrying out testing at central, regional or local level (keep as appropriate)
Indication of reference laboratory carrying out confirmatory testing (avian influenza national reference laboratory, OIE or Community reference laboratory for avian influenza)
12. Reporting system/protocol used for avian influenza surveillance results (include results where available)
13. Follow-up investigations of positive results for subtypes H5 and H7.
B. where available information on surveillance for avian influenza in wild birds to assess risk factors for avian influenza introduction into poultry:
1. Type of surveillance:
— Serological surveillance
— Virological surveillance
— Targeted avian influenza subtypes
2. Sampling criteria
3. Targeting of wild bird species (indicate species names in Latin)
4. Targeting of selected areas
5. Information referred to in point 6 and points 8 to 12 of Part I.A.
II. Avian influenza surveillance to be carried out following the occurrence of an outbreak of that disease in a third country, territory, zone or compartment previously free from that disease, as referred to in Articles 8(2)(d) and 9(2)(b)
Surveillance for avian influenza must provide at least the confidence by a randomised representative sample of the populations at risk to demonstrate the absence of infection taking into account the specific epidemiological circumstances in relation to the occurred outbreak(s).
ANNEX V
(as referred to in Article 11(a))
INFORMATION TO BE SUBMITTED BY A THIRD COUNTRY VACCINATING AGAINST AVIAN INFLUENZA ( 26 )
I. Requirements for vaccination plans carried out in a third country, territory, zone or compartment as referred to in Article 11
1. Country, territory, zone or compartment (keep as appropriate)
2. Disease history (previous outbreaks in poultry or cases in wild birds of HPAI/LPAI)
3. Description of the reasons for the decision on the introduction of vaccination
4. Risk assessment based on:
— Avian influenza outbreak within that third country, territory, zone or compartment (keep as appropriate)
— Avian influenza outbreak in a nearby country
— Other risk factors such as certain areas, type of poultry husbandry or categories of poultry or other captive birds
5. Geographical area where vaccination is carried out
6. Number of establishments in vaccination area
7. Number of establishments where vaccination is carried out, if different from number in point 6
8. Species and categories of poultry or other captive birds in vaccination territory, zone or compartment
9. Approximate number of poultry or other captive birds in the establishments referred to in point 7
10. Summary of the vaccine characteristics
11. Authorisation, handling, manufacture, storage, supply, distribution and sale of avian influenza vaccines on national territory
12. Implementation of a DIVA strategy
13. Envisaged duration of vaccination campaign
14. Provisions and restrictions on the movements of vaccinated poultry and poultry products derived from vaccinated poultry or vaccinated other captive birds
15. Clinical and laboratory tests carried out in the establishments vaccinated and/or located in the vaccination area (e.g. efficacy and pre-movement testing etc.)
16. Means of record keeping (e.g. for the detailed information referred to point 15) and registration of holdings where vaccination is carried out.
II. Surveillance for third countries, territories, zones or compartments that carry out vaccination against avian influenza as referred to in Article 11
Where vaccination is carried out in a third country, territory, zone or compartment all commercial establishments that are vaccinated against avian influenza must be required to undergo laboratory testing and the following information, in addition to the information referred to in Part I.A to Annex IV, shall be submitted:
1. Number of vaccinated establishments in area per category
2. Number of vaccinated establishments to be sampled per poultry category
3. Use of sentinel birds (indicate species and number of sentinel birds used per shed)
4. Number of samples taken per establishment and/or shed
5. Data on vaccine efficacy.
ANNEX VI
(as referred to in Article 12(1)(b), Article 12(2)(c)(ii) and Article 13(1)(a))
CRITERIA FOR RECOGNISED NEWCASTLE DISEASE VACCINES
I. General criteria
1. Vaccines must comply with the standards set out in the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals of the World Organisation for Animal Health (OIE) in the Chapter on Newcastle disease.
2. Vaccines must be registered by the competent authorities of the third country concerned before being allowed to be distributed and used. For such registration, the competent authorities of the third country concerned must rely on a complete file containing data on the efficacy and innocuity of the vaccine; for imported vaccines the competent authorities may rely on data checked by the competent authorities of the country where the vaccine is produced, as far as these checks have been carried out in conformity with OIE standards.
3. In addition, imports or production and distribution of the vaccines must be controlled by the competent authorities of the third country concerned.
4. Before distribution is allowed, each batch of vaccines must be tested on innocuity, in particular regarding attenuation or inactivation and absence of undesired contaminating agents, and on efficacy on behalf of the competent authorities.
II. Specific criteria
Live attenuated Newcastle disease vaccines must be prepared from a Newcastle disease virus strain for which the master seed has been tested and shown to have an intracerebral pathogenicity index (ICPI) of:
(a) less than 0,4, if not less than 107 EID50 are administered to each bird in the ICPI test; or
(b) less than 0,5, if not less than 108 EID50 are administered to each bird in the ICPI test.
ANNEX VII
(as referred to in Article 13)
ADDITIONAL HEALTH REQUIREMENTS
I. For poultry, day-old chicks and hatching eggs coming from a third country, Territory, zone or compartment where vaccines used against Newcastle disease do not meet THE criteria of Annex VI
1. Where the third country, territory, zone or compartment does not prohibit the use of Newcastle disease vaccines that do not meet the specific criteria set out in Annex VI the following additional health requirements shall apply to:
(a) poultry, including day-old chicks must not have been vaccinated with such vaccines for at least 12 months preceding the date of import to the Community;
(b) the flock or flocks must have undergone a virus isolation test for Newcastle disease not earlier than two weeks before the date of import into the Community or, in the case of hatching eggs, not earlier than two weeks before the date of collection of the eggs:
(i) carried out in an official laboratory;
(ii) on a random sample of cloacal swabs from at least 60 birds in each flock;
(iii) in which no avian paramyxoviruses with an Intracerebral Pathogenicity Index of more than 0,4 have been found.
(c) poultry must have been kept in isolation under official surveillance on the establishment of origin during the two-week period referred to in (b);
(d) poultry must not have been in contact with poultry not meeting the requirements set out in (a) and (b) during a period of 60 days before the date of import into the Community or, in the case of hatching eggs, during a period of 60 days before the date of collection of the eggs.
2. Where day-old chicks are imported from a third country, territory, zone or compartment as referred to in point 1, the day-old chicks and the hatching eggs from which the day-old chicks are derived must not have been in contact in the hatchery or during transport with poultry or hatching eggs not meeting the requirements set out in point 1(a) to (d).
II. For meat of poultry
Meat of poultry must come from slaughter poultry that:
(a) has not been vaccinated with live attenuated vaccines prepared from a Newcastle disease virus master seed showing a higher pathogenicity than lentogenic strains of the virus within the period of 30 days preceding slaughter;
(b) underwent a virus isolation test for Newcastle disease, carried out in an official laboratory at the time of slaughter on a random sample of cloacal swabs from at least 60 birds in each flock concerned and in which no avian paramyxoviruses with an Intracerebral Pathogenicity Index (ICPI) of more than 0,4 were found;
(c) has not been in contact in 30 days preceding the date of slaughter with poultry that does not fulfil the conditions set out in (a) and (b).
ANNEX VIII
(as referred to in Article 14(1)(a))
BREEDING AND PRODUCTIVE POULTRY OTHER THAN RATITES, HATCHING EGGS AND DAY-OLD CHICKS OTHER THAN OF RATITES
I. Requirements applicable before import
1. Breeding and productive poultry other than ratites, hatching eggs and day-old chicks other than of ratites for import into the Community shall only come from establishments which have been approved by the competent authority of the third country concerned in accordance with conditions that are at least as strict as those laid down in Annex II to Directive 90/539/EEC and where such approval has not been suspended or withdrawn.
2. Where breeding and productive poultry other than ratites, hatching eggs and day-old chicks other than of ratites and/or their flocks of origin are to undergo testing to meet the requirements of the relevant veterinary certificates laid down in this Regulation, sampling for testing and the testing itself must be carried out in accordance with Annex III.
3. Hatching eggs for import into the Community shall bear the name of the third country of origin and the word ‘hatching’ that is more than 3mm high in one of the official languages of the Community.
4. Each package of hatching eggs as referred to in point 3 shall contain only eggs of a single species, category and type of poultry from the same third country, territory, zone or compartment of origin and consignor, and shall bear at least the following particulars:
(a) the information shown on the eggs as provided for in point 3;
(b) the species of poultry from which the eggs come;
(c) the consignor's name or business name and address.
5. Each box of imported day-old chicks shall contain only a single species, category and type of poultry from the same third country, territory, zone or compartment of origin, hatchery and consignor and shall bear at least the following particulars:
(a) the name of the third country, territory, zone or compartment of origin;
(b) the species of poultry to which the day-old chicks belong;
(c) the distinguishing number of the hatchery;
(d) the consignor's name or business name and address.
II. Requirements applicable after imports
1. Imported breeding and productive poultry other than ratites and day-old chicks other than of ratites shall be kept on the establishment(s) of destination from their date of arrival:
(a) for a period of at least six weeks; or
(b) where the birds are slaughtered before the expiry of the period referred to in (a), until the day of slaughter.
However, the period provided for in (a) may be reduced to three weeks, provided that sampling and testing in accordance with Annex III have been carried out with favourable results.
2. Breeding and productive poultry other than ratites which have been hatched from imported hatching eggs shall be kept for at least three weeks from the date of hatching in the hatchery or for at least three weeks on the establishment(s) to which the poultry has been sent after hatching.
Where day-old chicks are not reared in the Member State which imported the hatching eggs, they shall be transported directly to the final destination (as specified in points 1.10 and 1.11 of the health certificate, Model 2 in Annex IV to Directive 90/539/EEC) and kept there for at least three weeks from the date of hatching.
3. During the relevant periods, as referred to in points 1 and 2, imported breeding and productive poultry and day-old chicks and breeding and productive poultry other than ratites which have hatched from imported hatching eggs shall be kept in isolation in poultry houses where no other flocks are present.
However, they may be introduced into poultry houses where breeding and productive poultry and day-old chicks are already present.
In that case, the relevant periods referred to in points 1 and 2 shall commence from the date of introduction of the last imported bird and no poultry present shall be moved from the poultry houses before the end of those periods.
4. Imported hatching eggs shall be hatched in separate incubators and hatchers.
However, imported hatching eggs may be introduced into incubators and hatchers where other hatching eggs are already present.
In that case, the periods referred to in points 1 and 2 shall commence from the date of introduction of the last imported hatching egg.
5. No later than the date of expiry of the relevant periods as provided for in point 1 or 2, imported breeding and productive poultry and day-old chicks shall undergo a clinical examination carried out by the official veterinarian and, where necessary, samples shall be taken to monitor their state of health.
ANNEX IX
(as referred to in Article 14(1)(b))
RATITES FOR BREEDING AND PRODUCTION, HATCHING EGGS AND DAY-OLD CHICKS THEREOF
I. Requirements applicable before import
1. Imported ratites for breeding and production (ratites) shall be identified by neck-tags and/or microchips bearing the ISO code of the third country of origin. Such microchips must comply with ISO standards.
2. Imported hatching eggs of ratites shall be marked with a stamp indicating the ISO code of the third country of origin and the approval number of the establishment of origin.
3. Each package of hatching eggs as referred to in point 2 shall contain only eggs of ratites from the same third country, territory, zone or compartment of origin and consignor, and shall bear at least the following particulars:
(a) the information shown on the eggs as provided for in point 2;
(b) a clearly visible and legible indication that the consignment contains hatching eggs of ratites;
(c) the consignor’s name or business name and address.
4. Each box of imported day-old chicks of ratites for breeding and production shall contain only ratites from the same third country, territory, zone or compartment of origin, establishment and consignor, and shall bear at least the following particulars:
(a) the ISO code of the third country of origin and the approval number of the establishment of origin;
(b) a clearly visible and legible indication that the consignment contains day-old chicks of ratites;
(c) the consignor’s name or business name and address.
II. Requirements applicable after import
1. After the import controls have been carried out in accordance with Directive 91/496/EEC, consignments of ratites and hatching eggs and day-old chicks thereof shall be transported directly to the final destination.
2. Imported ratites and day-old chicks thereof shall be kept on the establishment(s) of destination from their date of arrival:
(a) for a period at least six weeks; or
(b) where the birds are slaughtered before the expiry of the period referred to in (a), until the day of slaughter.
3. Ratites which have hatched from imported hatching eggs shall be kept for a period of at least three weeks from the date of hatching in the hatchery or for at least three weeks on the establishment(s) to which they have been sent after hatching.
Where day-old chicks of ratites are not reared in the Member State which imported the hatching eggs, they shall be transported directly to the final destination (as specified in points I.10 and I.11 of the health certificate, Model 2, in Annex IV to Council Directive 2009/158/EC ( 27 )) and kept there for at least three weeks from the date of hatching.
4. During the relevant periods as referred to in points 2 and 3, imported ratites and ratites which have hatched from imported hatching eggs shall be kept in isolation in poultry houses where no other ratites or poultry are present.
However, they may be introduced into poultry houses where other ratites or poultry are already present. In that case, the periods referred to in points 2 and 3 shall commence from the date of introduction of the last imported ratite and no ratites or poultry present shall be moved from the poultry housing before the end of those periods.
5. Imported hatching eggs shall be hatched in separate incubators and hatchers.
However, imported hatching eggs may be introduced into incubators and hatchers where other hatching eggs are already present. In that case, the periods referred to in points 2 and 3 shall commence from the date of introduction of the last imported hatching egg and the measures as provided for in those points shall apply.
6. No later than the date of expiry of the relevant periods as referred to in point 2 or 3, imported ratites and day-old chicks thereof shall undergo a clinical examination carried out by an official veterinarian and, where necessary, samples shall be taken to monitor their state of health.
III. Requirements for ratites for breeding and production and day-old chicks thereof from Asia and Africa applicable on their import into the Community
The protective measures for Crimean-Congo haemorrhagic fever set out in Part I of Annex X shall apply to ratites for breeding and production and day-old chicks thereof coming from third countries, territories, zones or compartments in Asia and Africa.
All ratites testing positive to the competitive ELISA test for antibodies to Crimean Congo haemorrhagic fever provided for therein shall be destroyed.
All birds of the same consignment shall be retested by the competitive ELISA test 21 days after the date of the original sampling. Where any bird tests positive all birds in the same consignment shall be destroyed.
IV. Requirements for ratites for breeding and production from a third country, territory or zone considered to be infected with Newcastle disease
The following rules shall apply to ratites and hatching eggs thereof coming from a third country, territory or zone considered as infected with Newcastle disease and to day-old chicks that have hatched from such eggs:
(a) before the date the isolation period begins, the competent authority shall check the isolation facilities as referred to in point 4 of Part II of this Annex to verify whether they are satisfactory;
(b) during the relevant periods as referred to in points 2 and 3 of Part II of this Annex a virus isolation test for Newcastle disease shall be carried out on a cloacal swab or faeces sample from each ratite;
(c) where ratites are to be sent to a Member State, the status of which has been established in accordance with Article 12(2) of Directive 90/539/EEC, a serological test shall be carried out on each ratite, in addition to the virus isolation test provided for in point (b) of this Part;
(d) negative results of the tests provided for in points (b) and (c) shall be available before any bird is released from isolation.
ANNEX X
(as referred to in Article 17)
PROTECTIVE MEASURES IN RELATION TO CRIMEAN-CONGO HAEMORRHAGIC FEVER
I. For ratites
The competent authority shall ensure that the ratites are isolated in rodent-proof, tick-free surroundings for at least 21 days prior to the date of import into the Community.
Before moving to the tick-free surroundings, the ratites shall be treated to ensure that all ectoparasites on them are destroyed. After 14 days in tick-free surroundings, the ratites shall undergo the competitive ELISA test for antibodies to Crimean-Congo haemorrhagic fever. Every animal put into isolation must test negative to the test. On the ratites' arrival in the Community, the treatment for ectoparasites and the serological test shall be repeated.
II. For ratites from which meat for import is derived
The competent authority shall ensure that the ratites are isolated in rodent-proof, tick-free surroundings for at least 14 days prior to the date of slaughter.
Before moving to the tick-free surroundings, the ratites shall either be examined to verify that they are tick-free or treated to ensure that all ticks on them are destroyed. The treatment used must be specified on the import certificate. Any treatment used shall not result in any detectable residues in the ratite meat.
Each batch of ratites shall be examined for ticks prior to slaughter. If any ticks are detected, the entire batch shall again be put into pre-slaughter isolation.
ANNEX XI
(as referred to in Article 18(2))
Model veterinary certificate for transit/storage of specified pathogen-free eggs, meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds, eggs and egg products

![Part II: CertificationCOUNTRYTransit/storage of specified pathogen-free eggs, meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds, eggs and egg productsII. Health informationII.a. Certificate reference numberII.b.II.1. Health attestationI, the undersigned official veterinarian, hereby certify that specified pathogen-free eggs, the meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds, eggs and egg products (1) described in this certificate:II.1.1 come from a third country, territory, zone or compartment appearing in Part 1 of Annex I to Regulation (EC) No 798/2008, and(2) II.1.2 complies (comply) with the relevant animal health conditions laid down in the animal health attestation in the model certificates in Annex I to Regulation (EC) No 798/2008.NotesPart I:Box I.8: provide the code for the zone or the compartment of origin, if necessary, as defined under code of column 2 of Part 1 of Annex I to Regulation (EC) No 798/2008.Box I.11: Name, address and approval number of the establishment of dispatch.Box I.15: Indicate the registration number(s) of railway wagons and lorries, the names of ships and, if known, the flight numbers of aircraft. In the case of transport in containers or boxes, the total number of these and their registration and where there is a serial number of the seal it has to be indicated in box I.23.Box I.19: use the appropriate Harmonised System (HS) code of the World Customs Organisation: 02.07; 02.08.90; 04.07; 04.08 or 21.06.10Part II(1) Specified pathogen-free eggs, meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds, eggs and egg products as laid down in Part 1 of Annex I to Regulation (EC) No 798/2008.(2) In the case of specified pathogen-free eggs [SPF], meat of poultry [POU], meat of ratites [RAT], wild game-bird meat [WGM], minced meat and mechanically separated meat of poultry [POU-MI/MSM], minced meat and mechanically separated meat of ratites [RAT-MI/MSM], wild game-bird minced meat and mechanically separated meat [WGM-MI/MSM], eggs [E] or egg products [EP].Official veterinarianName (in capital letters):Qualification and title:Date:Signature:Stamp:](./../../../resource.html?uri=celex:02008R0798-20130326.ENG.xhtml.L_2009124EN.01002001.tif.jpg)
ANNEX XII
(as referred to in Article 20)
CORRELATION TABLE
|
This Regulation |
Decision 2006/696/EC |
Decision 94/438/EC |
Decision 93/342/EEC |
|
Article 1(1) first subparagraph |
Article 1 first subparagraph |
||
|
Article 1(1) second subparagraph |
Article 5 |
||
|
Article 1(2) |
Article 1 second subparagraph |
||
|
Article 1(3) |
Annex I and II (part1) |
||
|
Article 2 (1-5) |
Article 2 (a-e) |
||
|
Article 2 (6) |
Article 2 (m) |
||
|
Article 2 (7) |
Article 2 (j) |
||
|
Article 2 (8) |
Article 2 (k) |
||
|
Article 2 (9) |
Article 2 (l) |
||
|
Article 2 (10) |
|||
|
Article 2 (11) |
|||
|
Article 2 (12) (a-c) |
Article 2 (g) |
||
|
Article 2 (12) (d) |
|||
|
Article 2 (13) |
Article 2 (h) |
||
|
Article 2 (14) |
Article 2 (f) |
||
|
Article 2 (15) |
|||
|
Article 2 (16) |
|||
|
Article 2 (17) |
|||
|
Article 2 (18) |
|||
|
Article 2 (19) |
|||
|
Article 2 (20) |
|||
|
Article 3 |
Article 5 |
||
|
Article 4 first subparagraph |
Article 5 and 3 |
||
|
Article 4 second subparagraph |
Annex I part 3 |
||
|
Article 4 third subparagraph |
Article 3 second subparagraph |
||
|
Article 5 |
Article 4 |
||
|
Article 6 |
|||
|
Article 7 (a) |
Article 2 (h) |
||
|
Article 7 (b) |
Article 2 (g) |
||
|
Article 7 (c) |
Article 2 (i) |
||
|
Article 8 |
|||
|
Article 9 |
|||
|
Article 10 |
|||
|
Article 11 |
|||
|
Article 12 |
Article 4 (1) (2) |
Article 4 (1) (2) |
|
|
Article 13 |
Article 4 (3) |
Article 4 (4) |
|
|
Article 14 (1) (a) |
Article 9 |
||
|
Article 14 (1) (b) |
Article 11 |
||
|
Article 14 (2) |
|||
|
Article 15 |
Article 18 |
||
|
Article 16 |
Article 8 |
||
|
Article 17 |
Article 16 (2) |
||
|
Article 18 (1) |
|||
|
Article 18 (2) |
Article 19 (b) |
||
|
Article 18 (3) |
Article 19 |
||
|
Article 19 |
Article 20 |
||
|
Article 20 |
|||
|
Article 21 |
|||
|
Article 22 |
|||
|
Annex I |
Annex I and II |
||
|
Annex II |
Annex I part 3 |
||
|
Annex III (I) (1-6) |
Annex I part 4 (A) |
||
|
Annex III (I) (7) |
|||
|
Annex III (II), (III) |
Annex I part 4 (B) |
||
|
Annex IV |
|||
|
Annex V |
|||
|
Annex VI |
Annex B |
||
|
Annex VII (I) |
Article 7 |
||
|
Annex VII (II) |
Annex |
||
|
Annex VIII (I) |
Article 9 |
||
|
Annex VIII (II) |
Article 10 |
||
|
Annex IX (I) |
Article 11 |
||
|
Annex IX (II) |
Article 12 |
||
|
Annex IX (III) |
Article 13 |
||
|
Annex IX (IV) |
Article 14 |
||
|
Annex X |
Annex V |
||
|
Annex XI |
Annex IV |
||
|
Annex XII |
( 1 ) OJ L 303, 31.10.1990, p. 6. Directive as last amended by Commission Decision 2007/729/EC (OJ L 294, 13.11.2007, p. 26).
( 2 ) OJ L 268, 24.9.1991, p. 56. Directive as last amended by Directive 2006/104/EC (OJ L 363, 20.12.2006, p. 352).
( 3 ) OJ L 125, 23.5.1996, p. 10. Directive as last amended by Directive 2006/104/EC.
( 4 ) OJ L 24, 30.1.1998, p. 9. Directive as last amended by Directive 2006/104/EC.
( 5 ) OJ L 18, 23.1.2003, p. 11.
( 6 ) OJ L 325, 12.12.2003, p. 1. Regulation as last amended by Commission Regulation (EC) No 1237/2007 (OJ L 280, 24.10.2007, p. 5).
( 7 ) OJ L 139, 30.4.2004, p. 55; corrected by OJ L 226, 25.6.2004, p. 22. Regulation as last amended by Commission Regulation (EC) No 1243/2007 (OJ L 281, 25.10.2007, p. 8).
( 8 ) OJ L 139, 30.4.2004, p. 206; corrected by OJ L 226, 25.6.2004, p. 83. Regulation as last amended by Council Regulation (EC) No 1791/2006 (OJ L 363, 20.12.2006, p. 1).
( 9 ) OJ L 295, 25.10.2006, p. 1. Decision as last amended by Regulation (EC) No 1237/2007.
( 10 ) OJ L 137, 8.6.1993, p. 24. Decision as last amended by Decision 2006/696/EC (OJ L 295, 25.10.2006, p. 1).
( 11 ) OJ L 181, 15.7.1994, p. 35; corrected by OJ L 187, 26.5.2004, p. 8.
( 12 ) OJ L 10, 14.1.2006, p. 16.
( 13 ) http://www.oie.int/eng/normes/mcode/en_sommaire.htm (latest edition).
( 14 ) http://www.oie.int/eng/normes/en_mmanual.htm?e1d10 (latest edition).
( 15 ) OJ L 299, 16.11.2007, p. 1. Regulation as last amended by Commission Regulation (EC) No 510/2008 (OJ L 149, 7.6.2008, p. 61).
( 16 ) OJ L 13, 16.1.1997, p. 28.
( 17 ) http://www.edqm.eu (latest edition).
( 18 ) Veterinary Laboratories Agency, New Haw, Weybridge, Surrey KT 153NB, United Kingdom.
( 19 ) http://www.oie.int/eng/normes/mcode/en_sommaire.htm
( 20 ) OJ L 296, 12.11.2009, p. 1.
( 21 ) OJ L 237, 31.8.2006, p. 1.
( 22 ) http://www.oie.int/eng/normes/mmanual/A_summry.htm
( 23 ) OJ L 260, 5.9.1992, p. 1. Directive as last amended by Directive 2006/104/EC (OJ L 363, 20.12.2006, p. 352).
( 24 ) OJ L 188, 8.7.1992, p. 34.
( 25 ) Please give as much detailed information as necessary to allow proper assessment of the programme.
( 26 ) Please give as much detailed information as necessary to allow proper assessment of the programme.
( 27 ) OJ L 343, 22.12.2009, p. 74.


