EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document 02007D0777-20101105
Commission Decision of 29 November 2007 laying down the animal and public health conditions and model certificates for imports of certain meat products and treated stomachs, bladders and intestines for human consumption from third countries and repealing Decision 2005/432/EC (notified under document number C(2007) 5777) (Text with EEA relevance) (2007/777/EC)
Consolidated text: Commission Decision of 29 November 2007 laying down the animal and public health conditions and model certificates for imports of certain meat products and treated stomachs, bladders and intestines for human consumption from third countries and repealing Decision 2005/432/EC (notified under document number C(2007) 5777) (Text with EEA relevance) (2007/777/EC)
Commission Decision of 29 November 2007 laying down the animal and public health conditions and model certificates for imports of certain meat products and treated stomachs, bladders and intestines for human consumption from third countries and repealing Decision 2005/432/EC (notified under document number C(2007) 5777) (Text with EEA relevance) (2007/777/EC)
2007D0777 — EN — 05.11.2010 — 004.001
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
COMMISSION DECISION of 29 November 2007 laying down the animal and public health conditions and model certificates for imports of certain meat products and treated stomachs, bladders and intestines for human consumption from third countries and repealing Decision 2005/432/EC (notified under document number C(2007) 5777) (Text with EEA relevance) (OJ L 312, 30.11.2007, p.49) |
Amended by:
|
|
|
Official Journal |
||
|
No |
page |
date |
||
|
L 207 |
24 |
5.8.2008 |
||
|
L 283 |
49 |
28.10.2008 |
||
|
L 314 |
97 |
1.12.2009 |
||
|
L 272 |
1 |
16.10.2010 |
||
Corrected by:
COMMISSION DECISION
of 29 November 2007
laying down the animal and public health conditions and model certificates for imports of certain meat products and treated stomachs, bladders and intestines for human consumption from third countries and repealing Decision 2005/432/EC
(notified under document number C(2007) 5777)
(Text with EEA relevance)
(2007/777/EC)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Directive 92/118/EEC of 17 December 1992 laying down animal health and public health requirements governing trade in and imports into the Community of products not subject to the said requirements laid down in specific Community rules referred to in Annex A (I) to Directive 89/662/EEC and, as regards pathogens, to Directive 90/425/EEC ( 1 ), and in particular Article 10(2)(c) thereof,
Having regard to Council Directive 2002/99/EC of 16 December 2002 laying down the animal health rules governing the production, processing, distribution and introduction of products of animal origin for human consumption ( 2 ), and in particular the introductory phrase of Article 8, the first paragraph of point 1 of Article 8, Article 8(4), Article 9(2)(b) and Article 9(4)(b) and (c) thereof,
Whereas:|
(1) |
Commission Decision 2005/432/EC of 3 June 2005 laying down the animal and public health conditions and model certificates for imports of meat products for human consumption from third countries and repealing Decisions 97/41/EC, 97/221/EC and 97/222/EC ( 3 ) lays down the animal and public health rules and certification requirements for the importation into the Community of consignments of certain meat products, including the lists of third countries and parts thereof from which imports of such products are authorised. |
|
(2) |
Decision 2005/432/EC, as amended by Commission Decision 2006/801/EC ( 4 ), takes into account the health requirements and definitions laid down in Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs ( 5 ), Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin ( 6 ) and Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption ( 7 ). |
|
(3) |
Annex I to Regulation (EC) No 853/2004 lays down separate definitions for meat products and for treated stomachs, bladders and intestines. |
|
(4) |
The specific treatments laid down for each third country by Decision 2005/432/EC are established basing on the treatments laid down by Directive 2002/99/EC in order to eliminate the potential animal health risk carried by the fresh meat used in the preparation of the meat products. From the animal health point of view, treated stomachs, bladders and intestines present the same animal health risk of the meat products. Therefore, they should be treated with the same specific treatments as provided for in Decision 2005/432/EC and consequently submitted to the harmonised veterinary certification for their import into the Community. |
|
(5) |
Animal health requirements for importation into the EU of casings are laid down in Decision 2003/779/EC ( 8 ). Therefore, the products covered by Decision 2003/779/EC should be excluded by the definition of meat products and treated stomachs, bladders and intestines laid down in this Decision. |
|
(6) |
Commission Decision 2004/432/EC of 29 April 2004 on the approval of residue monitoring plans submitted by third countries in accordance with Council Directive 96/23/EC ( 9 ) lists the third countries authorised to export to the Community on the basis of their approved residue monitoring plans. |
|
(7) |
Council Directive 97/78/EC ( 10 ) of 18 December 1997 laying down the principles governing the organisation of veterinary checks on products entering the Community from third countries lays down rules concerning veterinary checks on animal products introduced into the Community from third countries for the importation and transit of products of animal origin in the Community, including certain certification requirements. |
|
(8) |
It is necessary to lay down specific conditions for transit via the Community of consignments of meat products to and from Russia due to the geographical situation of Kaliningrad and taking into account climatic problems impeding the use of some ports at certain times of the year. |
|
(9) |
Commission Decision 2001/881/EC ( 11 ) of 7 December 2001 drawing up a list of border inspection posts agreed for veterinary checks on animals and animal products from third countries and updating the detailed rules concerning the checks to be carried out by the experts of the Commission specifies the Border Inspection Posts authorised to control the transit of consignments of meat products to and from Russia via the Community. |
|
(10) |
Annex II to Council Decision 79/542/EEC of 21 December 1976 drawing up a list of third countries or parts of third countries, and laying down animal and public health and veterinary certification conditions, for importation into the Community of certain live animals and their fresh meat ( 12 ), establishes the list of third countries or parts thereof from which imports of fresh meat of certain animals are authorised. Iceland is listed in Annex II to that Decision as a country authorised to export fresh meat of certain animals. Therefore, import of meat products and treated stomachs, bladders and intestines of those animals from Iceland should be allowed without the application of any specific treatment. |
|
(11) |
Annex 11 to the Agreement between the European Community and the Swiss Confederation on trade in agricultural products ( 13 ) lays down the animal health, public health and zootechnical measures applicable to trade in live animals and animal products. Treatments applicable to meat products and treated stomachs, bladders and intestines from the Swiss Confederation should be in accordance with that agreement. Therefore, it is not necessary to set out these treatments in the Annex to this Decision. |
|
(12) |
Annex IX to Regulation (EC) No 999/2001 of the European Parliament and the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmisible spongiform encephalopaties ( 14 ) has been amended by Regulation (EC) No 722/2007 of the Commission of 25 June 2007 amending Annexes II, V, VI, VIII, IX and XI to Regulation (EC) No 999/2001 ( 15 ) and by Regulation (EC) No 1275/2007 ( 16 ) amending Annex IX to Regulation (EC) No 999/2001 of the European Parliament and of the Council laying down rules for the prevention, control and eradication of certain transmisissible encephalopathies. New requirements with regard to the BSE status of third countries to export meat products and treated intestines to the Community should be included in the certificate. |
|
(13) |
Commission Decision 2007/453/EC of 29 June 2007 establishing the BSE status of Member States or third countries or regions thereof according to their BSE risk ( 17 ) lists countries or regions in three groups: negligible BSE risk, controlled BSE risk and undetermined BSE risk. A reference to that list should be made in the certificate. |
|
(14) |
In the interest of clarity of Community legislation, it is appropriate to repeal Decision 2005/432/EC and replace it by the present Decision. |
|
(15) |
The measures provided for in this Decision are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS DECISION:
Article 1
Subject matter and scope
1. This Decision lays down animal and public health rules for imports into the Community and the transit and storage in the Community, of consignments of:
(a) meat products, as defined in point 7.1 of Annex I to Regulation (EC) No 853/2004; and
(b) treated stomachs, bladders and intestines, as defined in point 7.9 of that Annex, which have undergone one of the treatments laid down in Annex II part 4 to this Decision.
Those rules shall include the lists of third countries and parts thereof from which such imports shall be authorised and the model public and animal health certificates and rules on the origin and treatments required for those imports.
2. This Decision shall apply without prejudice to Decision 2004/432/EC and Decision 2003/779/EC.
Article 2
Conditions concerning species and animals
Member States shall ensure that only consignments of meat products and treated stomachs, bladders and intestines, derived from meat or meat products from the following species or animals, are imported into the Community:
(a) poultry including fowl, turkeys, guinea fowl, ducks, geese, quails, pigeons, pheasants and partridges reared or kept in captivity for breeding, the production of meat or eggs for consumption or for restocking supplies of game;
(b) domestic animals of the following species: bovine animals, including Bubalus bubalis and Bison bison, swine, sheep, goats and solipeds;
(c) rabbits and hares, and farmed game, as defined in point 1.6 of Annex I to Regulation (EC) No 853/2004;
(d) wild game, as defined in point 1.5 of Annex I to Regulation (EC) No 853/2004.
Article 3
Animal health requirements concerning the origin and treatment of the meat products and treated stomachs, bladders and intestines
Member States shall authorise imports into the Community of meat products and treated stomachs, bladders and intestines that:
(a) comply with the conditions concerning origin and treatment set out in Annex I(1) or (2); and
(b) originate in the following third countries and parts thereof:
(i) in the case of meat products and treated stomachs, bladders and intestines not subject to a specific treatment as referred to in point 1(b) of Annex I, the third countries listed in Part 2 of Annex II and the parts thereof listed in Part 1 of that Annex;
(ii) in the case of meat products and treated stomachs, bladders and intestines subject to a specific treatment as referred to in point 2(a)(ii) of Annex I, the third countries listed in Parts 2 and 3 of Annex II and the parts thereof listed in Part 1 of that Annex.
Article 4
Public health requirements concerning the fresh meat used in the production of the meat products and treated stomachs, bladders and intestines to be imported into the Community and animal and public health certificates
Member States shall ensure that:
(a) only consignments of meat products and treated stomachs, bladders and intestines that are obtained from fresh meat, as defined in point 1.10 of Annex I to Regulation (EC) No 853/2004 that complies with the Community public health requirements, are imported into the Community;
(b) only consignments of meat products and treated stomachs, bladders and intestines complying with the requirements of the model public and animal health certificate set out in Annex III are imported into the Community;
(c) that certificate accompanies such consignments and is duly completed and signed by the official veterinarian of the third country of dispatch.
Article 5
Consignments of meat products and treated stomachs, bladders and intestines in transit or storage in the Community
Member States shall ensure that consignments of meat products and treated stomachs, bladders and intestines, introduced into the Community and which are destined for a third country either by transit immediately or following storage, in accordance with Article 12(4) or Article 13 of Directive 97/78/EC, and not intended for importation into the Community, shall comply with the following requirements:
(a) they come from the territory of a third country or a part thereof listed in Annex II and have undergone the minimum treatment for the import of meat products and treated stomachs, bladders and intestines of the species provided for therein;
(b) they comply with the specific animal health conditions for the species concerned set out in the model animal and public health certificate in Annex III;
(c) they are accompanied by an animal health certificate drawn up in accordance with the model set out in Annex IV, duly signed by an official veterinarian of the third country concerned;
(d) they are certified as acceptable for transit or storage, as appropriate, on the common veterinary entry document by the official veterinarian of the border inspection post of introduction into the Community.
Article 6
Derogation for certain destinations in Russia
1. By way of derogation from Article 5, Member States shall authorise the transit by road or by rail through the Community, between designated Community border inspection posts listed in the Annex to Decision 2001/881/EC, of consignments of meat products and treated stomachs, bladders and intestines coming from and destined to Russia directly or via another third country provided that they comply with the following requirements:
(a) the consignment shall be sealed with a serially numbered seal by the official veterinarian of the competent authority of the border inspection post of introduction to the Community;
(b) the documents accompanying the consignment and referred to in Article 7 of Directive 97/78/EC shall be stamped ‘ONLY FOR TRANSIT TO RUSSIA VIA THE EC’ on each page by the official veterinarian of the competent authority of the border inspection post of introduction to the Community;
(c) the procedural requirements provided for in Article 11 of Directive 97/78/EC shall be complied with;
(d) the consignment shall be certified as acceptable for transit on the common veterinary entry document by the official veterinarian of the competent authority of the border inspection post of introduction to the Community.
2. Member States shall not authorise the unloading or storage, as defined in Article 12(4) or Article 13 of Directive 97/78/EC, in the Community of such consignments.
3. Member States shall ensure that the competent authority makes regular audits to ensure that the number of consignments and the quantities of meat products and treated stomachs, bladders and intestines, coming from or destined to Russia, leaving the Community matches the number and quantities entering the Community.
Article 7
Transitional provision
Consignments for which veterinary certificates were issued before 1 May 2008 in accordance with the models established by Decision 2005/432/EC shall be accepted for import into the Community until 1 June 2008.
Article 8
Repeal
Decision 2005/432/EC is repealed.
Article 9
Date of application
This Decision shall apply from 1 December 2007.
Article 10
Addresses
This Decision is addressed to the Member States.
ANNEX I
1. Meat products and treated stomachs, bladders and intestines originating in the third countries or parts thereof referred to in Article 3(b)(i) of this Decision shall:
(a) contain meat eligible for import into the Community as fresh meat, as defined in point 1.10 of Annex I to Regulation (EC) No 853/2004; and
(b) be derived from one or more of the species or animals which have undergone a non-specific treatment as set out in point A Part 4 of Annex II to this Decision.
2. Meat products and treated stomachs, bladders and intestines originating in the third countries or parts thereof, as referred to in Article 3(b)(ii), shall comply with the conditions set out in (a), (b) or (c) of this point:
(a) the meat products and/or treated stomachs, bladders and intestines must:
(i) contain meat and/or meat products derived from a single species or animal, as set out under the relevant column in Parts 2 and 3 of Annex II indicating the species or animal concerned; and
(ii) have undergone at least the specific treatment required for meat of that species or animal, as set out in Part 4 of Annex II;
(b) the meat products and/or treated stomachs, bladders and intestines must:
(i) contain fresh, processed or partly processed meat of more than one species or animal, as set out under the relevant column of Parts 2 and 3 of Annex II which are mixed prior to undergoing their final treatment, as set out in Part 4 of Annex II; and
(ii) have undergone the final treatment referred to in (i) that must be at least as severe as the most severe treatment set out in Part 4 of Annex II for meat of the species or animals concerned, as set out under the relevant column in Parts 2 and 3 of Annex II;
(c) the final meat products and/or treated stomachs, bladders and intestines must:
(i) be prepared by mixing previously treated meat or treated stomachs, bladders and intestines of more than one species or animal; and
(ii) have undergone the previous treatment referred to in (i) that must have been at least as severe as the relevant treatment set out in Part 4 of Annex II for the species or animal concerned as set out under the relevant column in Parts 2 and 3 of Annex II for each meat component of the meat product and treated stomachs, bladders and intestines.
3. The treatments set out in Part 4 of Annex II shall constitute the minimum acceptable processing conditions for animal health purposes for meat products and stomachs, bladders and intestines derived from the relevant species or animal originating in the third countries or parts thereof listed in Annex II.
However, in cases where import of offal is not authorised under Decision 79/542/EEC owing to Community animal health restrictions, it may be imported as a meat product or treated stomach, intestine or bladder or used in a meat product provided the relevant treatment referred to in Part 2 of Annex II is carried out and the Community public health requirements are fulfilled.
In addition, an establishment from a country listed in Annex II may be authorised to produce meat products and treated stomachs, bladders and intestines that have undergone treatments B, C or D, as referred to in Part 4 of Annex II, even where that establishment is located in a third country or part thereof that is not authorised for imports into the Community of fresh meat under the condition that the Community public health requirements are fulfilled.
ANNEX II
PART 1
Regionalised territories for the countries listed in parts 2 and 3
|
Country |
Territory |
Description of territory |
|
|
ISO code |
Version |
||
|
Argentina |
AR |
01/2004 |
Whole country |
|
AR-1 |
01/2004 |
The whole country, except the Provinces of Chubut, Santa Cruz and Tierra del Fuego for the species covered by Decision 79/542/EEC (as last amended) |
|
|
AR-2 |
01/2004 |
The Provinces of Chubut, Santa Cruz and Tierra del Fuego for the species covered by Decision 79/542/EEC (as last amended) |
|
|
Brazil |
BR |
01/2004 |
Whole country |
|
BR-1 |
01/2005 |
States of Rio Grande do Sul, Santa Catarina, Paraná, São Paulo and Mato Grosso do Sul |
|
|
BR-2 |
01/2005 |
Part of the State of Mato Grosso do Sul (except for the municipalities of Sonora, Aquidauana, Bodoqueno, Bonito, Caracol, Coxim, Jardim, Ladario, Miranda, Pedro Gomes, Porto Murtinho, Rio Negro, Rio Verde of Mato Grosso and Corumbá); State of Paraná; State of Sao Paulo; Part of the State of Minas Gerais (except the regional delegations of Oliveira, Passos, São Gonçalo de Sapucai, Setelagoas and Bambuí); State of Espíritu Santo; State of Rio Grande do Sul; State of Santa Catarina; State of Goias; Part of the State of Mato Grosso comprising: the regional unit of Cuiaba (except for the municipalities of San Antonio de Leverger, Nossa Senhora do Livramento, Pocone and Barão de Melgaço); the regional unit of Caceres (except for the municipality of Caceres); the regional unit of Lucas do Rio Verde; the regional unit of Rondonopolis (except for the municipality of Itiquiora); the regional unit of Barra do Garça and the regional unit of Barra do Burgres. |
|
|
BR-3 |
01/2005 |
States of Goiás, Minas Gerais, Mato Grosso, Mato Grosso do Sul, Paraná, Rio Grande do Sul, Santa Catarina and São Paulo |
|
|
China |
CN |
01/2007 |
Whole country |
|
CN-1 |
01/2007 |
Province of Shandong |
|
|
Malaysia |
MY |
01/2004 |
Whole country |
|
MY-1 |
01/2004 |
Peninsular (Western) Malaysia only |
|
|
Namibia |
NA |
01/2005 |
Whole country |
|
NA-1 |
01/2005 |
South of the cordon fences which extend from Palgrave Point in the west to Gam in the east |
|
|
South Africa |
ZA |
01/2005 |
Whole country |
|
ZA-1 |
01/2005 |
The whole country except: the part of the foot-and-mouth disease control area situated in the veterinary regions of Mpumalanga and Northern provinces, the district of Ingwavuma in the veterinary region of Natal and in the border area with Botswana east of longitude 28°, and the district of Camperdown in the province of KwaZuluNatal. |
|
PART 2
Third countries or parts thereof from which the introduction of meat products and treated stomachs, bladders and intestines into the Union is authorised
(See Part 4 of this Annex for the interpretation of codes used in the table)
|
ISO code |
Country of origin or part thereof |
1. Domestic bovine 2. Farmed cloven-hoofed game-(excluding swine) |
Domestic ovine/ caprine |
1. Domestic porcine 2. Farmed cloven-hoofed game (swine) |
Domestic soliped |
1. Poultry 2. Farmed feathered game (except ratites) |
Farmed ratites |
Domestic rabbit and farmed leporidae |
Wild cloven-hoofed game (excluding swine) |
Wild swine |
Wild soliped |
Wild leporidae (rabbits and hares) |
Wild game birds |
Wild land mamma-lian game (excluding ungulates, solipeds and leporidae) |
|
AR |
Argentina AR |
C |
C |
C |
A |
A |
A |
A |
C |
C |
XXX |
A |
D |
XXX |
|
Argentina AR-1 (1) |
C |
C |
C |
A |
A |
A |
A |
C |
C |
XXX |
A |
D |
XXX |
|
|
Argentina AR-2 (1) |
A (2) |
A (2) |
C |
A |
A |
A |
A |
C |
C |
XXX |
A |
D |
XXX |
|
|
AU |
Australia |
A |
A |
A |
A |
D |
D |
A |
A |
A |
XXX |
A |
D |
A |
|
BH |
Bahrain |
B |
B |
B |
B |
XXX |
XXX |
A |
C |
C |
XXX |
A |
XXX |
XXX |
|
BR |
Brazil |
XXX |
XXX |
XXX |
A |
D |
D |
A |
XXX |
XXX |
XXX |
A |
D |
XXX |
|
Brazil BR-1 |
XXX |
XXX |
XXX |
A |
XXX |
A |
A |
XXX |
XXX |
XXX |
A |
A |
XXX |
|
|
Brazil BR-2 |
C |
C |
C |
A |
D |
D |
A |
C |
XXX |
XXX |
A |
D |
XXX |
|
|
Brazil BR-3 |
XXX |
XXX |
XXX |
A |
A |
XXX |
A |
XXX |
XXX |
XXX |
A |
D |
XXX |
|
|
BW |
Botswana |
B |
B |
B |
B |
XXX |
A |
A |
B |
B |
A |
A |
XXX |
XXX |
|
BY |
Belarus |
C |
C |
C |
B |
XXX |
XXX |
A |
C |
C |
XXX |
A |
XXX |
XXX |
|
CA |
Canada |
A |
A |
A |
A |
A |
A |
A |
A |
A |
XXX |
A |
A |
A |
|
CH |
Switzerland (4) |
|||||||||||||
|
CL |
Chile |
A |
A |
A |
A |
A |
A |
A |
B |
B |
XXX |
A |
A |
XXX |
|
CN |
China |
B |
B |
B |
B |
B |
B |
A |
B |
B |
XXX |
A |
B |
XXX |
|
China CN-1 |
B |
B |
B |
B |
D |
B |
A |
B |
B |
XXX |
A |
B |
XXX |
|
|
CO |
Colombia |
B |
B |
B |
B |
XXX |
A |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
ET |
Ethiopia |
B |
B |
B |
B |
XXX |
XXX |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
GL |
Greenland |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
A |
XXX |
XXX |
XXX |
A |
A |
A |
|
HK |
Hong Kong |
B |
B |
B |
B |
D |
D |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
HR |
Croatia |
A |
A |
D |
A |
A |
A |
A |
A |
D |
XXX |
A |
A |
XXX |
|
IL |
Israel |
B |
B |
B |
B |
A |
A |
A |
B |
B |
XXX |
A |
A |
XXX |
|
IN |
India |
B |
B |
B |
B |
XXX |
XXX |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
IS |
Iceland |
A |
A |
B |
A |
A |
A |
A |
A |
B |
XXX |
A |
A |
XXX |
|
KE |
Kenya |
B |
B |
B |
B |
XXX |
XXX |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
KR |
South Korea |
XXX |
XXX |
XXX |
XXX |
D |
D |
A |
XXX |
XXX |
XXX |
A |
D |
XXX |
|
MA |
Morocco |
B |
B |
B |
B |
XXX |
XXX |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
ME |
Montenegro |
A |
A |
D |
A |
D |
D |
A |
D |
D |
XXX |
A |
XXX |
XXX |
|
MG |
Madagascar |
B |
B |
B |
B |
D |
D |
A |
B |
B |
XXX |
A |
D |
XXX |
|
MK |
former Yugoslav Republic of Macedonia (5) |
A |
A |
B |
A |
XXX |
XXX |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
MU |
Mauritius |
B |
B |
B |
B |
XXX |
XXX |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
MX |
Mexico |
A |
D |
D |
A |
D |
D |
A |
D |
D |
XXX |
A |
D |
XXX |
|
MY |
Malaysia MY |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
|
Malaysia MY-1 |
XXX |
XXX |
XXX |
XXX |
D |
D |
A |
XXX |
XXX |
XXX |
A |
D |
XXX |
|
|
NA |
Namibia (1) |
B |
B |
B |
B |
D |
A |
A |
B |
B |
A |
A |
D |
XXX |
|
NC |
New Caledonia |
A |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
A |
XXX |
XXX |
XXX |
XXX |
XXX |
|
NZ |
New Zealand |
A |
A |
A |
A |
A |
A |
A |
A |
A |
XXX |
A |
A |
A |
|
PY |
Paraguay |
C |
C |
C |
B |
XXX |
XXX |
A |
C |
C |
XXX |
A |
XXX |
XXX |
|
RS |
Serbia (6) |
A |
A |
D |
A |
D |
D |
A |
D |
D |
XXX |
A |
XXX |
XXX |
|
RU |
Russia |
C |
C |
C |
B |
A (3) |
XXX |
A |
C |
C |
XXX |
A |
XXX |
A |
|
SG |
Singapore |
B |
B |
B |
B |
D |
D |
A |
B |
B |
XXX |
A |
XXX |
XXX |
|
SZ |
Swaziland |
B |
B |
B |
B |
XXX |
XXX |
A |
B |
B |
A |
A |
XXX |
XXX |
|
TH |
Thailand |
B |
B |
B |
B |
A |
A |
A |
B |
B |
XXX |
A |
D |
XXX |
|
TN |
Tunisia |
C |
C |
B |
B |
A |
A |
A |
B |
B |
XXX |
A |
D |
XXX |
|
TR |
Turkey |
XXX |
XXX |
XXX |
XXX |
D |
D |
A |
XXX |
XXX |
XXX |
A |
D |
XXX |
|
UA |
Ukraine |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
A |
XXX |
XXX |
XXX |
A |
XXX |
XXX |
|
US |
United States |
A |
A |
A |
A |
A |
A |
A |
A |
A |
XXX |
A |
A |
XXX |
|
UY |
Uruguay |
C |
C |
B |
A |
D |
A |
A |
XXX |
XXX |
XXX |
A |
D |
XXX |
|
ZA |
South Africa (1) |
C |
C |
C |
A |
D |
A |
A |
C |
C |
A |
A |
D |
XXX |
|
ZW |
Zimbabwe (1) |
C |
C |
B |
A |
D |
A |
A |
B |
B |
XXX |
A |
D |
XXX |
|
(1) See Part 3 of this Annex for the minimum treatment requirements applicable to pasteurised meat products and biltong. (2) For meat products and treated stomachs, bladders and intestines prepared from fresh meat obtained from animals slaughtered after 1 March 2002. (3) Onlly for transit in accordance with Article 5. (4) In accordance with the Agreement between the European Community and the Swiss Confederation on trade in agricultural products. (5) The Former Yugoslav Republic of Macedonia; provisional code that does not prejudge in any way the definitive nomenclature for this country, which will be agreed following the conclusion of negotiations currently taking place on this subject in the United Nations. (6) Not including Kosovo as defined by United Nations Security Council Resolution 1244 of 10 June 1999. XXX No certificate laid down and meat products and treated stomachs, bladders and intestines containing meat of this species are not authorised. |
||||||||||||||
PART 3
Third countries or parts thereof not authorised for certain species under the non-specific treatment regime (A) but from where imports into the Community of biltong/jerky and pasteurised meat products are authorised
|
ISO code |
Country of origin or part thereof |
1. Domestic bovine 2. Farmed cloven-hoofed game (excluding swine) |
Domestic ovine/caprine |
1. Domestic porcine 2. Farmed cloven-hoofed game (swine) |
Domestic soliped |
1. Poultry 2. Farmed feathered game |
Ratites |
Domestic rabbit and farmed leporidae |
Wild cloven-hoofed game (excluding swine) |
Wild swine |
Wild soliped |
Wild leporidae (rabbits and hares) |
Wild game birds |
Wild land mammalian game (excluding ungulates, solipeds and leporidae) |
|
AR |
Argentina — AR |
F |
F |
XXX |
XXX |
XXX |
XXX |
A |
XXX |
XXX |
XXX |
A |
XXX |
XXX |
|
NA |
Namibia |
XXX |
XXX |
XXX |
XXX |
E |
E |
A |
XXX |
XXX |
A |
A |
E |
XXX |
|
Namibia NA-1 |
E |
E |
XXX |
XXX |
E |
E |
A |
XXX |
XXX |
A |
A |
E |
||
|
UY |
Uruguay |
E |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
XXX |
|
ZA |
South Africa |
XXX |
XXX |
XXX |
XXX |
E |
E |
A |
XXX |
XXX |
A |
A |
E |
XXX |
|
South Africa ZA-1 |
E |
E |
XXX |
XXX |
E |
E |
A |
E |
XXX |
A |
A |
E |
||
|
ZW |
Zimbabwe |
XXX |
XXX |
XXX |
XXX |
E |
E |
A |
XXX |
XXX |
E |
A |
E |
XXX |
|
XXX No certificate laid down and imports into the Community of biltong/jerky and pasteurised meat products are not authorised unless the country is authorised in Part 2 for treatment ‘A’ for the relevant species. |
||||||||||||||
PART 4
Interpretation of codes used in tables in parts 2 and 3
TREATMENTS REFERRED TO IN ANNEX I
Non-specific treatment:
|
A |
= |
No minimum specified temperature or other treatment is established for animal health purposes for meat products and treated stomachs, bladders and intestines. However, the meat of such meat products and treated stomachs, bladders and intestines must have undergone a treatment such that its cut surface shows that it no longer has the characteristics of fresh meat and the fresh meat used must also satisfy the animal health rules applicable to exports of fresh meat into the Community. |
Specific treatments listed in descending order of severity:
|
B |
= |
Treatment in a hermetically sealed container to an Fo value of three or more. |
|
C |
= |
A minimum temperature of 80 °C which must be reached throughout the meat and/or stomachs, bladders and intestines during the processing of the meat product and treated stomachs, bladders and intestines. |
|
D |
= |
A minimum temperature of 70 °C which must be reached throughout the meat and/or stomachs, bladders and intestines during the processing of meat products and treated stomachs, bladders and intestines, or for raw ham, a treatment consisting of natural fermentation and maturation of not less than nine months and resulting in the following characteristics: — Aw value of not more than 0,93, — pH value of not more than 6,0. |
|
E |
= |
In the case of ‘biltong’-type products, a treatment to achieve: — Aw value of not more than 0,93, — pH value of not more than 6,0. |
|
F |
= |
A heat treatment ensuring that a centre temperature of at least 65 °C is reached for a period of time as necessary to achieve a pasteurisation value (pv) equal to or above 40. |
ANNEX III
Model animal and public health certificate for certain meat products and treated stomachs, bladders and intestines intended for consignment to the European Union from third countries

![Part II: CertificationCOUNTRYMeat products/treated stomachs, bladders and intestines for importII.a. Certificate reference numberII.b.II.1. Animal Health AttestationI, the undersigned official veterinarian certify that:II.1.1. The meat product, treated stomachs, bladders and intestines (1) contains the following meat constituents and meet the criteria indicated below:Species (A)Treatment (B)Origin (C)(A) Insert the code for the relevant species of meat product, treated stomachs, bladders and intestines where BOV = domestic bovine animals (Bos taurus, Bison bison, Bubalus bubalis and their crossbreds); OVI = domestic sheep (Ovis aries) and goats (Capra hircus); EQI = domestic equine animals (Equus caballus, Equus asinus and their crossbreds), POR = domestic porcine animals (Sus scrofa); RAB = domestic rabbits, PFG = domestic poultry and farmed feathered game, RUF farmed non-domestic animals other than suidae and solipeds; RUW = wild non-domestic animals other than suidae and solipeds; SUW = wild non-domestic suidae: EQW = wild non-domestic solipeds, WLP = wild lagomorphs, WGB = wild game birds.(B) Insert A, B, C, D, E or F for the required treatment as specified and defined in Parts 2, 3 and 4 of Annex II to Decision 2007/777/EC.(C) Insert the ISO code of the country of origin and, in the case of regionalization by Community legislation for the relevant meat constituents, the region as indicated in Part 1 of Annex II to Decision 2007/777/EC (as last amended).(2) II.1.2. The meat product, treated stomachs, bladders and intestines described in point II.1.1 has been prepared from fresh meat from domestic bovine animals (Bos taurus, Bison bison, Bubalus bubalis and their crossbreds); domestic sheep (Ovis aries) and goats (Capra hircus); domestic equine animals (Equus caballus, Equus asinus and their crossbreds), domestic porcine animals (Sus scrofa); farmed non-domestic animals other than suidae and solipeds; wild non-domestic animals other than suidae and solipeds; wild non-domestic suidae; wild non-domestic solipeds and the fresh meat used in the production of the meat products:either [II.1.2.1. has undergone a non-specific treatment as specified and defined under point A in Part 4 of Annex II to Decision 2007/777/EC] and: (2)either [II.1.2.1.1. satisfies the relevant animal and public health requirements laid down in the appropriate health certificate(s) in Annex II, Part 2, to Council Decision 79/542/EEC and originates in a third country, or part thereof in the case of regionalisation under Community legislation, as described in the relevant column of part 2 of Annex II to Decision 2007/777/EC]. (2)or [II.1.2.1.1. originates in a Member State of the European Community] (2)or [II.1.2.1. meets any requirements agreed under Directive 2002/99/EC, is derived from animals coming from a holding not subject to restrictions for the specific diseases mentioned in the appropriate health certificate(s) in Annex II, Part 2, to Council Decision 79/542/EEC and within a 10 km radius of which no outbreaks of such diseases have occurred in the last 30 days and has undergone the specific treatment laid down for the third country of origin or part thereof for the meat of the species concerned in Parts 2 or 3 (as appropriate) of Annex II to Commission Decision 2007/777/EC] (2)(2) II.1.3. The meat product, treated stomachs, bladders and intestines described under point II.1.1 has been prepared from fresh meat of domestic poultry, including farmed or wild game birds, that:either [II.1.3.1. has undergone a non-specific treatment as specified and defined under point A in Part 4 of Annex II to Decision 2007/777/EC] and: (2)either [II.1.3.1.1. satisfies the animal health requirements laid down in Commission Decision 2006/696/EC,] (2)or [II.1.3.1.1. originates in a Member State of the European Community satisfying the requirements of Article 3 of Council Directive 2002/99/EC] (2)or [II.1.3.1. originates in a third country referred to in Annex II part 1 to Decision 2006/696/EC, comes from a holding not subject to restrictions for Avian Influenza or Newcastle disease within a 10 km radius of which no outbreaks of such diseases have occurred in the last 30 days and has undergone the specific treatment laid down for the third country of origin or part thereof for the meat of the species concerned in Parts 2 or 3 (as appropriate) of Annex II to Decision 2007/777/EC.] (2)](./../../../resource.html?uri=celex:02007D0777-20101105.ENG.xhtml.L_2007312EN.01006101.tif.jpg)
 [II.1.4. in the case of meat products, treated stomachs, bladders and intestines derived from fresh meat from lagomorphs and other land mammals:satisfies the relevant animal health and public health requirements laid down in Commission Decision 2000/585/EC and has not come from a holding subject to restrictions for animal diseases affecting the animals concerned within a 10 km radius of which no outbreaks of such diseases have occurred in the last 30 days;]II.1.5. the meat product, treated stomachs, bladders and intestines:II.1.5.1. [consists of meat and/or meat products derived from a single species, and has undergone the treatment satisfying the relevant conditions laid down in Annex II to Decision 2007/777/EC]or (2) II.1.5.1. [consists of meat of more than one species and, after such meat has been mixed, the entire product has subsequently undergone a treatment at least as severe as that required for the meat components of the meat product as laid down in Annex II to Decision 2007/777/EC;]or (2) II.1.5.1. [has been prepared from meat of more than one species and each meat component has previously undergone a treatment prior to mixing which meets the relevant treatment requirements for meat of that species as laid down in Annex II to 2007/777/EC]; (2)II.1.6. after treatment all precautions to avoid contamination have been taken(2) [II.1.7. Additional guarantees:in the case of poultry meat products which have not undergone a specific treatment and are destined for Member States or regions thereof which have been recognised in accordance with Article 12 of Council Directive 90/539/EEC, the poultry meat was derived from poultry which had not been vaccinated with a live vaccine against Newcastle disease within 30 days prior to slaughter;](2) II.2. Public Health AttestationI, the undersigned, declare that I am aware of the relevant provisions of Regulations (EC) No 178/2002, (EC) No 852/2004, (EC) No 853/2004 and (EC) No 999/2001 and certify that the meat products, treated stomachs, bladders and intestines described above were produced in accordance with those requirements, in particular that:II.2.1. they come from (an) establishment(s) implementing a programme based on the HACCP principles in accordance with Regulation (EC) No 852/2004;II.2.2. they have been produced from raw material which met the requirements of Sections I to VI of Annex III to Regulation (EC) No 853/2004;II.2.3.1. (2) the meat products have been obtained from domestic pig meat which either has been subject to an examination for trichinosis with negative results or has been subjected to a cold treatment in accordance with Commission Regulation (EC) No 2075/2005;II.2.3.2. (2) the meat products have been obtained from horse meat or wild boar meat which has been subject to an examination for trichinosis with negative results in accordance with Commission Regulation (EC) No 2075/2005;II.2.3.3. (2) the treated stomachs, bladders and intestines have been produced in accordance with Section XIII of Annex III, to Regualtion (EC) No 853/2004;II.2.4. they have been marked with an identification mark in accordance with Section I of Annex II to Regualtion (EC) No 853/2004;II.2.5. the label affixed on the packaging of meat products described above, bear(s) a mark to the effect that the meat products come wholly from fresh meat from animals slaughtered in slaughterhouses approved for exporting to the European Community or, from animals slaughtered in a slaughterhouse specially for the delivery of meat for the required treatment as laid down in Part 2 and 3 of Annex II of Decision 2007/777/EC;II.2.6. they satisfy the relevant criteria set out in Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs;](./../../../resource.html?uri=celex:02007D0777-20101105.ENG.xhtml.L_2007312EN.01006201.tif.jpg)



ANNEX IV
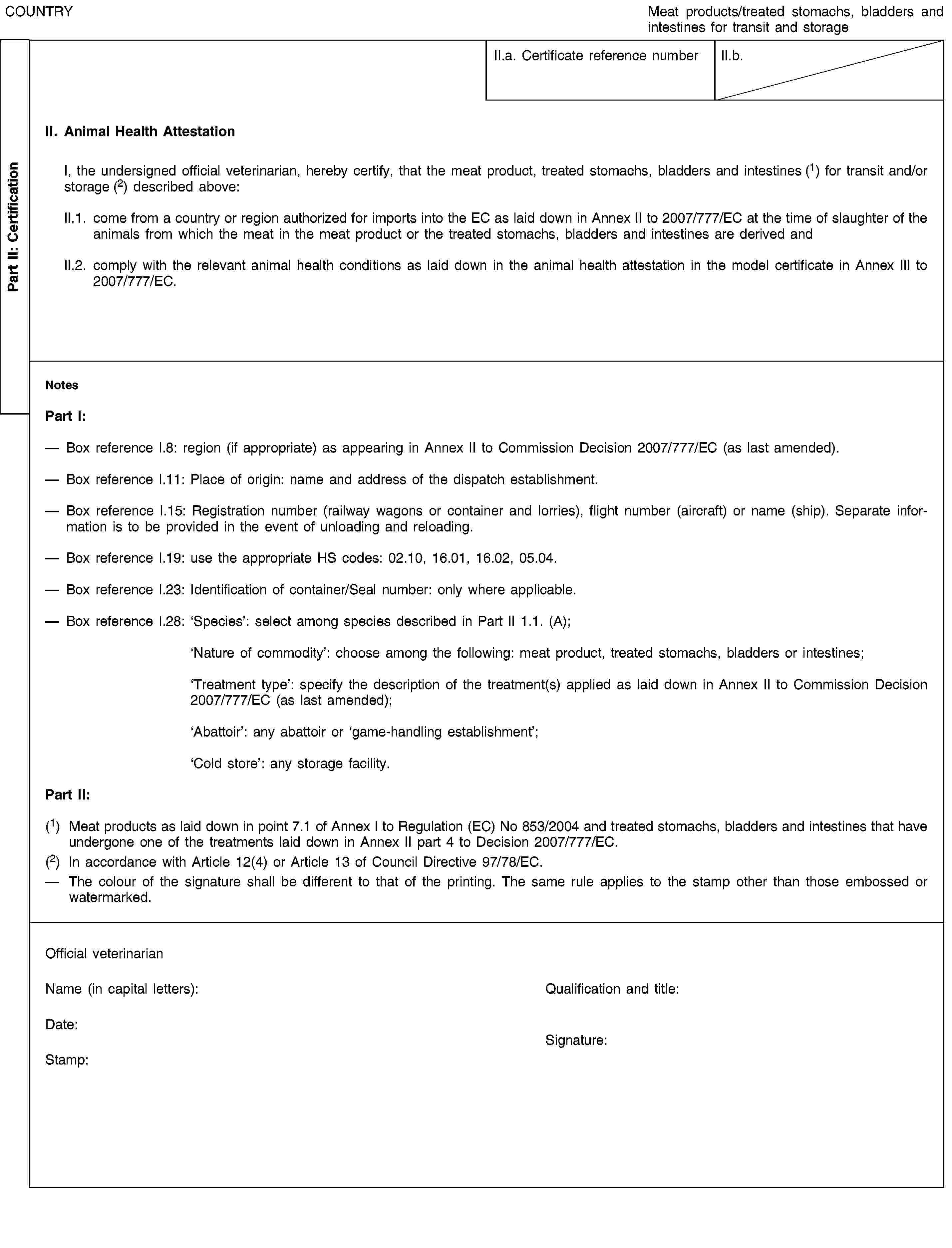
(Transit and/or storage)

( 1 ) OJ L 62, 15.3.1993, p. 49. Directive as last amended by Commission Regulation (EC) No 445/2004 (OJ L 72, 11.3.2004, p. 60).
( 2 ) OJ L 18, 23.1.2003, p. 11.
( 3 ) OJ L 151, 14.6.2005, p. 3. Decision as last amended by Commission Regulation (EC) No 1792/2006 (OJ L 362, 20.12.2006, p. 1).
( 4 ) OJ L 329, 25.11.2006, p. 26.
( 5 ) OJ L 139, 30.4.2004, p. 1; corrected version OJ L 226, 25.6.2004, p. 3.
( 6 ) OJ L 139, 30.4.2004, p. 55; corrected version OJ L 226, 25.6.2004, p. 22. Regulation as last amended by Council Regulation (EC) No 1791/2006 (OJ L 363, 20.12.2006, p. 1).
( 7 ) OJ L 139, 30.4.2004, p. 206; corrected version OJ L 226, 25.6.2004, p. 83. Regulation as last amended by Council Regulation (EC) No 1791/2006.
( 8 ) OJ L 285, 1.11.2003, p. 38. Decision as amended by Decision 2004/414/EC (OJ L 151, 30.4.2004, p. 56).
( 9 ) OJ L 154, 30.4.2004, p. 44. Decision as last amended by Decision 2007/362/EC (OJ L 138, 30.5.2007, p. 18).
( 10 ) OJ L 24, 30.1.1998, p. 9. Directive as last amended by Directive 2006/104/EC (OJ L 363, 20.12.2006, p. 352).
( 11 ) OJ L 326, 11.12.2006, p. 44. Decision as last amended by Decision 2007/276/EC (OJ L 116, 4.5.2007, p. 34).
( 12 ) OJ L 146, 14.6.1979, p. 15. Decision as last amended by Regulation (EC) No 1791/2006 (OJ L 363, 20.12.2006, p. 1).
( 13 ) OJ L 114, 30.4.2002, p. 132.
( 14 ) OJ L 147, 31.5.2001, p. 1. Regulation as last amended by Regulation (EC) No 727/2007 (OJ L 165, 27.6.2007, p. 8).
( 15 ) OJ L 164, 26.6.2007, p. 7.
( 16 ) OJ L 284, 30.10.2007, p. 8.
( 17 ) OJ L 172, 30.6.2007, p. 84.


