EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document 02006D0696-20071027
Commission Decision of 28 August 2006 laying down a list of third countries from which poultry, hatching eggs, day-old chicks, meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may be imported into and transit through the Community and the applicable veterinary certification conditions, and amending Decisions 93/342/EEC, 2000/585/EC and 2003/812/EC (notified under document number C (2006) 3821) (Text with EEA relevance) (2006/696/EC)
Consolidated text: Commission Decision of 28 August 2006 laying down a list of third countries from which poultry, hatching eggs, day-old chicks, meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may be imported into and transit through the Community and the applicable veterinary certification conditions, and amending Decisions 93/342/EEC, 2000/585/EC and 2003/812/EC (notified under document number C (2006) 3821) (Text with EEA relevance) (2006/696/EC)
Commission Decision of 28 August 2006 laying down a list of third countries from which poultry, hatching eggs, day-old chicks, meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may be imported into and transit through the Community and the applicable veterinary certification conditions, and amending Decisions 93/342/EEC, 2000/585/EC and 2003/812/EC (notified under document number C (2006) 3821) (Text with EEA relevance) (2006/696/EC)
2006D0696 — EN — 27.10.2007 — 001.001
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
COMMISSION DECISION of 28 August 2006 laying down a list of third countries from which poultry, hatching eggs, day-old chicks, meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may be imported into and transit through the Community and the applicable veterinary certification conditions, and amending Decisions 93/342/EEC, 2000/585/EC and 2003/812/EC (notified under document number C (2006) 3821) (Text with EEA relevance) (OJ L 295, 25.10.2006, p.1) |
Amended by:
|
|
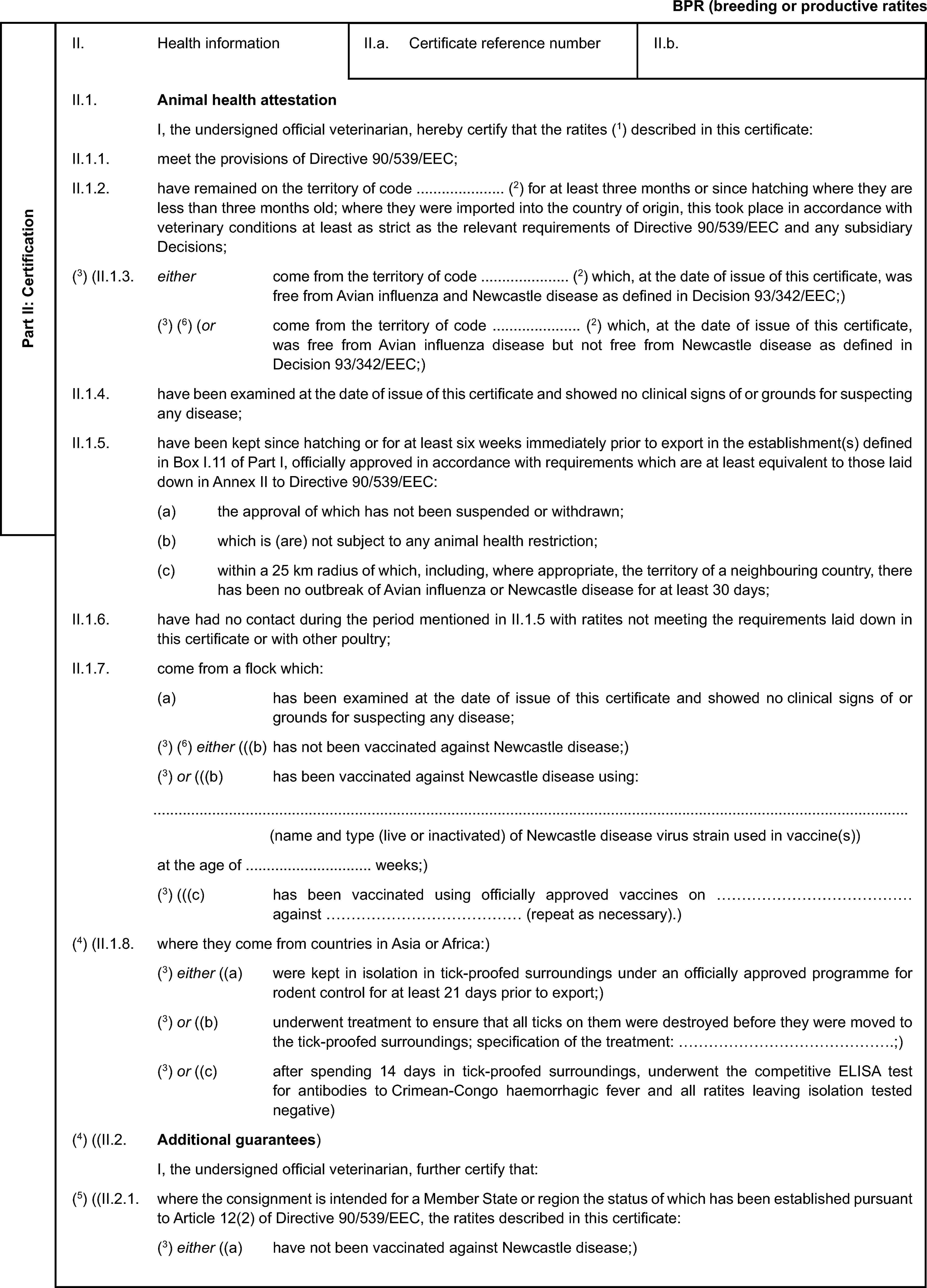
|
Official Journal |
||
|
No |
page |
date |
||
|
L 280 |
5 |
24.10.2007 |
||
COMMISSION DECISION
of 28 August 2006
laying down a list of third countries from which poultry, hatching eggs, day-old chicks, meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may be imported into and transit through the Community and the applicable veterinary certification conditions, and amending Decisions 93/342/EEC, 2000/585/EC and 2003/812/EC
(notified under document number C (2006) 3821)
(Text with EEA relevance)
(2006/696/EC)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Community,
Having regard to Council Directive 90/539/EEC of 15 October 1990 on animal health conditions governing intra-Community trade in, and imports from third countries of, poultry and hatching eggs ( 1 ), and in particular Articles 21(1) and 22(3), Article 23, Article 24(2) and Articles 26 and 27a thereof,
Having regard to Council Directive 91/496/EEC of 15 July 1991 laying down the principles governing the organisation of veterinary checks on animals entering the Community from third countries and amending Directives 89/662/EEC, 90/425/EEC and 90/675/EEC ( 2 ) and in particular Article 10 and Article 18 thereof,
Having regard to Council Directive 92/118/EEC of 17 December 1992 laying down animal health and public health requirements governing trade in and imports into the Community of products not subject to the said requirements laid down in specific Community rules referred to in Annex A (I) to Directive 89/662/EEC and, as regards pathogens, to Directive 90/425/EEC ( 3 ), and in particular Article 10(2) and the first subparagraph of Article 10(3)(a) thereof and the first indent of Chapter 2 of Annex II thereto,
Having regard to Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC ( 4 ), and in particular the fourth subparagraph of Article 29(1), thereof,
Having regard to Council Directive 97/78/EC of 18 December 1997 laying down the principles governing the organisation of veterinary checks on products entering the Community from third countries ( 5 ), and in particular Article 22(1) thereof,
Having regard to Council Directive 2002/99/EC of 16 December 2002 laying down the animal health rules governing the production, processing, distribution and introduction of products of animal origin for human consumption ( 6 ), in particular Article 8 and Article 9(2) and (4) thereof,
Having regard to Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin ( 7 ), and in particular Article 9 thereof,
Having regard to Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption ( 8 ), and in particular Article 11(1) thereof,
Whereas:|
(1) |
Commission Decisions 94/85/EC ( 9 ), 94/86/EC ( 10 ), 94/984/EC ( 11 ), 95/233/EC ( 12 ), 96/482/EC ( 13 ), 96/659/EC ( 14 ) 97/38/EC ( 15 ), 2000/609/EC ( 16 ), 2001/393/EC ( 17 ) and 2001/751/EC lay down Community rules on imports into and transit through the Community of poultry, hatching eggs, day-old chicks and meat of poultry, ratites and wild-game birds and eggs and egg products and specified pathogen-free eggs (the commodities concerned). Those Decisions generally only authorise the import and transit of the commodities concerned where they come from a third country or part thereof appearing on a list of approved third countries and where they comply with Community health conditions. |
|
(2) |
Council Regulation (EEC) No 2782/75 of 29 October 1975 on the production and marketing of eggs for hatching and of farmyard poultry chicks ( 18 ), Council Regulation (EEC) No 1907/90 of 26 June 1990 on certain marketing standards for eggs ( 19 ) and Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety ( 20 ) set out Community health rules of a more general scope and apply to the import into and transit through the Community of the commodities concerned. |
|
(3) |
Existing Community rules governing imports into and transit through the Community of the commodities concerned should be amended to take account of new public-health requirements laid down in Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs ( 21 ), Regulations (EC) No 853/2004 and (EC) No 854/2004 and Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs ( 22 ). |
|
(4) |
In addition, imports into the Community of hatching eggs, meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds, eggs and egg products are only authorised where they comply with residue control monitoring as provided for in Directive 96/23/EC. |
|
(5) |
In the interests of clarity and consistency of Community rules, it is appropriate that the definitions set out in Directive 90/539/EEC and Regulation (EC) No 853/2004 be taken into account for the purposes of this Decision. |
|
(6) |
With a view to harmonising Community conditions for imports into the Community of the commodities concerned, as well as making them more transparent and simplifying the legislative procedure for amending such conditions, when required, those conditions should be set out in the relevant model veterinary certificate. |
|
(7) |
In order to protect the Community from disease by ensuring that consignments in transit through or stored in the Community comply with the animal health import conditions applicable to approved third countries for the animals or products concerned, a specific model veterinary certificate should be drawn up for use for the transit and storage of the commodities concerned. |
|
(8) |
The World Organisation for Animal Health (OIE) and the Codex Alimentarius have laid down guidelines regarding the principles of certification which veterinarians have to follow. Those principles state that the certifying veterinarian should certify only matters within his or her own knowledge at the time of signing the certificate or that have been separately attested by an official of another competent authority. |
|
(9) |
In addition, Council Directive 96/93/EC of 17 December 1996 on the certification of animals and animal products ( 23 ) lays down standards of certification which are necessary to ensure valid certification and to prevent fraud. It is, therefore, appropriate to ensure that the rules and principles applied by third country certifying officers provide guarantees that are equivalent to those laid down in that Directive and that the model veterinary certificates laid down in this Decision reflect only such facts as may be attested at the time the certificate is issued. |
|
(10) |
The United Nations has established guidelines for a common framework and layout for commercial documents. Under the aegis of several international bodies dealing with the streamlining of procedures in international trade, new principles and rules are to be followed for issuing certificates for international transactions. The OIE and the Codex Alimentarius have laid down guidelines on the use of electronic certification with regard to certification procedures. |
|
(11) |
For the information of the authorised veterinarian, importers and the competent authorities in the Member State where the veterinary certificates are presented, the notes for the exporting country on the compiling of the certificates should include further details relating to the term of validity, the date of issue and the scope of the certificate. For those reasons, each model veterinary certificate should also include explanations of certain definitions, supplementary guarantees approved by the Commission under specific conditions and, where relevant, the health requirements for holdings and establishments. |
|
(12) |
With a view to standardising the layout of the veterinary certificates to be issued by the official veterinarian of the exporting country and in order to facilitate the use of any electronic means of transferring certificates, the model veterinary certificates laid down in this Decision should be harmonised, as well as the notes for compiling such certificates in the exporting country. |
|
(13) |
In order to harmonise and streamline import procedures at Community borders, consignments presented for import and transit should be accompanied by the relevant veterinary certificates. |
|
(14) |
Specific conditions for transit via the Community of consignments to and from Russia should be provided for owing to the geographical situation of Kaliningrad. |
|
(15) |
Owing to the animal and public health situation with respect to Crimean-Congo Haemorrhagic Fever in Africa and Asia, certain special conditions should be laid down for imports of ratites for breeding and production and day-old chicks thereof coming from those regions. |
|
(16) |
In the interests of clarity of Community legislation, Commission Decisions 94/85/EC, 94/86/EC, 94/984/EC, 95/233/EC, 96/482/EC, 96/659/EC97/38/EC, 2000/609/EC, 2001/393/EC and 2001/751/EC should be repealed and replaced by this Decision. |
|
(17) |
Commission Decision 93/342/EEC of 12 May 1993 laying down the criteria for classifying third countries with regard to avian influenza and Newcastle disease in relation to imports of live poultry and hatching eggs ( 24 ), Commission Decision 2000/585/EC of 7 September 2000 drawing up a list of third countries from which Member States authorise imports of rabbit meat and certain wild and farmed game meat, and laying down the animal and public health and the veterinary certification conditions for such imports ( 25 ) and Commission Decision 2003/812/EC of 17 November 2003 drawing up lists of third countries from which Member States are to authorise imports of certain products for human consumption subject to Council Directive 92/118/EEC ( 26 ) lay down certain conditions concerning a number of the commodities concerned. In the interests of clarity of Community legislation, it is appropriate that the relevant conditions be included in this Decision. Decisions 93/342/EEC, 2000/585/EC and 2003/812/EC should therefore be amended accordingly. |
|
(18) |
It is appropriate to provide for a transitional period to permit the Member States and the industry to take the necessary measures to comply with the applicable veterinary certification conditions laid down in this Decision. |
|
(19) |
The measures provided for in this Decision are in accordance with the opinion of the Standing Committee on the Food Chain and Animal Health, |
HAS ADOPTED THIS DECISION:
CHAPTER I
INTRODUCTORY PROVISIONS
Article 1
Subject matter and scope
This Decision lays down veterinary certification conditions for imports into and transit through the Communityof:
(a) poultry, hatching eggs and day-old chicks;
(b) meat, minced meatand mechanically separated meat, of poultry, ratites and wild game-birds;
(c) eggs and egg products and specified pathogen-free eggs.
However, this Decision shall not apply to poultry for exhibitions, shows or contests.
Article 2
Definitions
For the purposes of this Decision, the following definitions shall apply:
(a) ‘poultry’ means fowl, turkeys, guinea fowl, ducks, geese, quails, pigeons, pheasants, partridges and ratites (Ratitae), reared or kept in captivity for breeding, the production of meat or eggs for consumption or for restocking supplies of game;
(b) ‘hatching eggs’ means eggs for incubation, laid by poultry as defined in (a);
(c) ‘day-old chicks’ means all poultry less than 72 hours old, not yet fed; however, muscovy ducks (Cairina moschata) and their crosses may be fed;
(d) ‘breeding poultry’ means poultry 72 hours old or more, intended for the production of hatching eggs;
(e) ‘productive poultry’ means poultry 72 hours old or more, reared for the production of meat and/or eggs for consumption or for restocking supplies of game;
(f) ‘flock’ means all poultry of the same health status kept on the same premises or in the same enclosure and constituting a single epidemiological unit; as regards housed poultry, this definition includes all birds sharing the same airspace;
(g) ‘establishment’ means a facility or part of a facility which occupies a single site and devoted to one or more of the following activities:
(i) pedigree breeding establishment: an establishment which produces hatching eggs for the production of breeding poultry;
(ii) breeding establishment: an establishment which produces hatching eggs for the production of productive poultry;
(iii) rearing establishment:
— either a breeding poultry-rearing establishment which rears breeding poultry prior to the reproductive stage, or
— a productive poultry-rearing establishment which rears egg-laying productive poultry prior to the laying stage;
(h) ‘hatchery’ means an establishment which incubates and hatches eggs and supplies day-old chicks;
(i) ‘authorised veterinarian’ means a veterinarian instructed by the competent veterinary authority and under its responsibility to carry out the checks provided for in this Decision in a particular establishment;
(j) ‘meat’ means edible parts of the following animals:
(i) poultry, which, when relating to meat, means farmed birds, including birds that are farmed as domestic animals without being considered as such, with the exception of ratites;
(ii) wild game birds that are hunted for human consumption;
(iii) ratites;
(k) ‘Mechanically separated meat’ or ‘MSM’ means the product obtained by removing meat from flesh-bearing bones after boning or from poultry carcases, using mechanical means resulting in the loss or modification of the muscle fibre structure;
(l) ‘minced meat’ means boned meat that has been minced into fragments and contains less than 1 % salt;
(m) ‘specified pathogen-free eggs’ means hatching eggs which are derived from ‘chicken flocks free from specified pathogens’ as described in the European Pharmacopoeia and which are intended solely for diagnostic, research or pharmaceutical use.
Article 3
Veterinary certification
Veterinary certificates as required under Part 1 of Annexes I and II shall be compiled in accordance with the notes set out in Part 2 of those Annexes.
However, electronic certification and other agreed systems harmonised at Community level may be used.
CHAPTER II
POULTRY, HATCHING EGGS AND DAY-OLD CHICKS
SECTION 1
General provisions
Article 4
General conditions for import and transit
1. Poultry, hatching eggs and day-old chicks imported into and transiting through the Community shall comply with the conditions laid down in Articles 5 to 14.
2. Paragraph 1 shall not apply to single consignments of fewer than 20 units of poultry, hatching eggs or day-old chicks.
However, such single consignments may only be imported from third countries or parts thereof that are approved for such imports as they comply with the following conditions:
(a) the country or a part thereof is listed in columns 1 and 3 of the table in Part 1 of Annex I and column 4 of that table provides for a model veterinary certificate for the commodity concerned;
(b) they are not covered by an import ban;
(c) the importation conditions include the requirement for post-import isolation or quarantine. This provision does not apply to consignments of ratites or hatching eggs thereof.
Article 5
Place of origin
Poultry, hatching eggs and day-old chicks may only be imported into or transit through the Community from third countries or parts thereof listed in columns 1 and 3 of the table in Part 1 of Annex I where column 4 of that table provides for a model veterinary certificate for the commodity concerned.
Article 6
Health conditions and additional guarantees
1. Poultry, hatching eggs and day-old chicks shall meet the requirements laid down in the relevant veterinary certificate compiled using the corresponding model in Part 2 of Annex I, subject to the specific conditions set out in column 6 of the table in Part 1 of Annex I.
2. Where required by the Member State of destination under Community legislation, the additional guarantees for poultry, hatching eggs and day-old-chicks as specified for that Member State in column 5 of the table in Part 1 of Annex I shall be included in the veterinary certificate using the corresponding model in Part 2 of Annex I.
Article 7
Additional health requirements for poultry, hatching eggs and day-old chicks from third countries where vaccines used against Newcastle disease do not meet Community standards
1. Where third countries do not prohibit the use of vaccines against Newcastle disease not meeting the specific criteria set out in point 2 of Annex B to Decision 93/342/EEC, the following additional health requirements shall apply to poultry and day-old chicks imported there from:
(a) for at least 12 months preceding the date of export to the Community, they have not been vaccinated with such vaccines;
(b) not more than two weeks before the date of export to the Community or, in the case of hatching eggs, not earlier than two weeks before the date of collection of the eggs, the flocks have undergone a virus isolation test for Newcastle disease:
(i) carried out in an official laboratory;
(ii) on a random sample of cloacal swabs from at least 60 birds in each flock;
(iii) in which no avian paramyxoviruses with an Intracerebral Pathogenicity Index of more than 0,4 have been found;
(c) during the two-week period referred to in (b), they have been kept in isolation under official surveillance on the holding of origin;
(d) during a period of 60 days before the date of export to the Community or, in the case of hatching eggs, during a period of 60 days before the date of collection of the eggs, they have not been in contact with poultry not meeting the requirements in (a) and (b).
2. Where day-old chicks are imported from a third country as referred to in paragraph 1, the hatching eggs from which they have hatched have not been in contact in the hatchery or during transport with poultry or hatching eggs not meeting the requirements in (a) to (d).
Article 8
Transport of poultry
1. Poultry shall not be loaded onto a means of transport carrying other poultry of a lower health status.
2. In the course of transport to the Community, poultry shall not be moved by road or rail through and not be unloaded in a third country or a part thereof that is not approved for imports into the Community of such poultry.
3. In the course of air transport, poultry shall not be unloaded in a third country or a part thereof that is not approved for imports of such poultry into the Community.
SECTION 2
Breeding and productive poultry other than ratites and hatching eggs and day-old chicks other than of ratites
Article 9
Requirements for i mports
1. Imported breeding and productive poultry other than ratites and hatching eggs and day-old chicks other than of ratites may only come from establishments which have been approved by the competent authority of the third country concerned in accordance with conditions that are at least as strict as those laid down in Annex II to Directive 90/539/EEC and where such approval has not been suspended or withdrawn.
2. Where breeding and productive poultry other than ratites and hatching eggs and day-old chicks other than of ratites and/or their flocks of origin are to undergo testing to meet the requirements of the relevant veterinary certificates laid down in this Decision, sampling for testing and the testing itself must be carried out in accordance with the methods referred to in Part 4(A) of Annex I.
3. Imported hatching eggs shall bear the name of the third country of origin and one of the printed indications set out in Annex III in type at least 3 mm high.
4. Each package of hatching eggs as referred to in paragraph 3 shall contain only eggs of a single species, category and type of poultry from the same third country of origin and consignor, and shall bear at least the following particulars:
(a) the information shown on the eggs as provided for in paragraph 3;
(b) the species of poultry from which the eggs come;
(c) the consignor's name or business name and address.
5. Each box of imported day-old chicks shall contain only a single species, category and type of poultry from the same third country of origin, hatchery and consignor and shall bear at least the following particulars:
(a) the name of the third country of origin;
(b) the species of poultry to which the day-old chicks belong;
(c) the distinguishing number of the hatchery;
(d) the consignor's name or business name and address.
Article 10
Requirements after import
1. Imported breeding and productive poultry other than ratites and day-old chicks other than of ratites shall be kept on the holding(s) of destination from their date of arrival:
(a) for a period of at least six weeks; or
(b) where the birds are slaughtered before the expiry of the period referred to in (a), until the day of slaughter.
However, the period provided for in (a) may be reduced to three weeks, provided that sampling and testing in accordance with the procedures set out in Part 4(B) of Annex I have been carried out with favourable results.
2. Breeding and productive poultry other than ratites hatched from imported hatching eggs shall be kept for at least three weeks from the date of hatching in the hatchery or on the holding(s) to which the poultry has been sent after hatching.
Where day-old chicks are not reared in the Member State which imported the hatching eggs, they shall be transported directly to the final destination specified in point 9.2 of the health certificate Model 2 in Annex IV to Directive 90/539/EEC and kept there for at least three weeks from the date of hatching.
3. During the relevant period as provided for in paragraphs 1 and 2, imported breeding and productive poultry and day-old chicks and breeding and productive poultry other than ratites hatched from imported hatching eggs shall be kept in isolation in poultry houses where no other flocks are present.
However, they may be introduced into poultry houses where breeding and productive poultry and day-old chicks are already present.
In that case, the periods referred to in paragraphs 1 and 2 shall start running from the date of introduction of the last imported bird and no poultry present shall be moved from the housing before the end of those periods.
4. Imported hatching eggs shall be hatched in separate incubators and hatchers.
However, imported hatching eggs may be introduced into incubators and hatchers where other hatching eggs are already present.
In that case, the periods referred to in paragraphs 1 and 2 shall start running from the date of introduction of the last imported hatching egg.
5. No later than the date of expiry of the relevant period as provided for in paragraph 1 or 2, imported breeding and productive poultry and day-old chicks shall undergo a clinical examination carried out by an authorised veterinarian and, where necessary, samples shall be taken to monitor their state of health.
SECTION 3
Ratites for breeding and production and hatching eggs and day-old chicks thereof
Article 11
Requirements for imports
1. Imported ratites for breeding and production shall be identified by neck-tags and/or microchips bearing the ISO code of the third country of origin.
Such microchips shall comply with ISO standards.
2. Imported hatching eggs of ratites for breeding and production shall be marked with a stamp indicating the ISO code of the third country of origin and the approval number of the establishment of origin.
3. Each package of hatching eggs as referred to in paragraph 2 shall contain only eggs of ratites from the same third country of origin and consignor, and shall bear at least the following particulars:
(a) the information shown on the eggs as provided for in paragraph 2;
(b) a clearly visible and legible indication that the consignment contains hatching eggs of ratites;
(c) the consignor's name or business name and address.
4. Each box of imported day-old chicks of ratites for breeding and production shall contain only ratites from the same third country of origin, establishment and consignor, and shall bear at least the following particulars:
(a) the ISO code of the third country of origin and the approval number of the establishment of origin;
(b) a clearly visible and legible indication that the consignment contains day-old chicks of ratites;
(c) the consignor's name or business name and address.
5. After the import controls have been carried out, consignments of ratites and hatching eggs and day-old chicks thereof shall be transported directly to the final destination.
Article 12
Requirements after import
1. Imported ratites for breeding and production (ratites) and day-old chicks thereof shall be kept on the holding(s) of destination from their date of arrival:
(a) for a period of at least six weeks; or
(b) where the birds are slaughtered before the expiry of the period referred to in (a), until the day of slaughter.
2. Ratites hatched from imported hatching eggs shall be kept for a period of at least three weeks from the date of hatching in the hatchery or on the holding(s) to which they have been sent after hatching.
3. During the relevant period as provided for in paragraphs 1 and 2, imported ratites and ratites hatched from imported hatching eggs shall be kept in isolation in houses where no other ratites or poultry are present.
However, they may be introduced into houses where other ratites or poultry are already present.
In that case, the periods provided for in paragraphs 1 and 2 shall start running from the date of introduction of the last imported ratite and no ratites or poultry present shall be moved from the housing before the end of those periods.
4. Imported hatching eggs shall be hatched in separate incubators and hatchers.
However, imported hatching eggs may be introduced into incubators and hatchers where other hatching eggs are already present.
In that case, the periods provided for in paragraphs 1 and 2 shall start running from the date of introduction of the last imported hatching egg and the measures as provided for in paragraphs 1 and 2 shall apply.
5. No later than the date of expiry of the relevant period as provided for in paragraph 1 or 2, imported ratites and day-old chicks thereof shall undergo a clinical examination carried out by an authorised veterinarian and, where necessary, samples shall be taken to monitor their state of health.
6. Where ratites, hatching eggs and day-old chicks thereof and/or their flocks of origin are to undergo testing in accordance with the requirements of the veterinary certificates laid down in Annex I to this Decision, sampling for testing for Newcastle disease and the testing itself shall be carried out in accordance with Annexes I and II to Commission Decision 92/340/EEC ( 27 ).
Article 13
Requirements for ratites for breeding and production and day-old chicks thereof from Asia and Africa
The protective measures for Crimean-Congo haemorrhagic fever set out in Part 1 of Annex V shall apply, on their arrival in the Community, to ratites for breeding and production and day-old chicks thereof coming from third countries in Asia and Africa.
All ratites testing positive to the competitive ELISA test for antibodies to Crimean Congo haemorrhagic fever provided for therein shall be destroyed.
All contact birds within the group shall be retested by the competitive ELISA test 21 days after the original sampling. Where any bird tests positive, the whole contact group shall be destroyed.
Article 14
Requirements for ratites for breeding and production from a country considered to be infected with Newcastle disease
The following rules shall apply to ratites and hatching eggs thereof coming from a third country considered as infected with Newcastle disease and day-old chicks that have hatched from such eggs:
(a) before the date the isolation period begins, the competent authority shall check the isolation facilities as referred to in Article 12(3) to see whether they are satisfactory;
(b) during the relevant period as provided for in Article 12(1) and (2), a virus isolation test for Newcastle disease shall be carried out on a cloacal swab or faeces sample from each ratite;
(c) where ratites are to be sent to a Member State or region the status of which has been established in accordance with Article 12(2) of Directive 90/539/EEC, a serological test shall be carried out on each ratite, in addition to the virus isolation test provided for in (b);
(d) negative results of the tests provided for in (b) and (c) shall be available before any bird is released from isolation.
CHAPTER III
MEAT, MINCED MEAT AND MECHANICALLY SEPARATED MEAT, OF POULTRY, RATITES AND WILD GAME-BIRDS, EGGS AND EGG PRODUCTS AND SPECIFIED PATHOGEN-FREE EGGS
SECTION 1
Imports
Article 15
Meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds
Meat, minced meat and mechanically separated meat, of poultry, ratites and wild game-birds may only be imported into the Community from a third country or a part thereof listed in columns 1 and 3 of the table in Part 1 of Annex II where column 4 of that table provides for a model veterinary certificate for the meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds concerned.
Article 16
Additional guarantees and additional health requirements for ratite meat and wild game-bird meat, minced meat and mechanically separated meat thereof
1. Ratite meat and wild game-bird meat, minced meat and mechanically separated meat thereof, may only be imported into the Community from a third country or a part thereof that is not subject to any restrictions relating to avian influenza and Newcastle disease.
2. Additional health requirements as laid down in Part 2 of Annex V concerning protection measures in relation to Crimean-Congo haemorrhagic fever shall apply for ratite meat from Africa and Asia imported into or transiting through the Community
3. Member States not vaccinating against Newcastle disease may demand additional guarantees concerning vaccination against that disease for ratite meat imported into or transiting through the Community.
Article 17
Eggs and egg products
Eggs and egg products may only be imported into the Community from a third country or a part thereof listed in columns 1 and 3 of the table in Part 1 of Annex II where column 4 of that table provides for a model veterinary certificate for the eggs and egg products concerned.
Article 18
Specified pathogen-free eggs
1. Specified pathogen-free eggs may only be imported into the Community from a third country or a part thereof listed in columns 1 and 3 of the table in Part 1 of Annex I where column 4 of that table provides for a model veterinary certificate for the specified pathogen-free eggs concerned.
2. Imported specified pathogen-free eggs as referred to in paragraph 1 shall be marked with a stamp bearing the ISO code of the third country of origin and the approval number of the establishment of origin.
3. Each package of specified pathogen-free eggs shall contain only eggs from the same third country of origin, establishment and consignor, and shall bear at least the following particulars:
(a) the information shown on the eggs as provided for in paragraph 2;
(b) a clearly visible and legible indication that the consignment contains specified pathogen-free eggs;
(c) the consignor's name or business name and address.
4. After the import controls have been carried out, consignments of specified pathogen-free eggs shall be transported directly to their final destination.
SECTION 2
Transit and storage
Article 19
Conditions for transit/storage
Meat, minced meat and mechanically separated meat, of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs may only transit through or be stored in the Community where they:
(a) comply with the relevant import conditions for the commodity concerned in Articles 15, 16, 17 or 18;
(b) come from a third country or a part thereof listed in Annex I or II;
(c) are accompanied by a veterinary certificate drawn up in accordance with the model set out in Annex IV.
Article 20
Derogation for transit
1. By way of derogation from Article 19, the Member States shall authorise transit by road or by rail through the Community, between border inspection posts in Latvia, Lithuania and Poland designated in accordance with Commission Decision 2001/881/EC ( 28 ), of consignments of meat minced meat and mechanically separated meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs coming from and bound for Russia directly or via another third country, provided that:
(a) the consignment is sealed with a serially numbered seal by the official veterinarian at the border inspection post of entry;
(b) the documents accompanying the consignment and referred to in Article 7 of Directive 97/78/EC are stamped ‘‘ONLY FOR TRANSIT TO RUSSIA VIA THE EC’’ on each page by the official veterinarian at the border inspection post of entry;
(c) the procedural requirements provided for in Article 11 of Directive 97/78/EC are complied with;
(d) the consignment is certified as acceptable for transit on the common veterinary entry document issued by the official veterinarian at the border inspection post of entry.
2. Consignments as referred to in paragraph 1 of this Article may not be unloaded or put into storage as referred to in Article 12(4) or Article 13 of Directive 97/78/EC within Community territory.
3. Regular audits shall be conducted by the competent authority to ensure that the number of consignments and the quantities of products leaving the Community territory match the number and quantities entering.
CHAPTER IV
TRANSITIONAL AND FINAL PROVISIONS
Article 21
Amendments to Decision 93/342/EEC
Decision 93/342/EEC is amended as follows:
(a) In Article 4(4), the second subparagraph is deleted.
(b) Annex E is deleted.
Article 22
Amendments to Decision 2000/585/EC
Decision 2000/585/EC is amended as follows:
(a) Article 1 is deleted;
(b) Article 2 is replaced by the following:
‘Article 2
Member States shall authorise imports of the following meat only:
(a) meat of wild leporidae, defined as wild rabbits and hares, not containing offal, except for unskinned and uneviscerated leporidae;
(b) meat of farmed rabbits;
(c) meat of wild land mammals other than ungulates and leporidae, not containing offal.
Such meat imports may only come from third countries or parts thereof listed in Annex I and subject to the conditions laid down in the veterinary certificate following the relevant model in Annex III, in accordance with Annex II.
The exporting third country shall meet the specific requirements referred to in Annex II and set out in Annex IV and shall certify this by completing Section V of each health certificate following the model in Annex III.’;
(c) Annex II is replaced by Annex VI to this Decision;
(d) In Annex III, Models D and I are deleted.
Article 23
Amendments to Decision 2003/812/EC
In Decision 2003/812/EC, Parts IV and V of the Annex are deleted.
Article 24
Repeals
Decisions 94/85/EC, 94/86/EC, 94/984/EC, 95/233/EC, 96/482/EC, 96/659/EC, 97/38/EC, 2000/609/EC, 2001/393/EC, 2001/751/EC are repealed.
Article 25
Transitional provisions
Poultry, hatching eggs, day-old chicks, meat, minced meat and mechanically separated meat of poultry, ratites and wild game-birds, eggs and egg products and specified pathogen-free eggs for which the relevant veterinary certificates have been issued in accordance with Decisions 94/85/EC, 94/86/EC, 94/984/EC, 95/233/EC, 96/482/EC, 97/38/EC, 2000/609/EC, 2001/393/EC, 2001/751/EC may be imported into or transit through the Community until six months after the day following that of its publication in the Official Journal of the European Union.
Article 26
Applicability
This Decision shall apply from six months after the day following that of the publication of this Decision in the Official Journal of the European Union.
Article 27
Addressees
This Decision is addressed to the Member States.
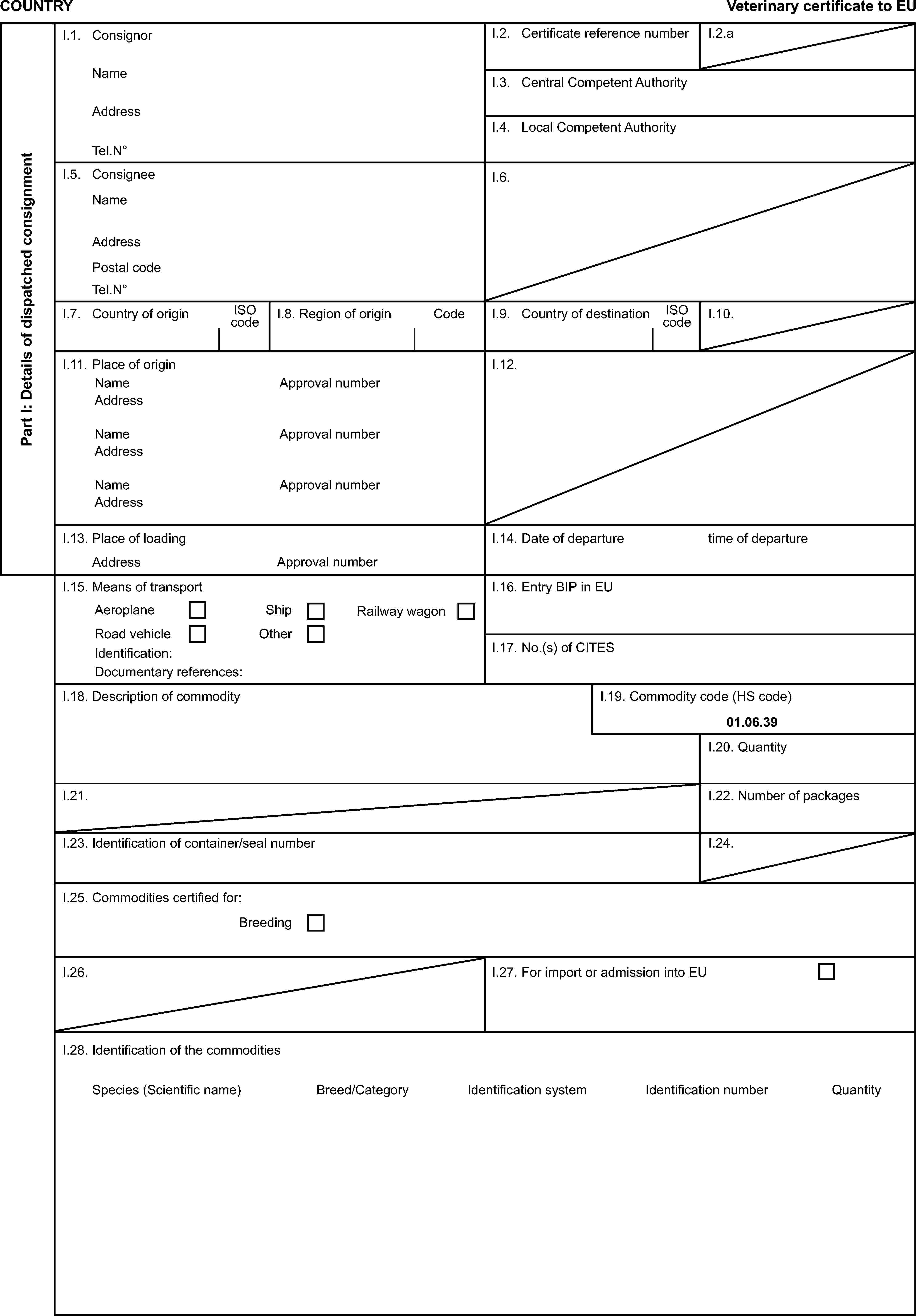
ANNEX I
POULTRY, RATITES INCLUDING HATCHING EGGS OF THESE SPECIES AND SPECIFIED PATHOGEN-FREE EGGS
PART 1
List of third countries or parts thereof (1)
|
Country |
Code of territory |
Description of territory |
Veterinary certificate |
Specific conditions |
|
|
Model(s) |
Additional Guarantees |
||||
|
1 |
2 |
3 |
4 |
5 |
6 |
|
AR – Argentina |
AR-0 |
SPF |
|||
|
AU – Australia |
AU-0 |
BPP, DOC, HEP, SPF, SRP |
|||
|
BPR |
I |
||||
|
DOR |
II |
||||
|
HER |
III |
||||
|
BG – Bulgaria (2) |
BG-0 |
BPP, DOC, HEP, SPF, SRP |
|||
|
BR – Brazil |
BR-0 |
SPF |
|||
|
BR-1 |
States of Mato Grosso, Paraná, Rio Grande do Sul, Santa Catarina and São Paulo |
BPP, DOC, HEP, SRP |
|||
|
BR-2 |
States of Rio Grande do Sul, Santa Catarina, Paraná, São Paulo and Mato Grosso do Sul |
BPR, DOR, HEP, HER, SRA |
|||
|
BW – Botswana |
BW-0 |
SPF |
|||
|
BPR |
I |
||||
|
DOR |
II |
||||
|
HER |
III |
||||
|
CA – Canada |
CA-0 |
BPR, BPP, DOC, DOR, HEP, HER, SRA, SPF, SRP |
|||
|
CH – Switzerland |
CH-0 |
||||
|
CL – Chile |
CL-0 |
BPR, BPP, DOC, DOR, HEP, HER, SPF, SRA, SRP |
|||
|
HR – Croatia |
HR-0 |
BPR, BPP, DOR, DOC, HEP, HER, SPF, SRA, SRP |
|||
|
GL – Greenland |
GL-0 |
SPF |
|||
|
IL – Israel |
IL-0 |
BPR, BPP, DOC, DOR, HEP, HER, SPF, SRP |
|||
|
IS – Iceland |
IS-0 |
SPF |
|||
|
MG – Madagascar |
MG-0 |
SPF |
|||
|
MX – Mexico |
MX-0 |
SPF |
|||
|
NA – Namibia |
NA-0 |
SPF |
|||
|
BPR |
I |
||||
|
DOR |
II |
||||
|
HER |
III |
||||
|
NZ – New Zealand |
NZ-0 |
BPR, BPP, DOC, DOR, HEP, HER, SPF, SRA, SRP |
|||
|
PM – St Pierre and Miquelon |
PM-0 |
SPF |
|||
|
RO – Romania (2) |
RO-0 |
BPR, BPP, DOC, DOR, HEP, HER, SPF, SRA, SRP |
|||
|
TH – Thailand |
TH-0 |
SPF |
|||
|
TN – Tunisia |
TN-0 |
DOR, BPR, BPP, HER, SPF |
|||
|
TR – Turkey |
TR-0 |
SPF |
|||
|
US – United States |
US-0 |
BPR, BPP, DOC, DOR, HEP, HER, SPF SRA, SRP |
|||
|
UY – Uruguay |
UY-0 |
SPF |
|||
|
ZA – South Africa |
ZA-0 |
SPF |
|||
|
BPR |
I |
||||
|
DOR |
II |
||||
|
HER |
III |
||||
|
(1) Without prejudice to specific certification requirements provided for in Community agreements with third countries. (2) Only applicable until this Acceding State becomes a Member State of the European Union. (3) Certificates in accordance with the agreement between the European Community and the Swiss Confederation on trade in agricultural products (OJ L 114, 30.4.2002, p. 132). |
|||||
PART 2
Model veterinary certificates
Models:
|
‘BPP’ |
: |
Model veterinary certificate for breeding or productive poultry other than ratites |
|
‘BPR’ |
: |
Model veterinary certificate for breeding or productive ratites |
|
‘DOC’ |
: |
Model veterinary certificate for day-old chicks other than of ratites |
|
‘DOR’ |
: |
Model veterinary certificate for day-old chicks of ratites |
|
‘HEP’ |
: |
Model veterinary certificate for hatching eggs of poultry other than ratites |
|
‘HER’ |
: |
Model veterinary certificate for hatching eggs of ratites |
|
‘SPF’ |
: |
Model veterinary certificate for specified pathogen-free (SPF) eggs |
|
‘SRP’ |
: |
Model veterinary certificate for slaughter poultry and poultry for restocking game supplies other than ratites |
|
‘SRA’ |
: |
Model veterinary certificate for slaughter ratites |
Additional guarantees (AG):
|
‘I’ |
: |
Guarantees for breeding and productive ratites coming from regions free from avian influenza but not free from Newcastle disease, certified in accordance with model BPR |
|
‘II’ |
: |
Guarantees for day-old chicks of ratites coming from regions free from avian influenza but not free from Newcastle disease, certified in accordance with model DOR |
|
‘III’ |
: |
Guarantees for hatching eggs of ratites coming from third countries free from avian influenza and free or not free from Newcastle disease certified in accordance with model HER |
Notes:
(a) Veterinary certificates based on the models in Part 2 of this Annex or Part 2 of Annex II and following the layout of the model that corresponds to the commodity concerned shall be issued by the exporting third country. They shall contain, in the order appearing in the model, the attestations that are required for any third country and, where applicable, those additional health requirements required for the exporting third country or part thereof.
Where additional guarantees are required by the EU Member State of destination for the commodity concerned, these shall also be entered on the original of the veterinary certificate.
(b) A separate, single certificate must be presented for each consignment of the commodity concerned, exported to the same destination from a territory appearing in columns 2 and 3 of Part 1 of this Annex or in columns 2 and 3 of Part 1 of Annex II and transported in the same railway wagon, lorry, aircraft or ship.
(c) The original of certificates shall consist of a single page printed on both sides or, where more text is required, such that all the pages form a whole and cannot be separated.
(d) The certificate shall be drawn up in at least one official language of the EU Member State where the border inspection takes place and in one official language of the EU Member State of destination. However, those Member States may allow another Community language instead of their own, accompanied, if necessary, by an official translation.
(e) Where additional pages are attached to the certificate for the purposes of identifying the items making up the consignment, such additional pages shall also be considered to form part of the original of the certificate, provided the signature and stamp of the certifying official veterinarian appear on each page.
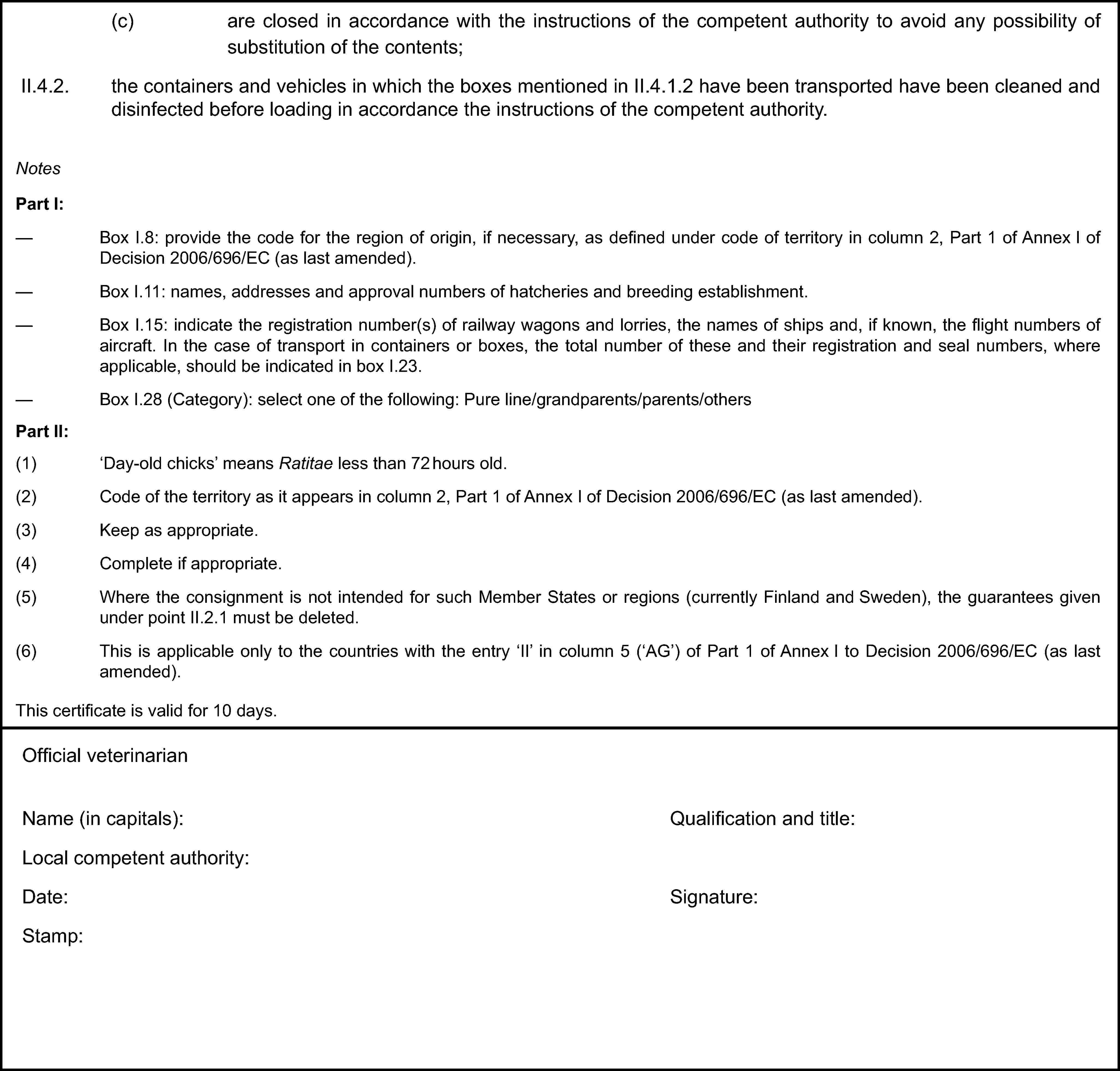
(f) Where the certificate, including any additional pages as provided for in (e), comprises more than one page, each page shall be numbered ‘–x(page number) of y(total number of pages)–’ on the bottom and shall bear the code number of the certificate allocated by the competent authority on the top.
(g) The original of the certificate must be completed and signed by an official veterinarian not more than 24 hours prior to loading of the consignment for export to the Community. To that end, the competent authorities of the exporting country shall ensure that principles of certification equivalent to those laid down in Directive 96/93/EC are followed.
The colour of the signature shall be different from that of the printing. The same rule shall apply to stamps other than embossed stamps or watermarks.
(h) The original of the certificate must accompany the consignment as far as the EU border inspection post.
(i) The certificate shall be valid for 10 days from the date of issue, unless otherwise stated.
In the case of transport by ship, the term of validity shall be extended by the time taken by the voyage. To that end, the original of a declaration by the ship's master, drawn up in accordance with the addendum to Part 3 of this Annex, shall be attached to the veterinary certificate.
(j) Poultry shall not be transported with other poultry that is either not intended for the European Community or of a lower health status.
(k) During transport to the European Community, poultry shall not be unloaded in the territory of a third country or part of a third country that is not approved for imports of poultry into the Community.
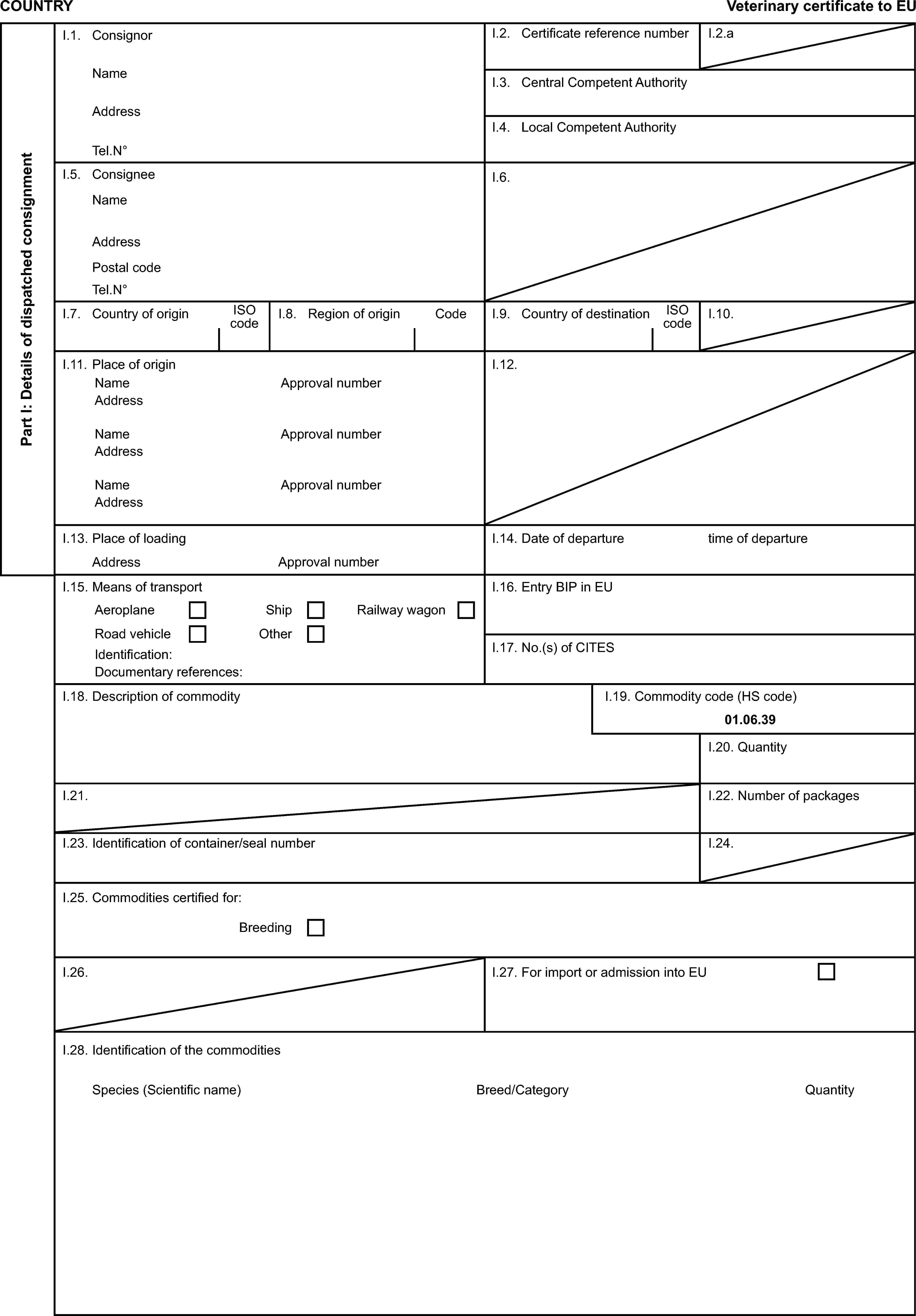
Model veterinary certificate for breeding or productive poultry other than ratites (BPP)




Model veterinary certificate for breeding or productive ratites (BPR)


Model veterinary certificate for day-old chicks other than of ratites (DOC)




Model veterinary certificate for day-old chicks of ratites (DOR)


Model veterinary certificate for hatching eggs of poultry other than ratites (HEP)
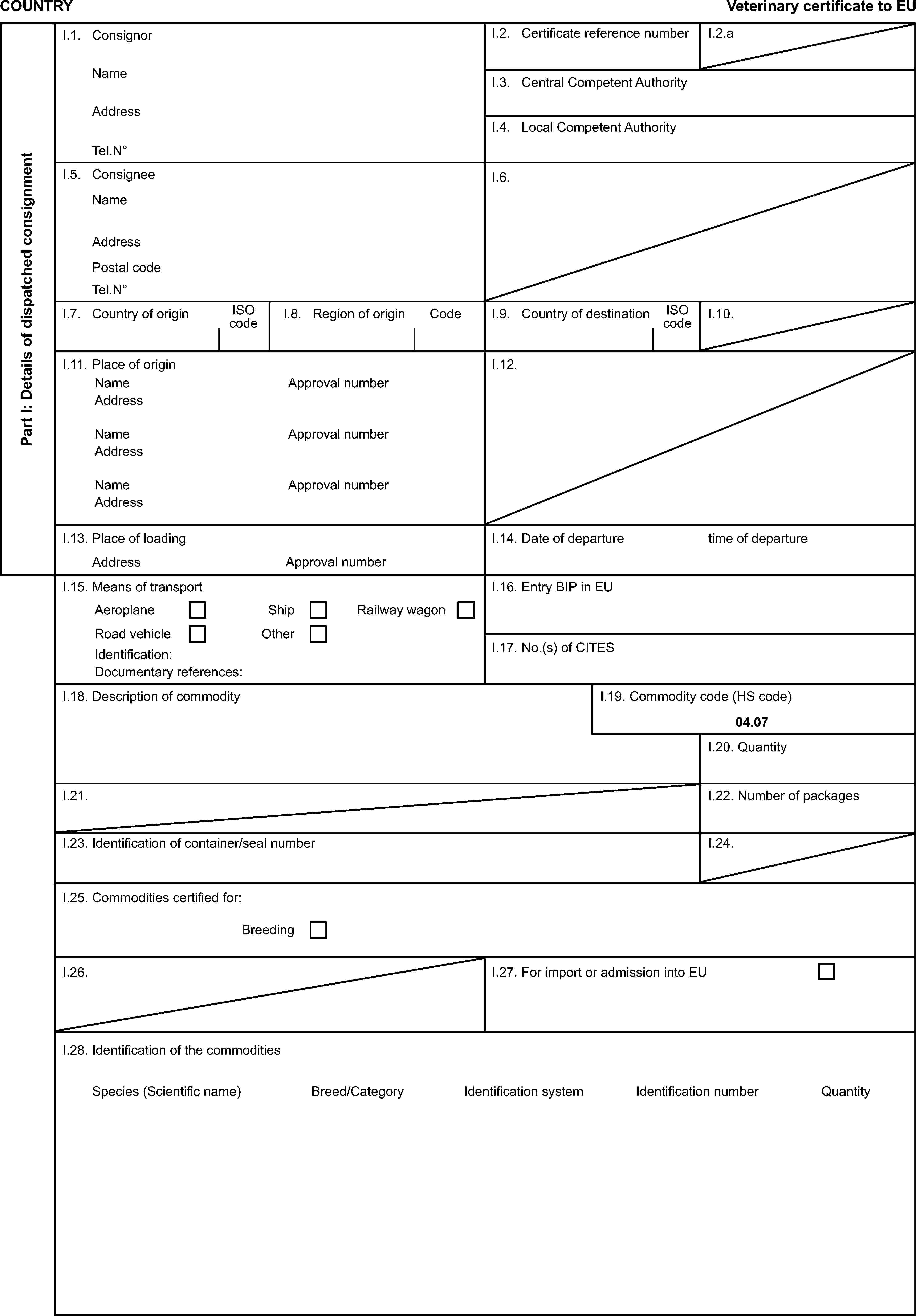



Model veterinary certificate for hatching eggs of ratites (HER)




Model veterinary certificate for specified pathogen-free eggs (SPF)



Model veterinary certificate for slaughter poultry and poultry for restocking game supplies other than ratites (SRP)




Model veterinary certificate for slaughter ratites (SRA)
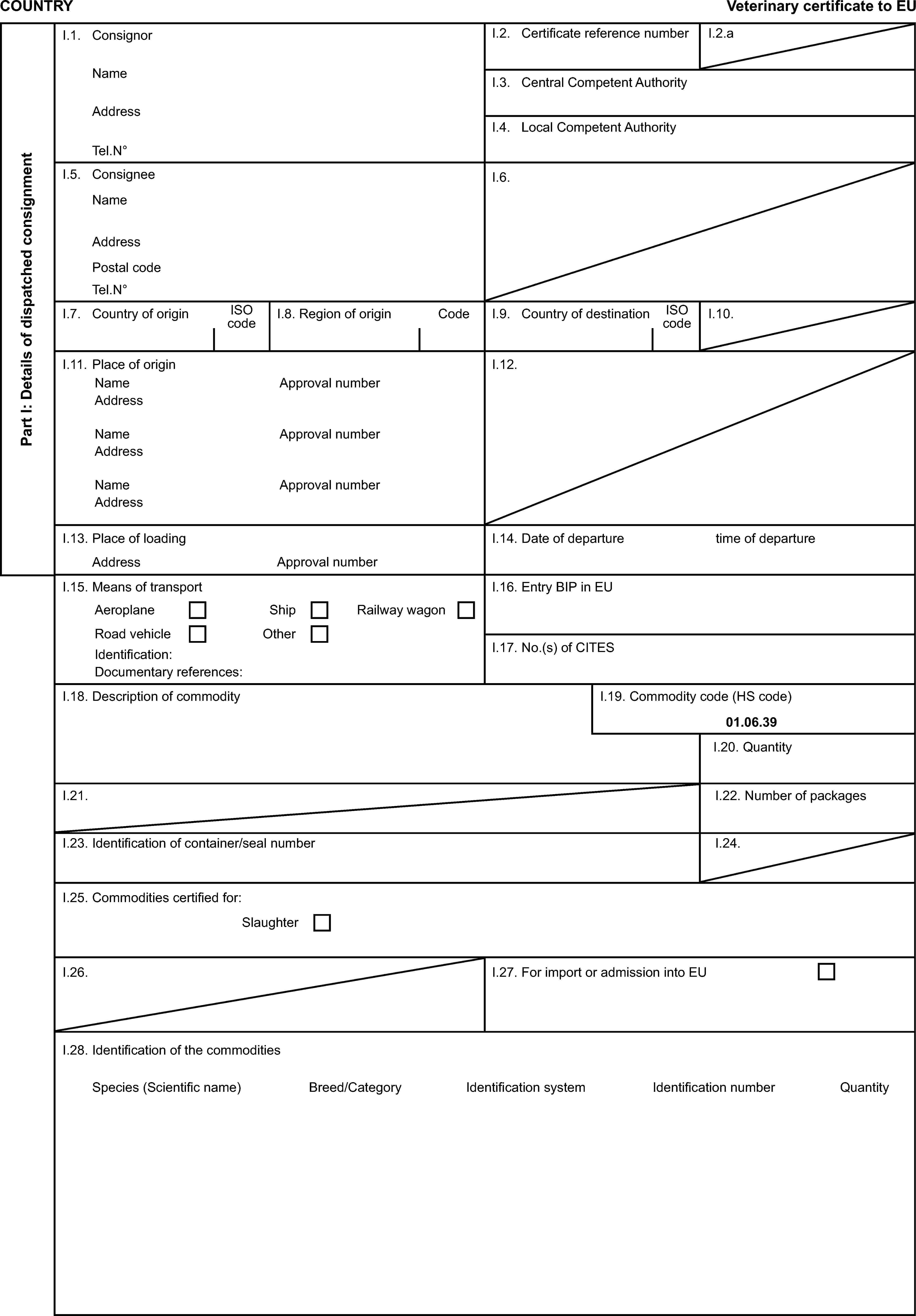


PART 3
Addendum for transport of poultry by sea
(To be completed and attached to the veterinary certificate where transport to the European Community border includes transport by ship, even for part of the journey.)

PART 4
A. Methods for standardisation of materials and procedures for veterinary tests for imports of poultry and hatching eggs
1. Newcastle disease
The sampling and testing methods must comply with the methods described in the Annex to Decision 92/340/EEC on testing of poultry for Newcastle disease prior to movement, in accordance with Article 12 of Directive 90/539/EEC.
2. Salmonella pullorum
— The sampling methods must comply with the methods described in Chapter III of Annex II to Directive 90/539/EEC.
— The testing methods must comply with the methods described in the latest version of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, published by OIE.
3. Salmonella gallinarum
— The sampling methods must comply with the methods described in Chapter III of Annex II to Directive 90/539/EEC.
— The testing methods must comply with the methods described in the latest version of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, published by OIE.
4. Salmonella arizonae
Serological examination: 60 birds to be sampled at the point of lay. Testing must be carried out in accordance with the methods described in the latest version of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, published by OIE.
5. Mycoplasma gallisepticum
— The sampling methods must comply with the methods described in Chapter III of Annex II to Directive 90/539/EEC.
— The testing methods must comply with the methods described in the latest version of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, published by OIE.
6. Mycoplasma meleagridis
The sampling methods must comply with the methods described in Chapter III of Annex II to Directive 90/539/EEC.
B. Sampling and testing procedures for Newcastle disease and avian influenza after import
During the period referred to in Article 10(1), the official/authorised veterinarian shall take samples from the imported poultry for virological examination, to be tested as follows:
— between the 7th and the 15th day of the isolation period, cloacal swabs must be taken from all birds where the consignment contains fewer than 60 birds, and from 60 birds where consignments contain more than 60 birds,
— testing of samples for avian influenza and Newcastle disease must be carried out in official laboratories designated by the competent authority, using diagnostic procedures in accordance with Annex III to Council Directive 92/66/EEC ( 29 ) and Annex III to Council Directive 92/40/EEC ( 30 ),
— samples may be pooled, subject to a maximum of five samples from individual birds in each pool,
— virus isolates must be sent without delay to the national reference laboratory.
ANNEX II
MEAT, MINCED MEAT, MECHANICALLY SEPARATED MEAT, EGGS AND EGG PRODUCTS
PART 1
List of third countries or parts thereof (1)
|
Country |
Code of territory |
Description of territory |
Veterinary certificate |
Specific conditions |
||
|
Model(s) |
Additional guarantees |
|||||
|
1 |
2 |
3 |
4 |
5 |
6 |
|
|
AL – Albania |
AL-0 |
EP, E |
||||
|
AR – Argentina |
AR-0 |
EP, E, POU, RAT |
||||
|
WGM |
III |
|||||
|
AU – Australia |
AU-0 |
EP, E |
||||
|
POU |
I |
|||||
|
RAT |
II |
|||||
|
BG – Bulgaria (2) |
BG-0 |
EP, E, POU, RAT, WGM |
||||
|
BR – Brazil |
BR-0 |
— |
||||
|
BR-1 |
States of Rio Grande do Sul, Santa Catarina, Paraná, São Paulo and Mato Grosso do Sul |
RAT |
||||
|
BR-2 |
Distrito Federal and States of Goiás, Minas Gerais, Mato Grosso, Mato Grosso do Sul, Paraná, Rio Grande do Sul, Santa Catarina and São Paulo |
WGM |
III |
|||
|
EP, E, POU |
||||||
|
BW – Botswana |
BW-0 |
RAT, EP, E |
II |
|||
|
CA – Canada |
CA-0 |
WGM |
III |
|||
|
EP, E, POU, RAT |
||||||
|
CH – Switzerland |
CH-0 |
EP, E, POU, RAT, WGM |
||||
|
CL – Chile |
CL-0 |
WGM |
III |
|||
|
EP, E, POU, RAT, SRA |
||||||
|
CN – China (People's Republic of) |
CN-0 |
EP, E |
||||
|
CN-1 |
Municipality of Shanghai, excluding county of Chongming, and, in the Province of Shangdong, the districts (prefectures) of Weifang, Linyi and Qingdao |
POU |
I |
|||
|
GL – Greenland |
GL-0 |
EP, WGM |
||||
|
HK – Hong Kong |
HK-0 |
EP |
||||
|
HR – Croatia |
HR-0 |
EP, E, POU, RAT, WGM |
||||
|
IL – Israel |
IL-0 |
WGM |
III |
|||
|
EP, E POU, RAT |
||||||
|
IN – India |
IN-0 |
EP |
||||
|
IS – Iceland |
IS-0 |
EP, E |
||||
|
KR – Korea (Rep) |
KR-0 |
EP, E |
||||
|
MG – Madagascar |
MG-0 |
EP, E, WGM |
||||
|
MY – Malaysia |
MY-0 |
— |
||||
|
MY-1 |
Western Peninsular |
EP, E |
||||
|
MK – Former Yugoslav Republic of Macedonia (3) |
MK-0 |
EP |
||||
|
MX – Mexico |
MX-0 |
EP |
||||
|
NA – Namibia |
NA-0 |
RAT, EP, E |
II |
|||
|
NC – New Caledonia |
NC-0 |
EP |
||||
|
NZ – New Zealand |
NZ-0 |
WGM |
III |
|||
|
EP, E, POU, RAT |
||||||
|
RO – Romania (2) |
RO-0 |
EP, E, POU, RAT, WGM |
||||
|
RU – Russian Federation |
RU-0 |
EP |
||||
|
XM – Montenegro |
XM-0 |
Whole custom territory () |
EP |
|||
|
XS – Serbia (4) |
XS-0 |
Whole custom territory () |
EP |
|||
|
SG – Singapore |
SG-0 |
EP |
||||
|
TH – Thailand |
TH-0 |
WGM |
III |
|||
|
EP, E, POU, RAT |
||||||
|
TN – Tunisia |
TN-0 |
WGM |
III |
|||
|
EP, E, POU, RAT |
||||||
|
TR – Turkey |
TR-0 |
EP, E |
||||
|
US – United States |
US-0 |
WGM |
III |
|||
|
EP, E, POU, RAT |
||||||
|
UY – Uruguay |
UY-0 |
EP, E, RAT |
||||
|
ZA – South Africa |
ZA-0 |
RAT, EP, E |
II |
|||
|
ZW – Zimbabwe |
ZW-0 |
RAT, EP, E |
II |
|||
|
(1) Without prejudice to specific certification requirements provided for in Community agreements with third countries. (2) Only applicable until this Acceding State becomes a Member State of the Community. (3) The former Yugoslav Republic of Macedonia; provisional code that does not affect the definitive denomination of the country to be attributed after the conclusion of the negotiations currently taking place in the United Nations. (4) Not including Kosovo as defined by United Nations Security Council Resolution 1 244 of 10 June 1999. (5) Serbia and Montenegro are Republics with individual customs forming a State Union and therefore are listed separately. |
||||||
PART 2
Model veterinary certificates
Model(s):
|
‘POU’ |
: |
Model veterinary certificate for meat of poultry |
|
‘POU-MI/MSM’ |
: |
Model veterinary certificate for minced meat and mechanically separated meat of poultry |
|
‘RAT’ |
: |
Model veterinary certificate for meat of farmed ratites for human consumption |
|
‘RAT-MI/MSM’ |
: |
Model veterinary certificate for minced meat and mechanically separated meat of farmed ratites for human consumption |
|
‘WGM’ |
: |
Model veterinary certificate for wild game-bird meat |
|
‘WGM-MI/MSM’ |
: |
Model veterinary certificate for wild game-bird minced meat and mechanically separated meat |
|
‘E’ |
: |
Model public health certificate for eggs |
|
‘EP’ |
: |
Model public-health certificate for egg products |
Additional guarantees (AG):
|
‘I.’ |
: |
additional guarantees covering poultry meat certified in accordance with model POU |
|
‘II.’ |
: |
additional guarantees covering meat of farmed ratites for human consumption certified in accordance with model RAT |
|
‘III.’ |
: |
additional guarantees for wild game-bird meat certified in accordance with model WGM |
Notes
(a) Veterinary certificates shall be issued by the exporting third country based on the models in Part 2 of Annex I or this Annex and following the layout of the model that corresponds to the commodity concerned. They shall contain, in the order appearing in the model, the attestations that are required for any third country and, where applicable, those additional health requirements required for the exporting third country or part thereof.
Where additional guarantees are required by the EU Member State of destination for the commodity concerned, these shall also be entered on the original of the veterinary certificate.
(b) A separate, single certificate must be presented for each consignment of the commodity concerned, exported to the same destination from a territory appearing in columns 2 and 3 of Part 1 of Annex I or this Annex and transported in the same railway wagon, lorry, aircraft or ship.
(c) The original of certificates shall consist of a single page printed on both sides or, where more text is required, such that all the pages form a whole and cannot be separated.
(d) The certificate shall be drawn up in at least one official language of the EU Member State where the border inspection takes place and in one official language of the EU Member State of destination. However, those Member States may allow another Community language instead of their own, accompanied, if necessary, by an official translation.
(e) Where additional pages are attached to the certificate for the purposes of identifying the items making up the consignment, such additional pages shall also be considered to form part of the original of the certificate, provided the signature and stamp of the certifying official veterinarian appear on each page.
(f) Where the certificate, including any additional pages as provided for in (e), comprises more than one page, each page shall be numbered ‘–x(page number) of y(total number of pages)–’ on the bottom and shall bear the code number of the certificate allocated by the competent authority on the top.
(g) The original of the certificate must be completed and signed by an official veterinarian no more than 24 hours prior to loading of the consignment for export to the Community. To that end, the competent authorities of the exporting country shall ensure that principles of certification equivalent to those laid down in Directive 96/93/EC are followed.
The colour of the signature shall be different from that of the printing. The same rule shall apply to stamps other than embossed stamps or watermarks.
(h) The original of the certificate must accompany the consignment as far as the EU border inspection post.
(i) The certificate shall be valid for 10 days from the date of issue, unless otherwise stated.
In the case of transport by ship, the term of validity shall be extended by the time taken by the voyage. To that end, the original of a declaration by the ship's master, drawn up in accordance with the addendum to Part 3 of Annex I, shall be attached to the veterinary certificate.
Specific conditions referred to in column 6
|
Code of territory |
Veterinary certificate |
Period/dates during which imports into the Community are authorised or not authorised in relation to dates of slaughter/killing of animals from which the meat was obtained |
||
|
Model |
AG |
|||
Model veterinary certificate for meat of poultry (POU)



Model veterinary certificate for minced meat and mechanically separated meatof poultry (POU-MI/MSM)
(NOT YET ESTABLISHED)
Model veterinary certificate for meat of farmed ratites for human consumption (RAT)




Model veterinary certificate for minced meat and mechanically separated meatof farmed ratites for human consumption (RAT-MI/MSM)
(NOT YET ESTABLISHED)
Model veterinary certificate for wild game-bird meat (WGM)



Model veterinary certificate for wild game-bird minced meat and mechanically separated meat(WGM-MI/MSM)
(NOT YET ESTABLISHED)
Model veterinary certificate for eggs (E)

![Part II: CertificationE (Eggs)II. Health informationII.a. Certificate reference numberII.b.II.1. Health attestationI, the undersigned official veterinarian/official inspector, declare that I am aware of the relevant provisions of Regulations (EC) Nos 178/2002, 852/2004, 853/2004 and 2160/2003 and hereby certify that the eggs described in this certificate have been obtained in accordance with those requirements, and in particular that:II.1.1. they come from (an) establishments(s) implementing a programme based on the HACCP principles in accordance with Regulation (EC) No 852/2004;II.1.2. they have been kept, stored, transported and delivered in accordance with the relevant conditions laid down in Section X, Chapter I of Annex III to Regulation (EC) No 853/2004;(1) II.1.3. they fulfil the requirements of Commission Regulation (EC) No 1688/2005 of 14 October 2005 implementing Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards special guarantees concerning Salmonella for consignments to Finland and Sweden of certain meat and eggs;II.1.4. the guarantees covering live animals and products thereof provided by the residue plans submitted in accordance with Directive 96/23/EC, and in particular Article 29 thereof, are fulfilled.(2) II.1.5. they fulfil the requirements in Article 10(6) of Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of Salmonella and other specified food-borne zoonotic agents. In particular:Eggs shall not be imported from flocks of laying hens in which Salmonella ssp. has been detected a result of the epidemiological investigation of a food-borne outbreak or if no equivalent guarantees have been provided.[From 1 January 2009 on, eggs shall also not be imported from flocks of laying hens with unknown health status, that are suspected of being infected or from flocks infected by Salmonella ssp. for which a target for reduction has been set in Community legislation (3) and on which monitoring equivalent to the monitoring laid down in the provisions in the Annex of Regulation (EC) No 1168/2006 is not applied, or if no equivalent guarantees have been provided.]NotesPart I:Box I.8: provide the code for the region of origin, if necessary, as defined under code of territory in column 2, Part 1 of Annex II of Decision 2006/696/EC [as last amended].Box I.11: Name, address and approval number of establishment of dispatch.Box I.15: Indicate the registration number(s) of railway wagons and lorries, the names of ships and, if known, the flight numbers of aircraft. In the case of transport in containers or boxes, the total number of these and their registration and seal numbers, where applicable, should be indicated in box I.23.Part II:(1) Delete if the consignment is not intended for export to Sweden or Finland.(2) Only applicable in case of import of eggs, Class A in accordance with Article 3 of Regulation (EC) No 1028/2006. Delete as appropriate.(3) Salmonella Enteritidis and Salmonella TyphimuriumOfficial veterinarian or official inspectorName (in capitals):Qualification and title:Local competent authority:Date:Stamp:Signature:](./../../../resource.html?uri=celex:02006D0696-20071027.ENG.xhtml.L_2007280EN.01000901.tif.jpg)
Model veterinary certificate for egg products(EP)

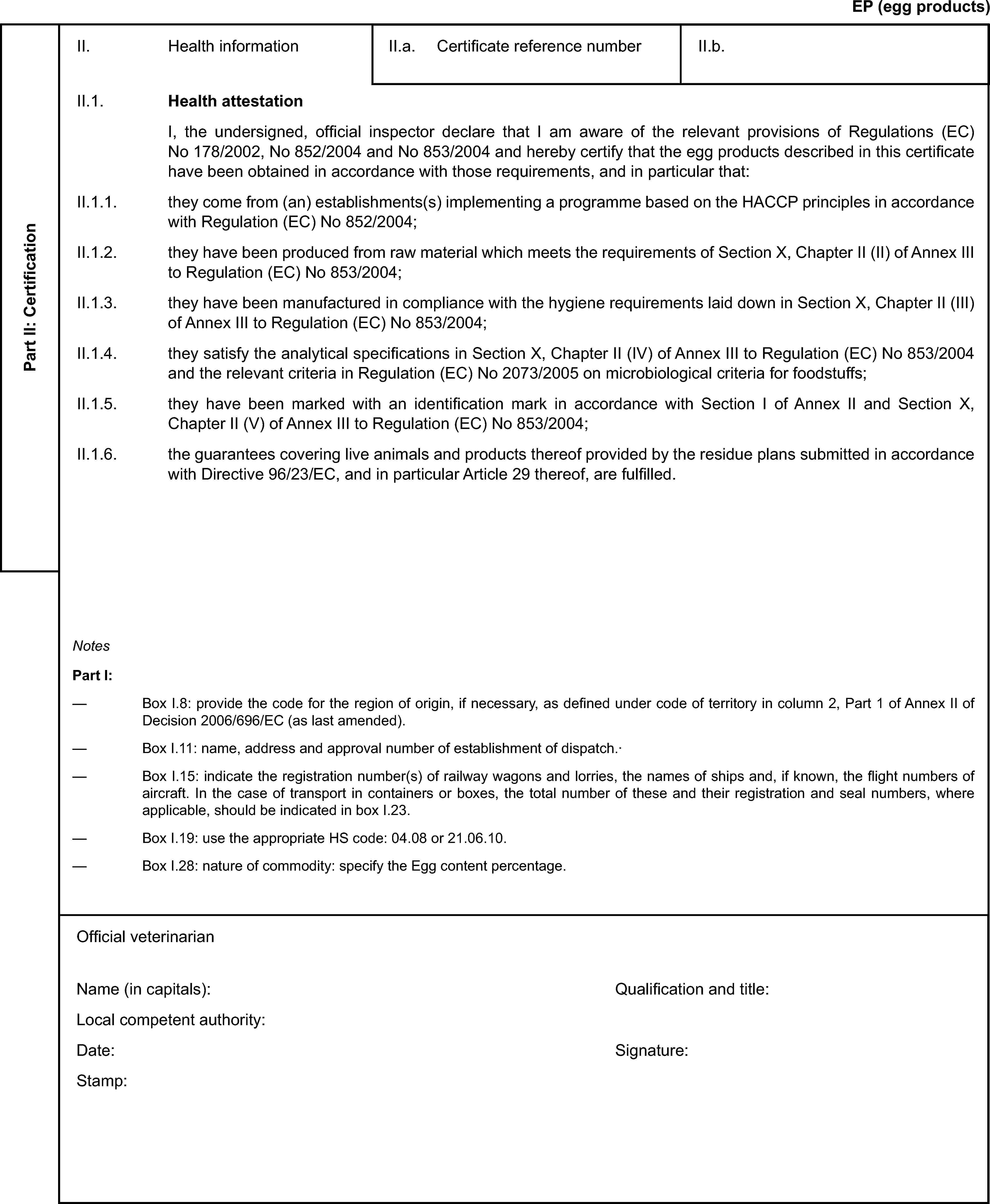
ANNEX III
Indications referred to in Article 9 (3)
— in Spanish: ‘para incubar’
— in Czech: ‘líhnutí’
— in Danish: ‘rugeæg’
— in German: ‘Brutei’
— in Estonian: ‘haue’
— in Greek: ‘προς εκκόλαψιν’
— in English: ‘hatching’
— in French: ‘à couver’
— in Italian: ‘cova’
— in Latvian: ‘inkubācija’
— in Lithuanian: ‘Skirti perinti’
— in Hungarian: ‘keltetésre’
— in Maltese: ‘tifqis’
— in Dutch: ‘broedei’
— in Polish: ‘do wylęgu’
— in Portuguese: ‘para incubação’
— in Slovak: ‘liahnutie’
— in Slovene: ‘valjenje’
— in Finnish: ‘haudottavaksi’
— in Swedish: ‘för kläckning’.
ANNEX IV
Model veterinary certificate for transit/storage of meat minced meat and mechanically separated meat of poultry, ratites and wild game-birds, specified pathogen-free eggs, eggs and egg products

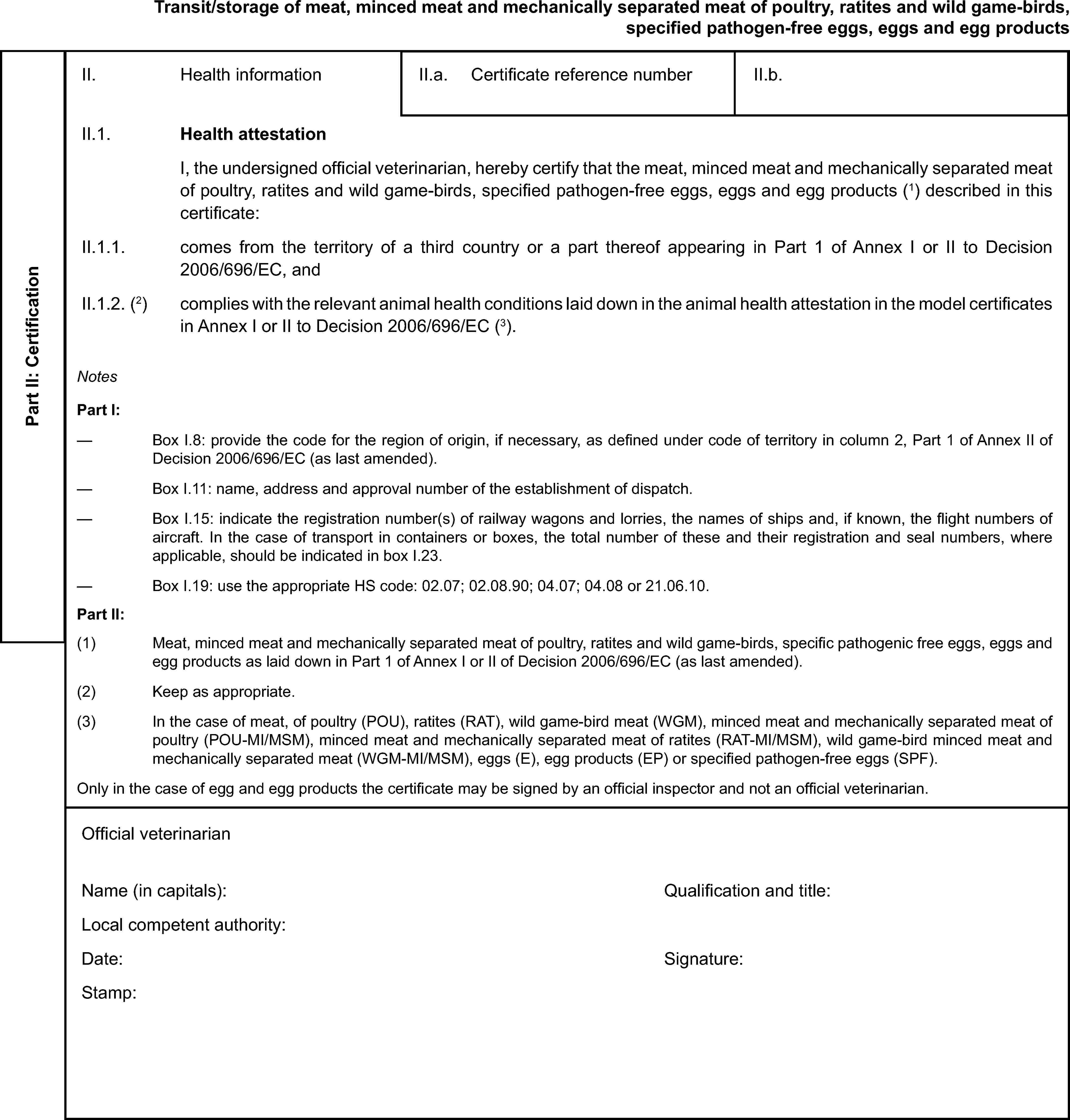
ANNEX V
PROTECTIVE MEASURES IN RELATION TO CRIMEAN-CONGO HAEMORRHAGIC FEVER
PART 1
For ratites
The competent authority shall ensure that the ratites are isolated in rodent-proof, tick-free surroundings for at least 21 days prior to export.
Before moving to the tick-free surroundings, the birds shall be treated to ensure that all ectoparasites on them are destroyed. After 14 days in tick-free surroundings, the ratites shall undergo the competitive ELISA test for antibodies to Crimean-Congo haemorrhagic fever. Every animal put into isolation must test negative to the test. On the animals' arrival in the Community, the treatment for ectoparasites and the serological test shall be repeated.
PART 2
For ratite meat
The competent authority shall ensure that the ratites are isolated in rodent-proof, tick-free surroundings for at least 14 days prior to slaughter.
Before moving to the tick-free surroundings, the birds shall either be examined to verify that they are tick-free or treated to ensure that all ticks on them are destroyed. The treatment used must be specified on the import certificate. Any treatment used shall not result in any detectable residues in the ratite meat.
Each batch of ratites shall be examined for ticks prior to slaughter. If any are detected, the entire batch shall again be put into pre-slaughter isolation.
ANNEX VI
‘ANNEX II
Animal and public-health conditions appearing in model veterinary certificate to be requested
|
Country |
Code of terri-tory |
Leporidae (rabbit and hare) |
Wild land mammals other than leporidae and ungulates |
|||||
|
Wild |
Domestic rabbit |
|||||||
|
MC (2) |
SC (3) |
MC (2) |
SC (3) |
MC (2) |
SC (3) |
|||
|
AR |
Argentina |
AR |
C |
H |
— |
|||
|
AU |
Australia |
AU |
C |
H |
E |
|||
|
BG |
Bulgaria (1) |
BG |
C |
H |
— |
|||
|
BR |
Brazil |
BR |
C |
H |
— |
|||
|
CA |
Canada |
CA |
C |
H |
E |
|||
|
CH |
Switzerland |
CH |
C |
H |
— |
|||
|
CL |
Chile |
CL |
C |
H |
— |
|||
|
GL |
Greenland |
GL |
C |
H |
E |
|||
|
HR |
Croatia |
HR |
C |
H |
— |
|||
|
IL |
Israel |
IL |
C |
H |
— |
|||
|
NZ |
New Zealand |
NZ |
C |
H |
E |
|||
|
RO |
Romania (1) |
RO |
C |
H |
E |
|||
|
RU |
Russia |
RU |
C |
H |
E |
|||
|
TH |
Thailand |
TH |
C |
H |
— |
|||
|
TN |
Tunisia |
TN |
C |
H |
— |
|||
|
US |
United States |
US |
C |
H |
— |
|||
|
Any other third country appearing in the list in Part 1 of Annex II to Council Decision 79/542/EEC, as last amended |
C |
H |
— |
|||||
|
(1) Only applicable until this Acceding State becomes a Member State of the Community. (2) MC: model certificate to be completed. The letters “C”, “H” and “E” appearing in the table refer to the model certificate described in Annex III to this Decision, to be used for each category of meat. A dash “—” indicates that imports of the meat are not authorised. (3) SC: specific conditions. The numbers appearing in the table refer to the specific conditions to be met by the exporting country, as described in Annex IV to this Decision. They must be inserted by the exporting country in Section V of the relevant model certificate set out in Annex III to this Decision.’ |
||||||||
( 1 ) OJ L 303, 31.10.1990, p. 6. Directive as last amended by the 2003 Act of Accession.
( 2 ) OJ L 268, 24.9.1991, p. 56. Directive as last amended by the 2003 Act of Accession.
( 3 ) OJ L 62, 15.3.1993, p. 49. Directive as last amended by Commission Regulation (EC) No 445/2004 (OJ L 72, 11.3.2004, p. 60).
( 4 ) OJ L 125, 23.5.1996, p. 10. Directive as last amended by Regulation (EC) No 882/2004 of the European Parliament and of the Council (OJ L 165, 30.4.2004, p. 1. (corrected by OJ L 191, 28.5.2004, p. 1).
( 5 ) OJ L 24, 30.1.1998, p. 9. Directive as last amended by Regulation (EC) No 882/2004.
( 6 ) OJ L 18, 23.1.2003, p. 11.
( 7 ) OJ L 139, 30.4.2004, p. 55. (corrected by OJ L 226, 25.6.2004, p. 22) Regulation as amended by Regulation (EC) No 2076/2005 (OJ L 338, 22.12.2005, p. 83.
( 8 ) OJ L 139, 30.4.2004, p. 206. (corrected by OJ L 226, 25.6.2004, p. 83). Regulation as amended by Regulation (EC) No 2076/2005.
( 9 ) OJ L 44, 17.2.1994, p. 31. Decision as last amended by Decision 2004/118/EC (OJ L 36, 7.2.2004, p. 34).
( 10 ) OJ L 44, 17.2.1994, p. 33. Decision as amended by Decision 96/137/EC (OJ L 31, 9.2.1996, p. 31).
( 11 ) OJ L 378, 31.12.1994, p. 11. Decision as last amended by Decision 2004/436/EC (OJ L 189, 27.5.2004, p. 47).
( 12 ) OJ L 156, 7.7.1995, p. 76. Decision as last amended by Decision 2004/118/EC.
( 13 ) OJ L 196, 7.8.1996, p. 13. Decision as last amended by Decision 2004/118/EC.
( 14 ) OJ L 302, 26.11.1996, p. 27. Decision as last amended by Decision 2001/751/EC (OJ L 281, 25.10.2001, p. 24).
( 15 ) OJ L 14, 17.1.1997, p. 61.
( 16 ) OJ L 258, 12.10.2000, p. 49. Decision as last amended by Decision 2005/804/EC (OJ L 303, 22.11.2005, p. 56).
( 17 ) OJ L 138, 22.5.2001, p. 31. Decision amended by Decision 2002/278/EC (OJ L 99, 16.4.2002, p. 14).
( 18 ) OJ L 282, 1.11.1975, p. 100. Regulation as last amended by the 2003 Act of Accession.
( 19 ) OJ L 173, 6.7.1990, p. 5. Regulation as last amended by Regulation (EC) No 1039/2005 (OJ L 172, 5.7.2005, p. 1).
( 20 ) OJ L 31, 1.2.2002, p. 1. Regulation as last amended by Commission Regulation (EC) No 575/2006 (OJ L 100, 8.4.2006, p. 3).
( 21 ) OJ L 139, 30.4.2004, p. 1. (corrected by OJ L 226, 25.6.2004, p. 3).
( 22 ) OJ L 338, 22.12.2005, p. 1.
( 23 ) OJ L 13, 16.1.1997, p. 28.
( 24 ) OJ L 137, 8.6.1993, p. 24. Decision as last amended by Decision 94/438/EC (OJ L 181, 15.7.1994, p. 35).
( 25 ) OJ L 251, 6.10.2000, p. 1. Decision as last amended by Decision 2004/413/EC (OJ L 151, 30.4.2004, p. 54. (corrected by OJ L 208, 10.6.2004, p. 51).
( 26 ) OJ L 305, 22.11.2003, p. 17. Decision as amended by Decision 2004/19/EC (OJ L 5, 9.1.2004, p. 84).
( 27 ) OJ L 188, 8.7.1992, p. 34.
( 28 ) OJ L 326, 11.12.2001, p. 44.
( 29 ) OJ L 260, 5.9.1992, p. 1.
( 30 ) OJ L 167, 22.6.1992, p. 1.


