EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document 01993D0197-20150626
Commission Decision of 5 February 1993 on animal health conditions and veterinary certification for imports of registered equidae and equidae for breeding and production (93/197/EEC)
Consolidated text: Commission Decision of 5 February 1993 on animal health conditions and veterinary certification for imports of registered equidae and equidae for breeding and production (93/197/EEC)
Commission Decision of 5 February 1993 on animal health conditions and veterinary certification for imports of registered equidae and equidae for breeding and production (93/197/EEC)
1993D0197 — EN — 26.06.2015 — 037.001
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
|
COMMISSION DECISION of 5 February 1993 on animal health conditions and veterinary certification for imports of registered equidae and equidae for breeding and production (OJ L 086 6.4.1993, p. 16) |
Amended by:
|
|
|
Official Journal |
||
|
No |
page |
date |
||
|
L 138 |
11 |
9.6.1993 |
||
|
L 238 |
45 |
23.9.1993 |
||
|
L 317 |
82 |
18.12.1993 |
||
|
L 187 |
11 |
22.7.1994 |
||
|
L 214 |
17 |
19.8.1994 |
||
|
L 190 |
9 |
11.8.1995 |
||
|
L 190 |
11 |
11.8.1995 |
||
|
L 304 |
49 |
16.12.1995 |
||
|
L 19 |
53 |
25.1.1996 |
||
|
L 19 |
56 |
25.1.1996 |
||
|
L 107 |
1 |
30.4.1996 |
||
|
L 3 |
9 |
7.1.1997 |
||
|
L 14 |
57 |
17.1.1997 |
||
|
L 62 |
39 |
4.3.1997 |
||
|
L 163 |
44 |
6.6.1998 |
||
|
L 286 |
53 |
23.10.1998 |
||
|
L 83 |
77 |
27.3.1999 |
||
|
L 87 |
13 |
31.3.1999 |
||
|
L 96 |
31 |
10.4.1999 |
||
|
L 243 |
12 |
15.9.1999 |
||
|
L 64 |
22 |
11.3.2000 |
||
|
L 43 |
38 |
14.2.2001 |
||
|
L 214 |
49 |
8.8.2001 |
||
|
L 215 |
55 |
9.8.2001 |
||
|
L 282 |
81 |
26.10.2001 |
||
|
L 288 |
50 |
1.11.2001 |
||
|
L 308 |
41 |
27.11.2001 |
||
|
L 206 |
20 |
3.8.2002 |
||
|
L 287 |
42 |
25.10.2002 |
||
|
L 185 |
41 |
24.7.2003 |
||
|
L 36 |
20 |
7.2.2004 |
||
|
L 74 |
19 |
12.3.2004 |
||
|
L 362 |
1 |
20.12.2006 |
||
|
L 117 |
85 |
11.5.2010 |
||
|
L 220 |
74 |
21.8.2010 |
||
|
L 158 |
74 |
10.6.2013 |
||
|
L 326 |
49 |
6.12.2013 |
||
|
L 167 |
52 |
6.6.2014 |
||
|
COMMISSION IMPLEMENTING DECISION (EU) 2015/1009 of 24 June 2015 |
L 161 |
22 |
26.6.2015 |
|
Amended by:
|
C 241 |
21 |
29.8.1994 |
||
|
|
L 001 |
1 |
.. |
|
|
L 236 |
33 |
23.9.2003 |
Corrected by:
COMMISSION DECISION
of 5 February 1993
on animal health conditions and veterinary certification for imports of registered equidae and equidae for breeding and production
(93/197/EEC)
THE COMMISSION OF THE EUROPEAN COMMUNITIES,
Having regard to the Treaty establishing the European Economic Community,
Having regard to Council Directive 90/426/EEC of 26 June 1990 on animal health conditions governing the movement and import from third countries of equidae ( 1 ), as last amended by Directive 92/36/EEC ( 2 ), and in particular Article 15 point (a) and Article 16 thereof,
Whereas by Council Decision 79/542/EEC ( 3 ), as last amended by Commission Decision 93/100/EEC ( 4 ), the list of third countries from which the Member States authorize imports of equidae in particular has been established;
Whereas it is also necessary to take into account the regionalization of certain third countries appearing on the abovementioned list, which is the subject of Commission Decision 92/160/EEC ( 5 ), as amended by Decision 92/161/EEC ( 6 );
Whereas the responsible national veterinary authorities have undertaken to notify the Commission and the Member States, by telegram, telex or telefax, within 24 hours of the confirmation of the occurrence of any infectious or contagious disease in equidae of lists A and B of the International Office of Epizootic Diseases or of the adoption of vaccination against any of them or, within an appropriate period, of any changes in the national import rules concerning equidae;
Whereas the conditions to be established for imports of equidae for breeding and production apply without prejudice to the requirements of Countil Directive 86/469/EEC ( 7 ) that no thyreostatic, estrogenic, androgenic or gestagenic substances are used for fattening purposes in equidae;
Whereas Member States import equidae in accordance with the provisions of Council Directive 91/496/EEC ( 8 ), as last amended by Decision 92/438/EEC ( 9 ), which laid down the principles governing the organization of veterinary checks on animals entering the Community from third countries;
Whereas the existence of equivalent health situations between certain third countries justifies establishing several health zones for the importation of equidae;
Whereas the different categories of equidae have their own features and their imports are authorized for different purposes; whereas, consequently specific health requirements must be established for imports of registered equidae and equidae for breeding and production;
Whereas, given the existence of different health situations, it is therefore necessary to establish several health certificates for registered equidae and for equidae for breeding and production;
Whereas the measures provided for in this Decision are in accordance with the opinion of the Standing Veterinary Committee,
HAS ADOPTED THIS DECISION:
Article 1
Without prejudice to Decision 92/160/EEC, Member States shall authorize imports of registered equidae and equidae for breeding and production:
— coming from third countries appearing in Annex I, and
— conforming to the requirements laid down in the appropriate specimen animal health certificate set out in Annex II.
Article 2
This Decision is addressed to the Member States.
ANNEX I
Sanitary Group A ( 10 )
Switzerland (CH), Falkland Islands (FK), Greenland (GL), Iceland (IS)
Sanitary Group B (10)
Australia (AU), Belarus (BY), ►M36 ————— ◄ Kyrgyzstan ( 11 ) ( 12 ) (KG), Montenegro (ME), former Yugoslav Republic of Macedonia ( 13 ) (MK), New Zealand (NZ), Serbia (RS), Russia (13) (RU), Ukraine (UA)
Sanitary Group C (13)
Canada (CA), China (13) (13) (CN), Hong Kong (13) (HK), India (13) (13) (IN), Japan (13) (JP), Korea Republic (13) (KR), Macao (13) (MO), Malaysia (peninsula) (13) (MY), Singapore (13) (SG), Thailand (13) (TH), United States of America (US)
Sanitary Group D (13)
Argentina (AR), Barbados (13) (BB), Bermuda (13) (BM), Bolivia (13) (BO), Brazil (13) (13) (BR), Chile (CL), Cuba (13) (CU), Jamaica (13) (JM), Mexico (13) (MX), Peru (13) (13) (PE), Paraguay (PY), Uruguay (UY)
Sanitary Group E (13)
United Arab Emirates (13) (AE), Bahrain (13) (BH), Algeria (DZ), Israel ( 14 ) (IL), Jordan (14) (JO), Kuwait (14) (KW), Lebanon (14) (LB), Morocco (MA), Mauritius (14) (MU), Oman (14) (OM), Qatar (14) (QA), Saudi Arabia (14) (14) (SA), Tunisia (TN), Turkey (14) (14) (TR)
Sanitary Group F (14)
Sanitary Group G (14)
Saint Pierre and Miquelon (PM)
ANNEX II
|
A. |
Health certificate for imports of registered equidae and equidae for breeding and production from third countries assigned to group A. |
|
B. |
Health certificate for imports of registered equidae and equidae for breeding and production from third countries assigned to group B. |
|
C. |
Health certificate for imports of registered equidae and equidae for breeding and production from third countries assigned to group C. |
|
D. |
Health certificate for imports of registered equidae and equidae for breeding and production from third countries assigned to group D. |
|
E. |
Health certificate for imports of registered equidae and equidae for breeding and production from third countries assigned to group E. |
|
F. |
Health certificate for imports of registered equidae and equidae for breeding and production form third countries assigned to Group F. |
|
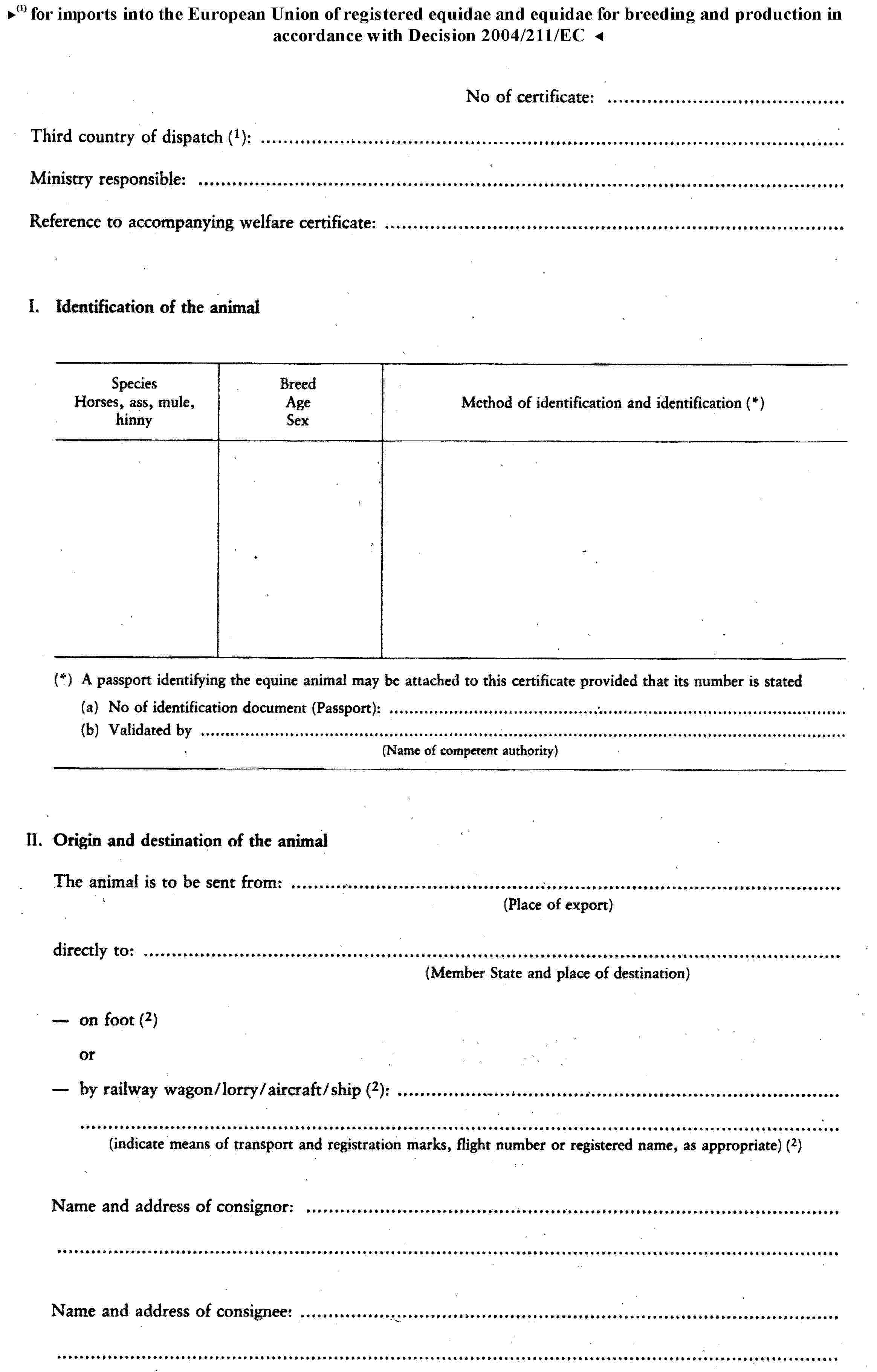
G. |
Health certificate for imports of registered equidae and equidae for breeding and production from third countries assigned to Group G. |
— A —
HEALTH CERTIFICATE
— B —
HEALTH CERTIFICATE

— C —
HEALTH CERTIFICATE
— D —
HEALTH CERTIFICATE
— E —
HEALTH CERTIFICATE



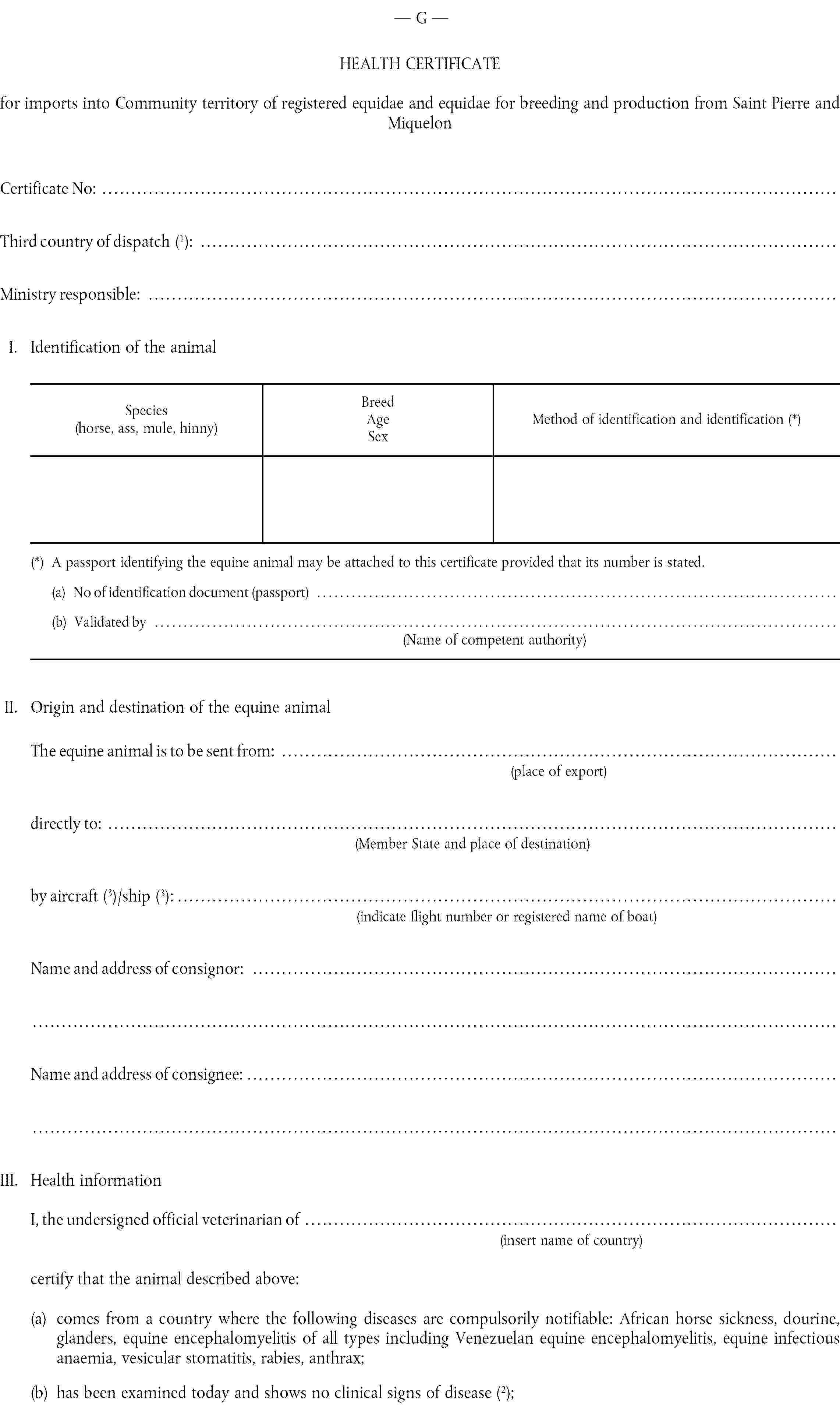

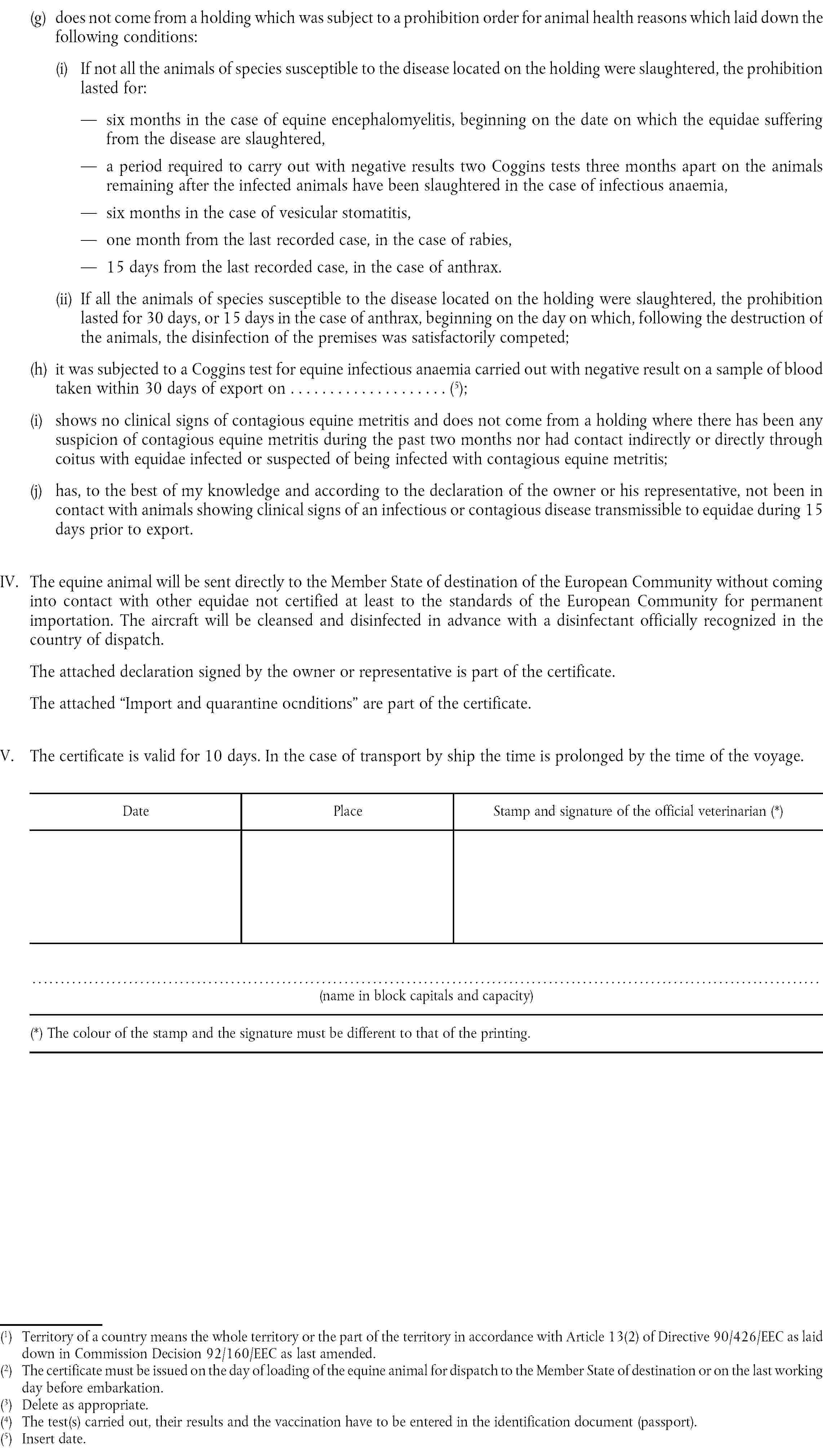



( 1 ) OJ No L 224, 18. 8. 1990, p. 42.
( 2 ) OJ No L 157, 10. 6. 1992, p. 28.
( 3 ) OJ No L 146, 14. 6. 1979, p. 15.
( 4 ) OJ No L 40, 17. 2. 1993, p. 23.
( 5 ) OJ No L 71, 18. 3. 1992, p. 27.
( 6 ) OJ No L 71, 18. 3. 1992, p. 29.
( 7 ) OJ No L 275, 16. 9. 1986, p. 36.
( 8 ) OJ No L 268, 24. 9. 1991, p. 56.
( 9 ) OJ No L 243, 25. 8. 1992, p. 27.
( 10 ) Sanitary group as indicated in column 5 of Annex I to Decision 2004/211/EC.
Third countries, territories or parts thereof assigned to that group shall use the Health Certificate with the same letter set out in Annex II to this Decision.
( 11 ) Part of the third country or territory in accordance with Article 13(2)(a) of Directive 90/426/EEC as indicated in columns 3 and 4 of Annex I to Decision 2004/211/EC.
( 12 ) Only registered horses.
( 13 ) Provisional code that does not affect the definitive denomination of the country to be attributed after the conclusion of the negotiations currently taking place in the United Nations.
( 14 ) ►M39 Hereafter understood as the State of Israel, excluding the territories under Israeli administration since June 1967, namely the Golan Heights, the Gaza Strip, East Jerusalem and the rest of the West Bank. ◄