EUR-Lex Access to European Union law

This document is an excerpt from the EUR-Lex website
Document 02016R0759-20161014
Commission Implementing Regulation (EU) 2016/759 of 28 April 2016 drawing up lists of third countries, parts of third countries and territories from which Member States are to authorise the introduction into the Union of certain products of animal origin intended for human consumption, laying down certificate requirements, amending Regulation (EC) No 2074/2005 and repealing Decision 2003/812/EC (Text with EEA relevance)
Consolidated text: Commission Implementing Regulation (EU) 2016/759 of 28 April 2016 drawing up lists of third countries, parts of third countries and territories from which Member States are to authorise the introduction into the Union of certain products of animal origin intended for human consumption, laying down certificate requirements, amending Regulation (EC) No 2074/2005 and repealing Decision 2003/812/EC (Text with EEA relevance)
Commission Implementing Regulation (EU) 2016/759 of 28 April 2016 drawing up lists of third countries, parts of third countries and territories from which Member States are to authorise the introduction into the Union of certain products of animal origin intended for human consumption, laying down certificate requirements, amending Regulation (EC) No 2074/2005 and repealing Decision 2003/812/EC (Text with EEA relevance)
2016R0759 — EN — 14.10.2016 — 001.001
This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document
|
COMMISSION IMPLEMENTING REGULATION (EU) 2016/759 of 28 April 2016 drawing up lists of third countries, parts of third countries and territories from which Member States are to authorise the introduction into the Union of certain products of animal origin intended for human consumption, laying down certificate requirements, amending Regulation (EC) No 2074/2005 and repealing Decision 2003/812/EC (OJ L 126 14.5.2016, p. 13) |
Amended by:
|
|
|
Official Journal |
||
|
No |
page |
date |
||
|
COMMISSION IMPLEMENTING REGULATION (EU) 2016/1793 of 10 October 2016 |
L 274 |
48 |
11.10.2016 |
|
COMMISSION IMPLEMENTING REGULATION (EU) 2016/759
of 28 April 2016
drawing up lists of third countries, parts of third countries and territories from which Member States are to authorise the introduction into the Union of certain products of animal origin intended for human consumption, laying down certificate requirements, amending Regulation (EC) No 2074/2005 and repealing Decision 2003/812/EC
(Text with EEA relevance)
CHAPTER 1
IMPORTS OF CERTAIN PRODUCTS OF ANIMAL ORIGIN
Article 1
Lists of third countries, parts of third countries and territories
The third countries, parts of third countries and territories from which Member States are to authorise the import of the following products of animal origin intended for human consumption are set out in the relevant Parts of Annex I:
(a) frogs' legs, Part I;
(b) snails, Part II;
(c) gelatine and collagen, Part III;
(d) raw materials for the production of gelatine and collagen, Part IV;
(e) treated raw materials for the production of gelatine and collagen, Part V;
(f) honey, royal jelly and other products of apiculture, Part VI;
(g) the following highly refined products, Part VII:
(i) chondroitin sulphate;
(ii) hyaluronic acid;
(iii) other hydrolysed cartilage products;
(iv) chitosan;
(v) glucosamine;
(vi) rennet;
(vii) isinglass;
(viii) amino acids that are authorised as food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council ( 1 ).
Article 2
Model certificates
1. The model certificates for imports into the Union of the products referred to in Article 1 are set out in Annex II as follows:
(a) frogs' legs, Part I;
(b) snails, Part II;
(c) gelatine, Part III;
(d) collagen, Part IV;
(e) raw materials for the production of gelatine and collagen, Part V;
(f) treated raw materials for the production of gelatine and collagen, Part VI;
(g) honey, royal jelly and other products of apiculture, Part VII;
(h) the following highly refined products, Part VIII:
(i) chondroitin sulphate;
(ii) hyaluronic acid;
(iii) other hydrolysed cartilage products;
(iv) chitosan;
(v) glucosamine;
(vi) rennet;
(vii) isinglass;
(viii) amino acids that are authorised as food additives in accordance with Regulation (EC) No 1333/2008.
Those certificates must be completed in accordance with the explanatory notes set out in Annex IV and the notes in the relevant certificate.
2. Electronic certification and other systems agreed between the Union and the third country concerned may be used.
CHAPTER 2
TRANSIT OF CERTAIN PRODUCTS OF ANIMAL ORIGIN
Article 3
Lists of third countries, parts of third countries and territories
The third countries, parts of third countries and territories from which Member States are to authorise the transit through the Union of raw materials and treated raw materials for the production of gelatine and collagen intended for human consumption bound for a third country, either by immediate transit or after storage in the Union in accordance with Article 12(4) and Article 13 of Council Directive 97/78/EC ( 2 ), are set out in Parts IV and V of Annex I to this Regulation, respectively.
Article 4
Model certificate
1. The model certificate for the transit through the Union of the raw materials and treated raw materials referred to in Article 3 is set out in Annex III.
That certificate must be completed in accordance with the notes set out in Annex IV and in the relevant model certificate.
2. Electronic certification and other systems harmonised at Union level may be used.
Article 5
Derogation for transit through Latvia, Lithuania and Poland
1. By way of derogation from Article 3, transit by road or by rail between the specific, designated border inspection posts in Latvia, Lithuania and Poland, listed and marked with special remark 13 in Annex I to Commission Decision 2009/821/EC ( 3 ), of consignments of the raw materials or treated raw materials referred to in Article 3 of this Regulation coming from and bound for Russia, directly or via another third country, shall be authorised where the following conditions are met:
(a) the consignment is sealed with a serially numbered seal by the official veterinarian at the border inspection post of entry;
(b) the documents accompanying the consignment, as provided for in Article 7 of Directive 97/78/EC, are stamped with the words ‘Only for transit to Russia via the EU’ on each page by the official veterinarian at the border inspection post of entry;
(c) the procedural requirements provided for in Article 11 of Directive 97/78/EC are complied with;
(d) the consignment is certified as acceptable for transit on the common veterinary entry document issued by the official veterinarian at the border inspection post of entry.
2. The consignments referred to in paragraph 1 shall not be unloaded or put into storage, as referred to in Article 12(4) or in Article 13 of Directive 97/78/EC, within the Union.
3. Regular audits shall be conducted by the competent authority to ensure that the number of consignments referred to in paragraph 1 and the corresponding quantities of products leaving the Union correspond with the number and quantities which have been introduced in the Union.
CHAPTER 3
FINAL PROVISIONS
Article 6
Amendment
Annex VI to Regulation (EC) No 2074/2005 is amended as follows:
(1) in Section I, Chapters I, II, III and VI are deleted;
(2) Appendices I, II, III and VI are deleted.
Article 7
Repeal
Decision 2003/812/EC is repealed.
Article 8
Transitional provisions
Consignments of products of animal origin in respect of which the relevant certificates have been issued in accordance with Regulation (EC) No 2074/2005 may continue to be introduced into the Union provided that the certificate was signed before 3 December 2016.
Article 9
Entry into force
This Regulation shall enter into force on the twentieth day following that of its publication in the Official Journal of the European Union.
This Regulation shall be binding in its entirety and directly applicable in all Member States.
ANNEX I
Lists of third countries, parts of third countries and territories as referred to in Article 1
PART I
FROGS' LEGS
Third countries and territories listed in the column ‘Countries’ of Annex II to Decision 2006/766/EC, except those for which a restriction is mentioned in the column ‘Restrictions’ of that Annex, and the following countries or territories:
|
COUNTRY ISO CODE |
COUNTRY/TERRITORY |
|
MK (1) |
former Yugoslav Republic of Macedonia |
|
(1) The former Yugoslav Republic of Macedonia; provisional code that does not prejudge in any way the definitive nomenclature for this country, which will be agreed following the conclusion of negotiations currently taking place on this subject in the United Nations. |
|
PART II
SNAILS
Third countries and territories listed in the column ‘Countries’ of Annex II to Decision 2006/766/EC, except those for which a restriction is mentioned in the column ‘Restrictions’ of that Annex, and the following countries/territories:
|
COUNTRY ISO CODE |
COUNTRY/TERRITORY |
|
MD |
Moldova |
|
MK (1) |
former Yugoslav Republic of Macedonia |
|
SY |
Syria |
|
(1) The former Yugoslav Republic of Macedonia; provisional code that does not prejudge in any way the definitive nomenclature for this country, which will be agreed following the conclusion of negotiations currently taking place on this subject in the United Nations. |
|
PART III
GELATINE AND COLLAGEN INTENDED FOR HUMAN CONSUMPTION
SECTION A
Gelatine and collagen derived from bovine, ovine, caprine, porcine and equine animals, both farmed and wild
Third countries and territories listed in column 1 of Part 1 of Annex II to Regulation (EU) No 206/2010 and the following countries or territories:
|
COUNTRY ISO CODE |
COUNTRY/TERRITORY |
|
KR |
Republic of Korea |
|
MY |
Malaysia |
|
PK |
Pakistan |
|
TW |
Taiwan |
SECTION B
Gelatine and collagen derived from poultry including ratites and feathered game
Third countries and territories listed in column 1 of Part 1 of Annex I to Regulation (EC) No 798/2008 and the following countries or territories:
|
COUNTRY ISO CODE |
COUNTRY/TERRITORY |
|
TW |
Taiwan |
SECTION C
Gelatine and collagen derived from fishery products
All third countries and territories listed in the column ‘Countries’ of Annex II to Decision 2006/766/EC, regardless of whether a restriction is mentioned in the column ‘Restrictions’ of that Annex.
SECTION D
Gelatine and collagen derived from leporidae and from wild land mammals not referred to in Section A
Third countries listed in column 1 of Part 1 of Annex I to Regulation (EC) No 119/2009.
PART IV
RAW MATERIALS FOR THE PRODUCTION OF GELATINE AND COLLAGEN INTENDED FOR HUMAN CONSUMPTION
SECTION A
Raw materials from bovine, ovine, caprine, porcine and equine animals, both farmed and wild
Third countries, territories and parts thereof listed in Part 1 of Annex II to Regulation (EU) No 206/2010 from which introduction into the Union of that category of fresh meat of the respective species is authorised as specified in that Part of that Annex, unless such introduction is limited by supplementary guarantees A or F as indicated in column 5.
SECTION B
Raw materials from poultry including ratites and feathered game
Third countries, parts of third countries and territories listed in Part 1 of Annex I to Regulation (EC) No 798/2008 from which imports of fresh poultry meat of the respective species is authorised as specified in that Part of that Annex.
SECTION C
Raw materials from fishery products
Third countries and territories listed in the column ‘Countries’ of Annex II to Decision 2006/766/EC, subject to the restrictions mentioned in the column ‘Restrictions’ of that Annex.
SECTION D
Raw materials from leporidae and from wild land mammals not referred to in Section A
Third countries listed in column 1 of Part 1 of Annex I to Regulation (EC) No 119/2009 from which imports of fresh meat of the respective species is authorised as specified in that Part of that Annex.
PART V
TREATED RAW MATERIALS FOR THE PRODUCTION OF GELATINE AND COLLAGEN INTENDED FOR HUMAN CONSUMPTION
SECTION A
Treated raw materials from bovine, ovine, caprine, porcine and equine animals, both farmed and wild
Third countries and territories and parts thereof listed in column 1 of Part 1 of Annex II to Regulation (EU) No 206/2010 and the following countries or territories:
|
COUNTRY ISO CODE |
COUNTRY/TERRITORY |
|
KR |
Republic of Korea |
|
MY |
Malaysia |
|
PK |
Pakistan |
|
TW |
Taiwan |
SECTION B
Treated raw materials from poultry including ratites and feathered game
Third countries and territories listed in column 1 of Part 1 of Annex I to Regulation (EC) No 798/2008 and the following countries or territories:
|
COUNTRY ISO CODE |
COUNTRY/TERRITORY |
|
TW |
Taiwan |
SECTION C
Treated raw materials from fishery products
All third countries and territories listed in the column ‘Countries’ of Annex II to Decision 2006/766/EC regardless of whether a restriction is mentioned in the column ‘Restrictions’ of that Annex.
SECTION D
Treated raw materials from leporidae and wild land mammals not referred to in Section A
Third countries listed in column 1 of Part 1 of Annex I to Regulation (EC) No 119/2009.
SECTION E
Treated raw materials referred to in Annex III to Regulation (EC) No 853/2004, Section XIV, Chapter I point 4(b)(iii) and Section XV, Chapter I, point 4(b)(iii)
Third countries, parts of third countries and territories referred to in Part IV of this Annex.
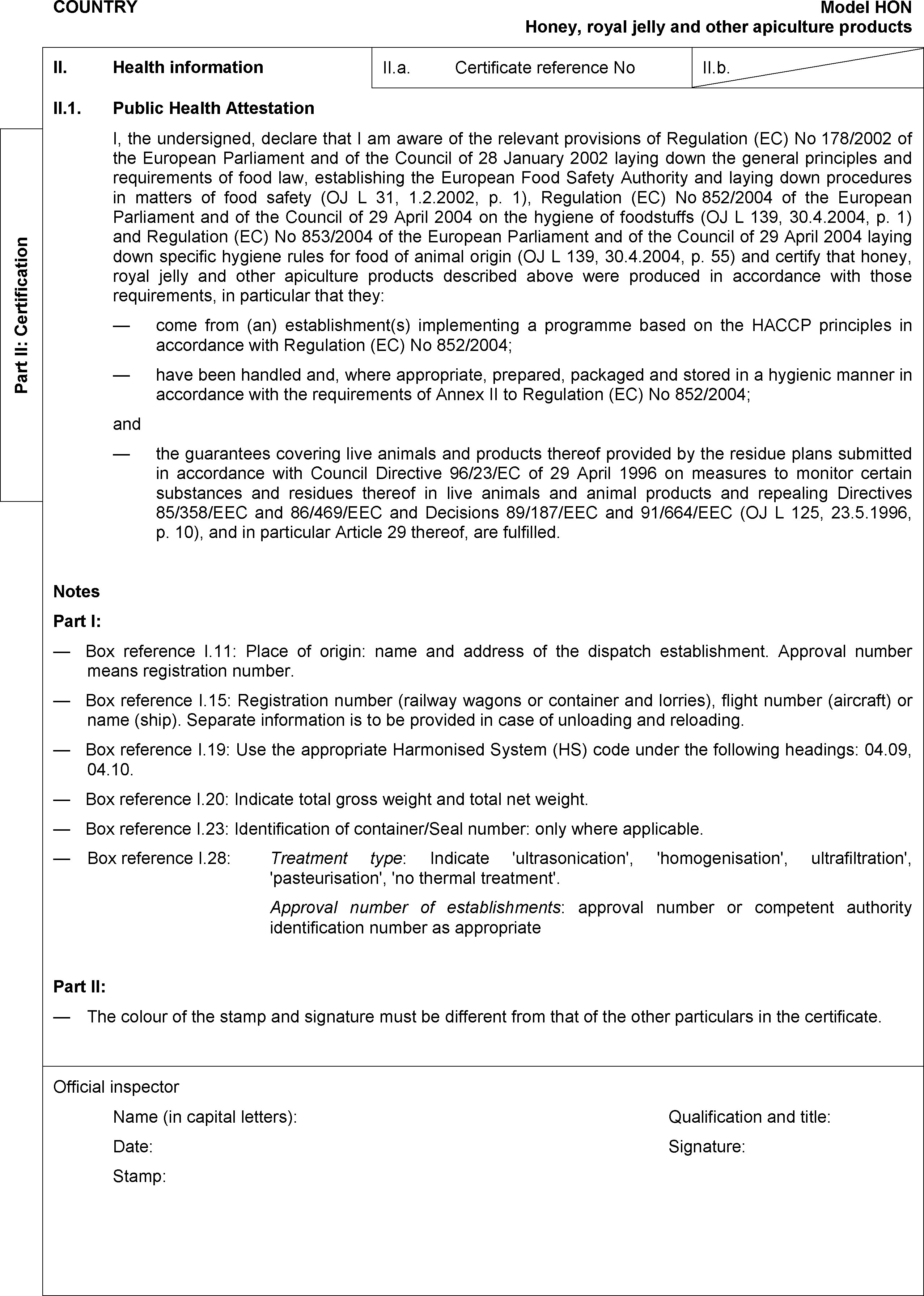
PART VI
HONEY, ROYAL JELLY AND OTHER PRODUCTS OF APICULTURE INTENDED FOR HUMAN CONSUMPTION
Third countries and territories listed in the column ‘Country’ in the Annex to Commission Decision 2011/163/EU ( 4 ) and marked with an ‘X’ in the column ‘Honey’ in that Annex.
PART VII
HIGHLY REFINED CHONDROITIN SULPHATE, HYALURONIC ACID, OTHER HYDROLYSED CARTILAGE PRODUCTS, CHITOSAN, GLUCOSAMINE, RENNET, ISINGLASS AND AMINO ACIDS FOR HUMAN CONSUMPTION
(a) In the case of raw materials derived from ungulates including equidae, third countries and territories listed in column 1 of Part 1 of Annex II to Regulation (EU) No 206/2010 and the following countries or territories:
|
COUNTRY ISO CODE |
COUNTRY/TERRITORY |
|
KR |
Republic of Korea |
|
MY |
Malaysia |
|
PK |
Pakistan |
|
TW |
Taiwan |
(b) In the case of the raw materials derived from fishery products, all third countries and territories listed in the column ‘Countries’ in Annex II to Decision 2006/766/EC, regardless of whether a restriction is mentioned in the column ‘Restrictions’ of that Annex.
(c) In the case of raw materials derived from poultry, third countries and territories listed in column 1 of Part 1 of Annex I to Regulation (EC) No 798/2008.
ANNEX II
Model certificates as referred to in Article 2
PART I
MODEL CERTIFICATE FOR IMPORTS OF CHILLED, FROZEN OR PREPARED FROGS' LEGS INTENDED FOR HUMAN CONSUMPTION



PART II
MODEL CERTIFICATE FOR IMPORTS OF CHILLED, FROZEN, SHELLED, COOKED, PREPARED OR PRESERVED SNAILS INTENDED FOR HUMAN CONSUMPTION

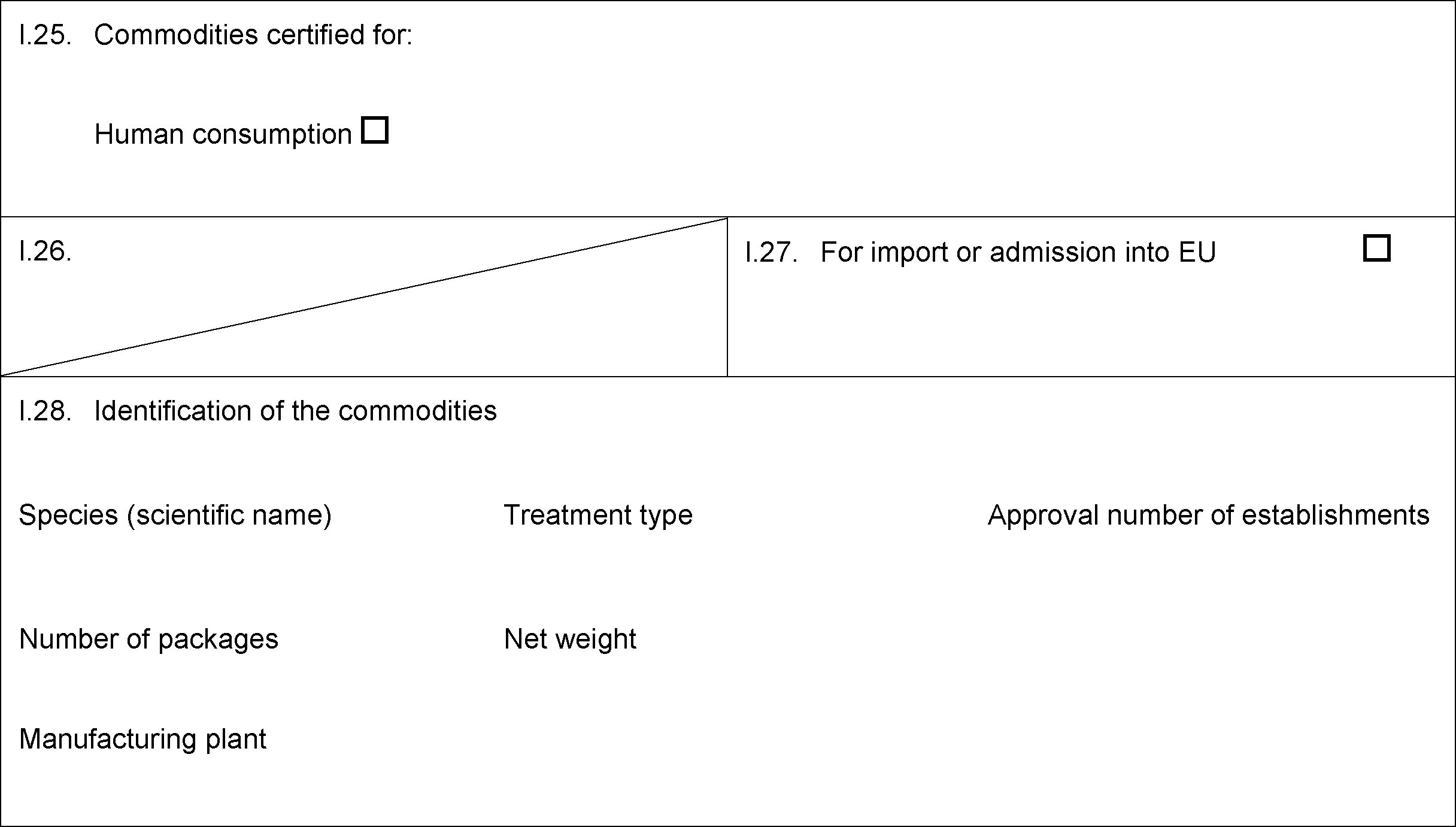

PART III
MODEL CERTIFICATE FOR IMPORTS OF GELATINE INTENDED FOR HUMAN CONSUMPTION
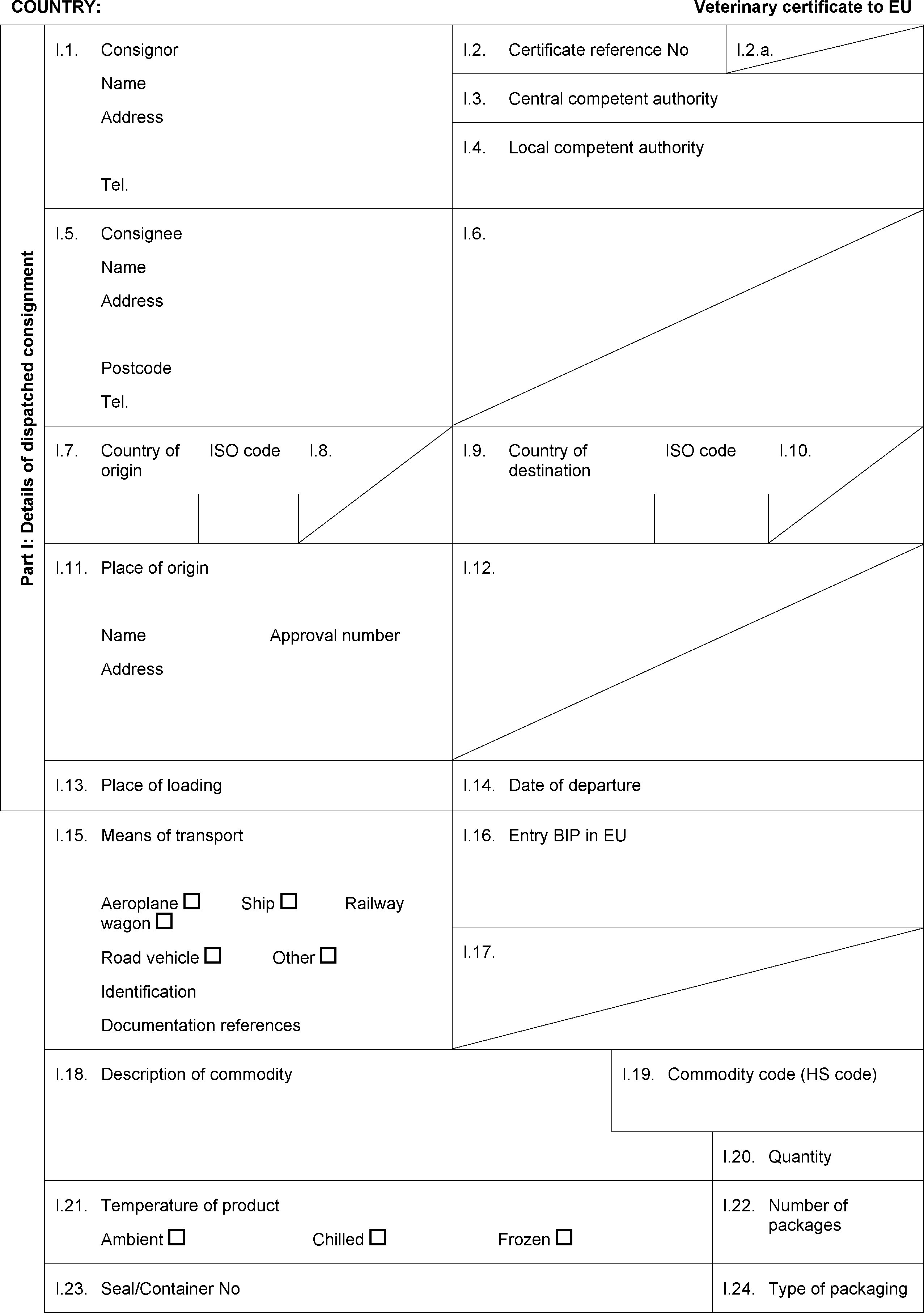




PART IV
MODEL CERTIFICATE FOR IMPORTS OF COLLAGEN INTENDED FOR HUMAN CONSUMPTION





PART V
MODEL CERTIFICATE FOR IMPORTS OF RAW MATERIALS FOR THE PRODUCTION OF GELATINE/COLLAGEN INTENDED FOR HUMAN CONSUMPTION ( 5 )
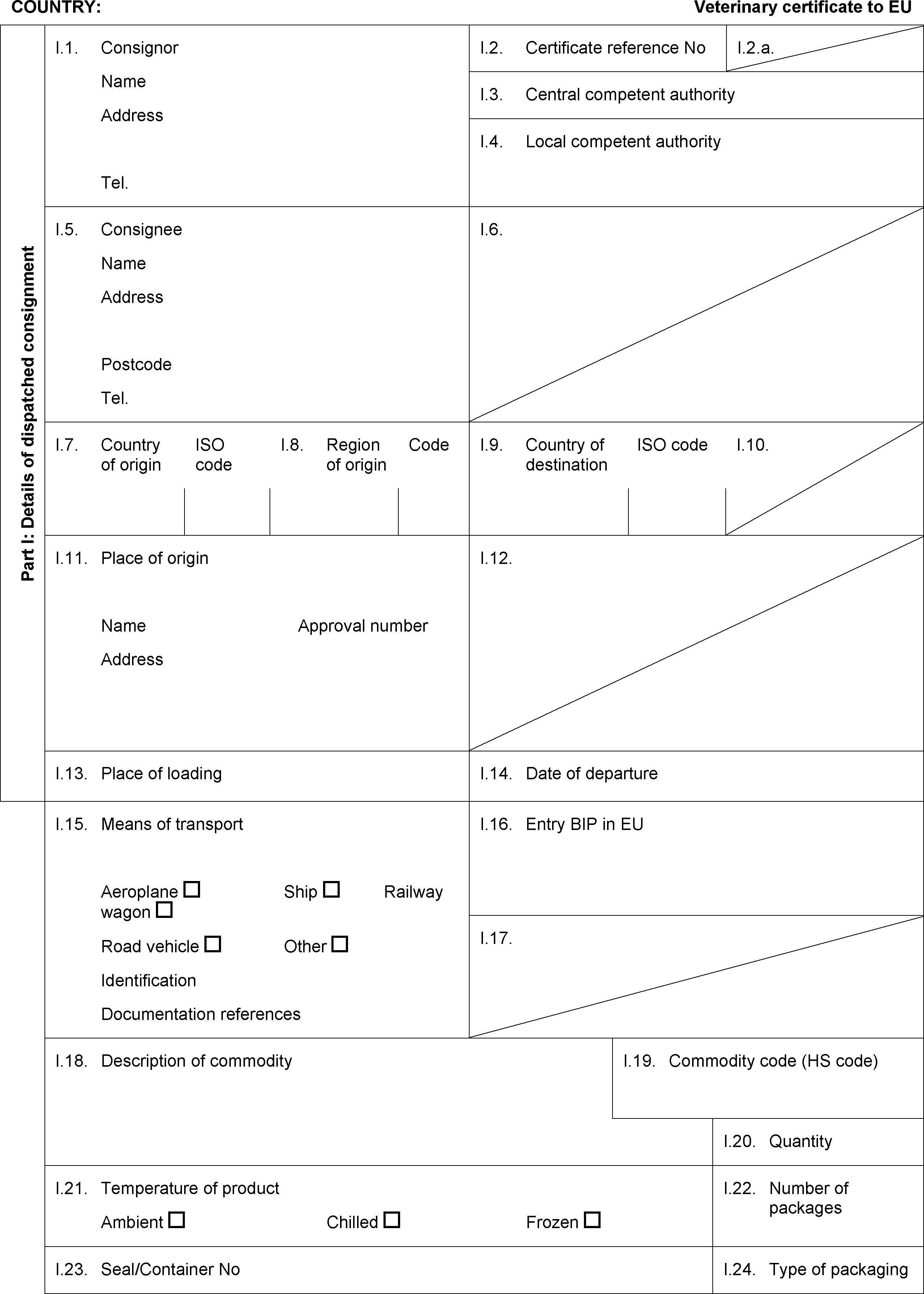
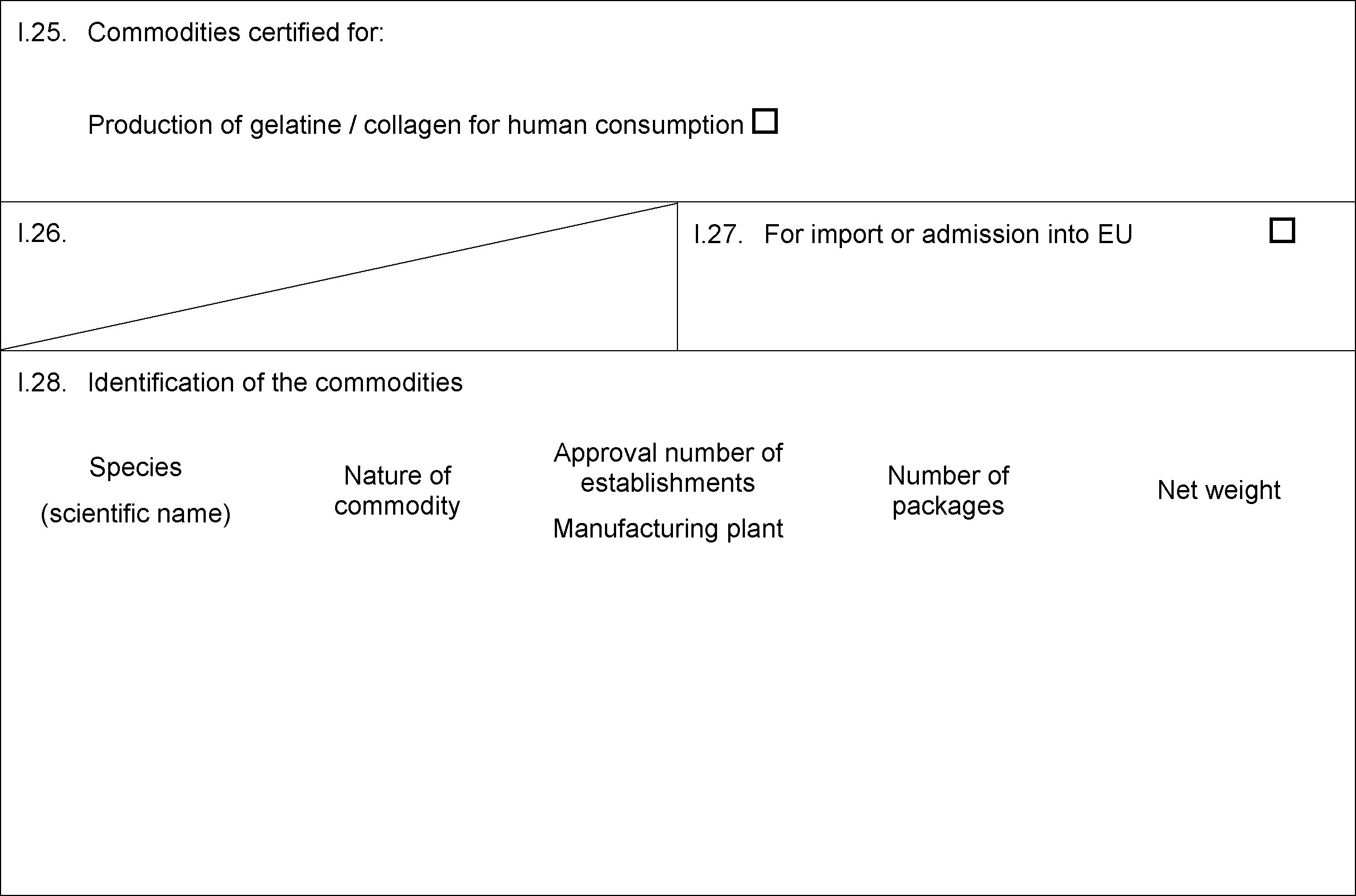





PART VI
MODEL CERTIFICATE FOR IMPORTS OF TREATED RAW MATERIALS FOR THE PRODUCTION OF GELATINE/COLLAGEN INTENDED FOR HUMAN CONSUMPTION
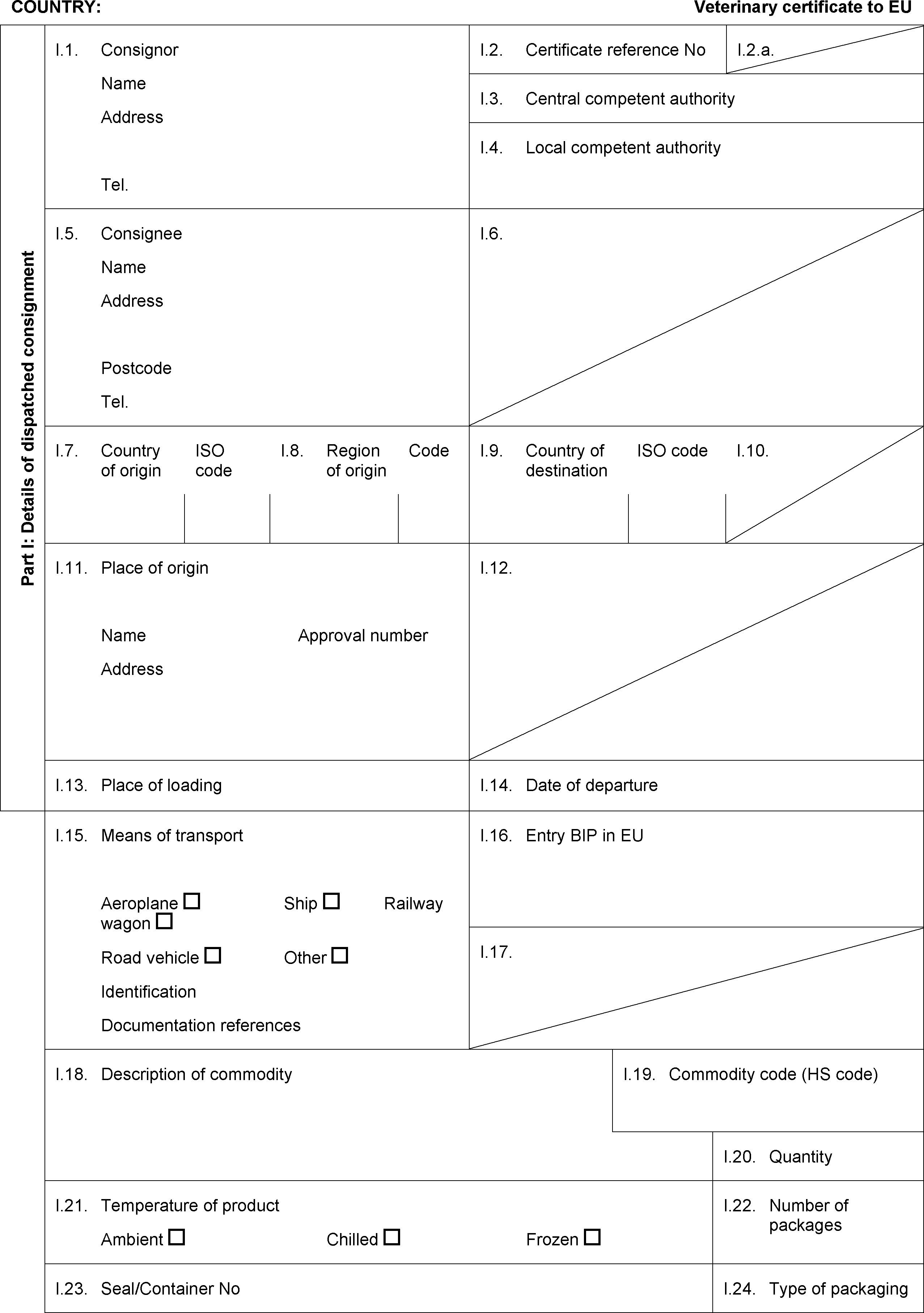

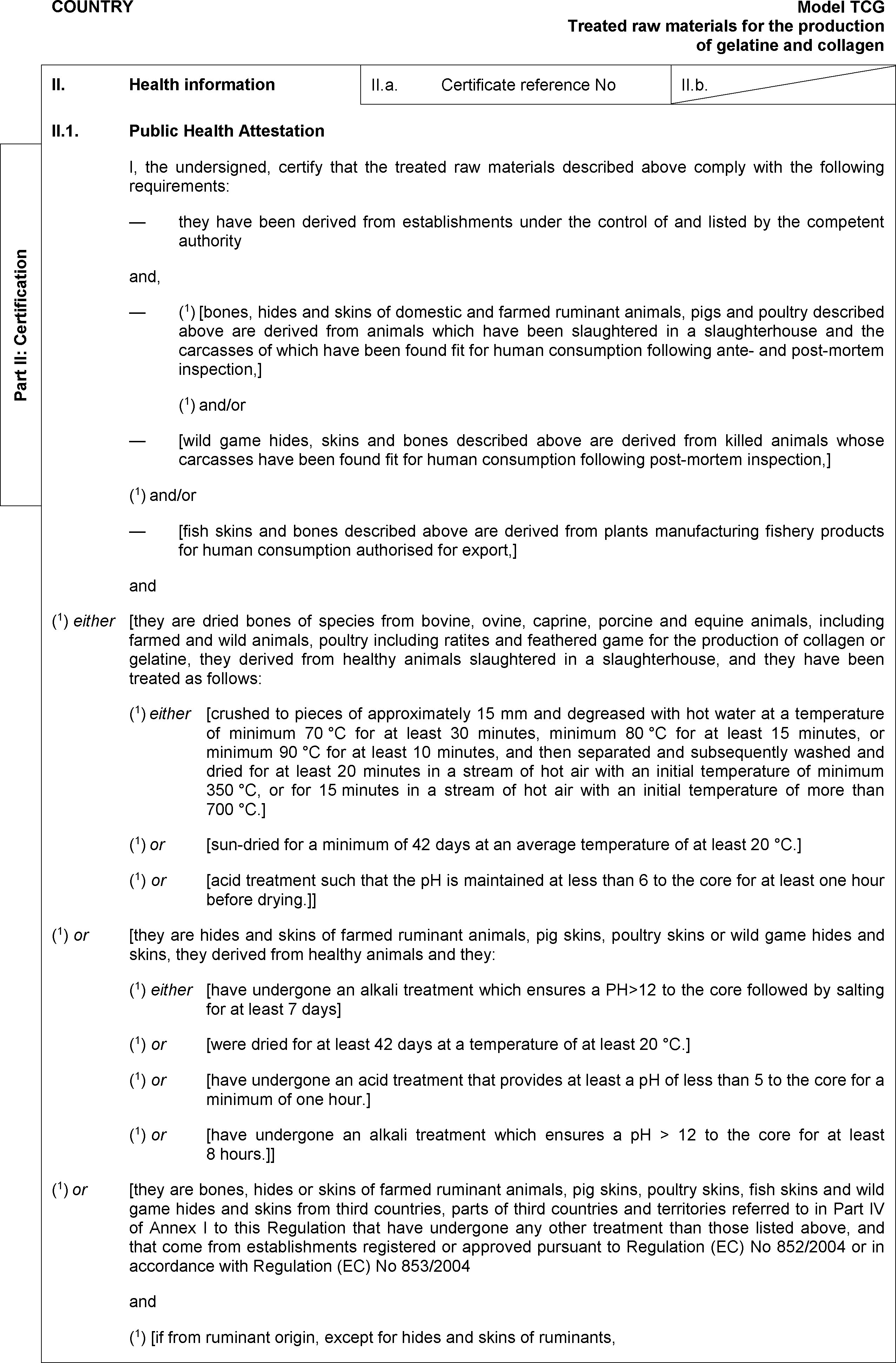



PART VII
MODEL CERTIFICATE FOR IMPORTS OF HONEY, ROYAL JELLY AND OTHER APICULTURE PRODUCTS INTENDED FOR HUMAN CONSUMPTION


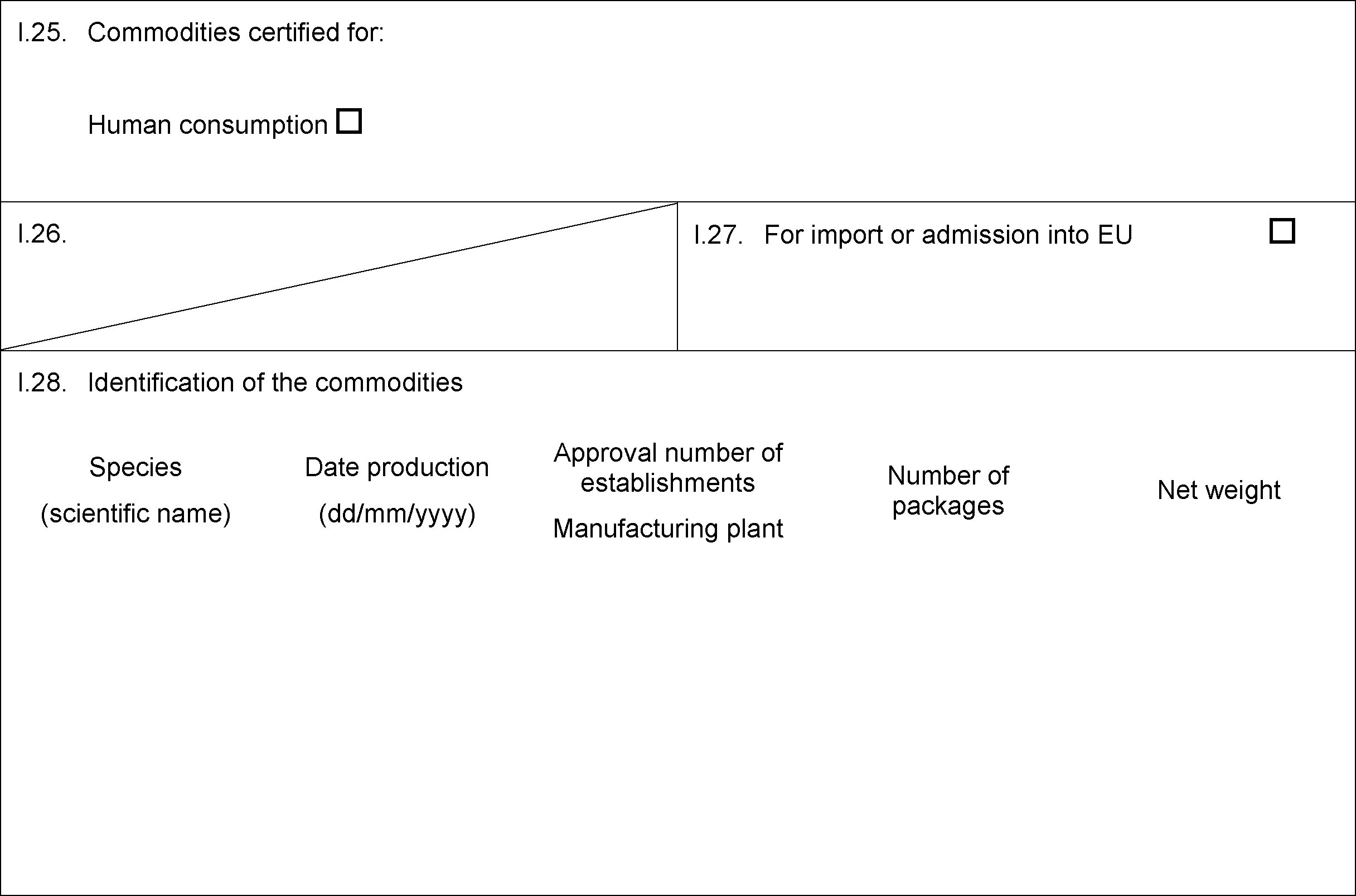
PART VIII
MODEL CERTIFICATE FOR IMPORTS OF HIGHLY REFINED CHONDROITIN SULPHATE, HYALURONIC ACID, OTHER HYDROLYSED CARTILAGE PRODUCTS, CHITOSAN, GLUCOSAMINE, RENNET, ISINGLASS AND AMINO ACIDS INTENDED FOR HUMAN CONSUMPTION


ANNEX III
MODEL CERTIFICATE FOR THE TRANSIT THROUGH THE UNION, IMMEDIATE TRANSIT OR AFTER STORAGE, FOR RAW MATERIALS OR TREATED RAW MATERIALS FOR THE PRODUCTION OF GELATINE/COLLAGEN FOR HUMAN CONSUMPTION

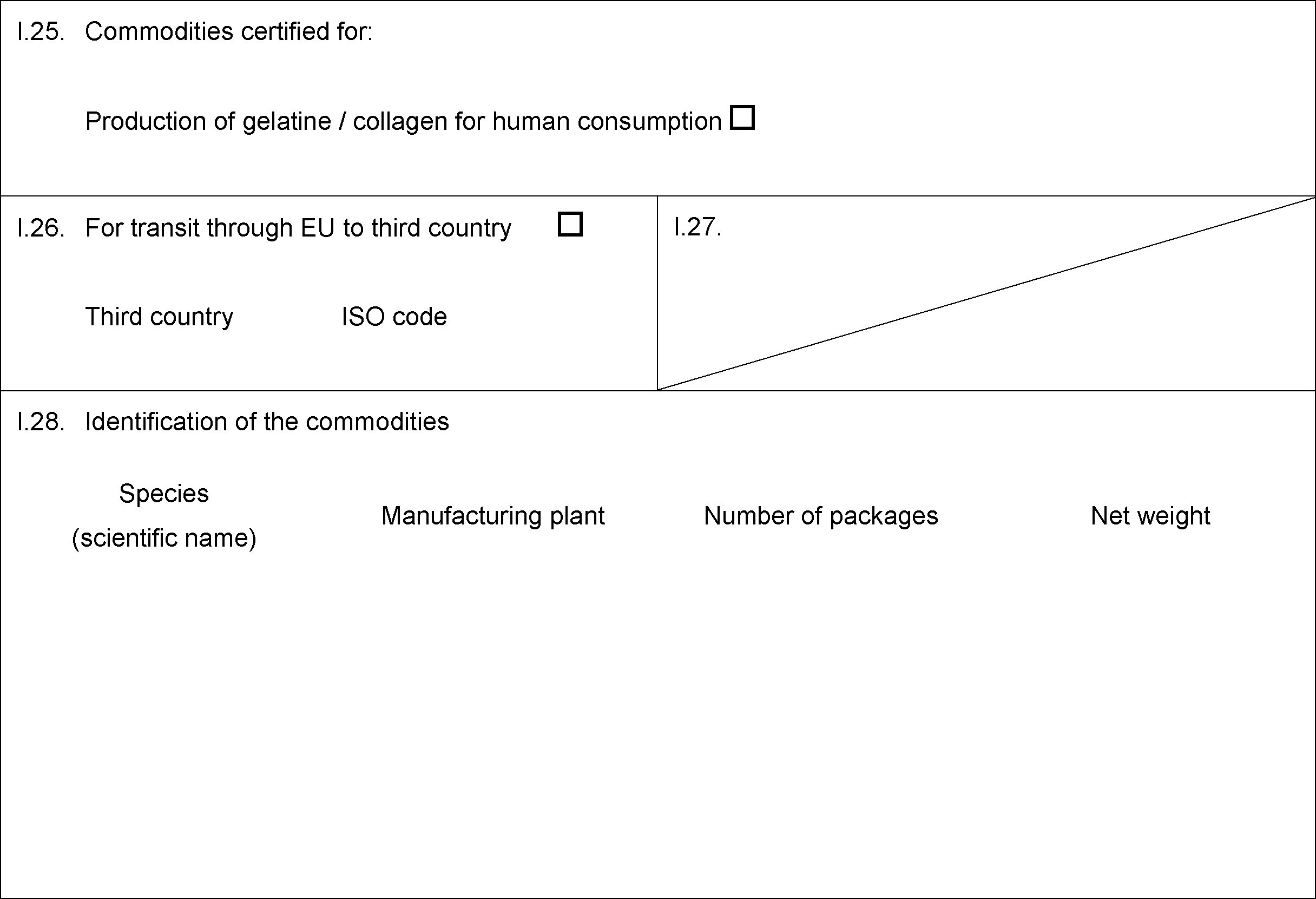


ANNEX IV
EXPLANATORY NOTES FOR COMPLETING THE CERTIFICATES
(referred to in Articles 2(1) and 4(1))
(a) Certificates shall be issued by the exporting third country, based on the models set out in Annexes II and III according to the layout of the model that corresponds to the products of animal origin concerned.
They shall contain, in the numbered order that appears in the model, the attestations that are required for any third country and, as the case may be, those supplementary guarantees that are required for the exporting third country or part thereof.
If the Member State of destination imposes, for the products of animal origin concerned, additional certification requirements, attestations to certify that those requirements are fulfilled shall also be incorporated in the original form of the certificate.
(b) Where the model certificate states that certain statements shall be kept as appropriate, statements which are not relevant, may be crossed out nd initialled and stamped by the certifying officer, or completely deleted from the certificate.
(c) A separate and unique certificate must be provided for the products of animal origin that are exported from a territory or territories or zone or zones of the same exporting country listed or referred to in Annex I which are consigned to the same destination and transported in the same railway wagon, lorry, aircraft or ship.
(d) The original of each certificate shall consist of a single sheet of paper, or, where more text is required it must be in such a form that all sheets of paper required are part of an integrated whole and indivisible.
(e) The certificate shall be drawn up in at least one of the official languages of the Member State of the border inspection post of entry of the consignment into the EU and of the Member State of destination. However, those Member States may authorise the certificate to be drawn up in the official language of another Member State, and accompanied, if necessary, by an official translation.
(f) If for reasons of identification of the items of the consignment (schedule in point I.28 of the model certificate), additional sheets of paper are attached to the certificate, those sheets of paper shall also be considered as forming part of the original of the certificate by the application of the signature and stamp of the certifying officer, on each of the pages.
(g) When the certificate, including additional sheets of paper referred to in (f), comprises more than one page, each page shall be numbered, (page number) of (total number of pages), at the end of the page and shall bear the certificate reference number that has been designated by the competent authority at the top of the pages.
(h) The original of the certificate must be completed and signed by an official veterinarian or by another designated official inspector where this is provided for in the model certificate. The competent authorities of the exporting third country shall ensure that rules of certification equivalent to those laid down in Council Directive 96/93/EC ( 6 ) are followed.
The colour of the signature shall be different from that of the printing. This requirement also applies to stamps other than those embossed or watermarked.
(i) The certificate reference number referred to in boxes I.2 and II.a. must be issued by the competent authority.
( 1 ) Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives (OJ L 354, 31.12.2008, p. 16).
( 2 ) Council Directive 97/78/EC of 18 December 1997 laying down the principles governing the organisation of veterinary checks on products entering the Community from third countries (OJ L 24, 30.1.1998, p. 9).
( 3 ) Commission Decision 2009/821/EC of 28 September 2009 drawing up a list of approved border inspection posts, laying down certain rules on the inspections carried out by Commission veterinary experts and laying down the veterinary units in Traces (OJ L 296, 12.11.2009, p. 1).
( 4 ) Commission Decision 2011/163/EU of 16 March 2011 on the approval of plans submitted by third countries in accordance with Article 29 of Council Directive 96/23/EC (OJ L 70, 17.3.2011, p. 40).
( 5 ) Unless covered by Part VI.
( 6 ) Council Directive 96/93/EC of 17 December 1996 on the certification of animals and animal products (OJ L 13, 16.1.1997, p. 28).


